Soft tissue coverage of lower extremity defects: pearls and pitfalls in the chronic wound population
Romina Deldar, Chamilka Merle, Christopher E.Attinger, Karen K.Evans
1Department of Plastic and Reconstructive Surgery, MedStar Georgetown University Hospital, Washington, DC 20007, USA.
2Department of General Surgery, MedStar Georgetown University Hospital, Washington, DC 20007, USA.
Abstract The incidence of chronic lower extremity (LE) wounds continues to increase.Lower limb amputations are associated with increased cardiovascular exertion, further decline in functional ability, and higher mortality rates.As such, there has been a shift towards limb salvage modalities.These include local debridement with advanced wound care, revascularization, bony reconstruction, and soft tissue reconstruction.Perioperative planning for soft tissue reconstruction requires careful consideration of several factors, including patient comorbidities, wound size and location, exposed underlying structures, and in the case of possible free flap, patency of donor and recipient vessels.This article reviews the perioperative factors that should be considered in preparation for successful soft tissue reconstruction of the LE.
Keywords: Free tissue transfer, chronic wounds, lower extremity, reconstruction
INTRODUCTION
Chronic wounds of the lower extremity (LE) can be defined as wounds that fail to heal within three months of onset.LE wounds are a relatively common condition, affecting 1% of the adult population and 3.6% of people older than age 65[1-3].This incidence continues to rise as a result of an aging population and increased atherosclerotic risk factors such as smoking, obesity, and diabetes mellitus (DM)[1].Diabetic foot ulcers (DFU) can cause a devastating impact on patient quality of life in terms of chronic pain, infection, decreased ambulation, social distress, and ultimately survival[4].
LE wound healing complications are a major concern in diabetic patients, with a lifetime incidence of DFU as high as 25%[5,6].Nearly 75% of all lower limb amputations are performed in diabetic patients[7].Following major LE amputation, 5-year mortality rates can reach 56.6%, which are higher than breast, colon, or prostate cancer[8].This is likely due to increased cardiovascular exertion, further decline in functional ability, and exacerbation of existing comorbidities[9].Moreover, major LE amputation increases the risk of contralateral amputation by up to 50% within two years[10-12].
Thus, recent advancements in the treatment of chronic LE wounds have focused on limb salvage modalities.These include local debridement, advanced wound care, revascularization, bony reconstruction, and soft tissue reconstruction.Planning for soft tissue reconstruction requires careful consideration of several factors, including patient comorbidities, size and location of the wound, exposed underlying structures, and in the case of the possible free flap, patency of donor and recipient vessels.This article aims to review the perioperative considerations, and local and free flap options that are essential to provide successful soft tissue coverage in the chronic LE wound population.
DISCUSSION
Preoperative optimization
Planning for soft tissue reconstruction requires optimization of patient comorbidities and wound bed preparation.This should entail a multidisciplinary collaboration amongst providers from different surgical and medical specialties.These include but are not limited to plastic surgery, orthopedic surgery, vascular surgery, podiatric surgery, internal medicine, endocrinology, cardiology, and hematology.
From a medical perspective, a thorough history detailing all pre-existing medical conditions should be obtained.In diabetic patients, tight glycemic control is essential.Patients with perioperative blood glucose levels greater than 200 mg/dL or hemoglobin A1c > 6.5% are more than three times likely to experience wound dehiscence[13,14].Close partnerships with endocrinologists and diabetes educators are important for long-term blood glucose maintenance.Our team’s goal is to maintain blood glucose levels perioperatively less than 200 mg/dL.The ideal perioperative HgbA1c is less than 7; however, if a patient’s HgbA1c is greater than 7, this is not a contraindication to free tissue transfer (FTT) because it may be falsely elevated perioperatively secondary to hyperglycemia from acute infection.Nutrition labs, including albumin and prealbumin, should also be obtained preoperatively as malnutrition has negative effects on the wound healing process[15,16].Hypoalbuminemia results in delayed tissue healing, reduced collagen synthesis, and a decrease in plasma colloid osmotic pressure, thereby causing tissue edema and leakage of interstitial fluid, which mediates bacterial propagation into wounds[15,17].Our institution previously found that in patients undergoing FTT, a preoperative albumin level less than 2.7 g/dL was associated with decreased flap healing outcomes[18].In general, a low prealbumin level (< 10 mg/dL) is associated with decreased free flap survival rate[19].
Smoking cessation can reduce the risk of impaired wound healing, infection, partial flap loss, and need for revision surgery[20].We encourage patients to refrain from tobacco use for at least four to eight weeks prior to reconstructive surgery; however, this may not be possible if the wound requires free flap immediately.Smoking is not a contraindication for free flap surgery in this population, but the patient must be educated that delayed healing is likely.In our practice, we also screen patients for inherited or acquired hypercoagulable traits that may predispose them to thrombosis.A hypercoagulable workup is a subject of debate among microsurgeons and is not routinely performed in many centers.Underlying hypercoagulability can lead to microvascular thrombosis and subsequent flap failure with high rates of nonsalvageability[21-23].In 2015, our institution implemented a risk-stratified algorithm for perioperative anticoagulation, whereby administration of heparin (subcutaneous or intravenous) following FTT is determined by the presence of thrombophilic risk factors for microvascular thrombosis[22].Prior to undergoing FTT, high-risk patients undergo a hypercoagulable workup, including a thrombophilia panel and thorough history taking, to assess for any personal or family history of thromboembolism.Table 1 outlines the components of a thorough thrombophilia screening panel.Our group previously reported that 61% of patients undergoing FTT to LE reconstruction had at least one hypercoagulable trait; the most common traits were plasminogen activator inhibitor-1 4G/5G variant and methylenetetrahydrofolate reductase (MTHFR) A1298C and C677T polymorphisms[23].In addition, in patients with documented thrombophilia, the use of a weight-based heparin drip titrated to a goal partial thromboplastin (PTT) of 50-70 s decreased flap failure from 19% to 3%[22].

Table 1.Thrombophilia screening panel
Wound bed preparation
From a surgical perspective, aggressive debridement and wound bed preparation is the first step in wound management.Serial excisional debridements are required until all infected or devitalized tissue and biofilm have been removed[24,25][Figure 1].Wide excision to a healthy wound bed is necessary, as there is up to a six-fold increase in amputation if the wound is closed with positive post-debridement cultures among diabetic patients undergoing LE FTT[26].Poor host defenses, high glucose levels, and periwound blood supply likely amplify the effect of residual bacterial colonization on the ultimate reconstructive outcome[27].
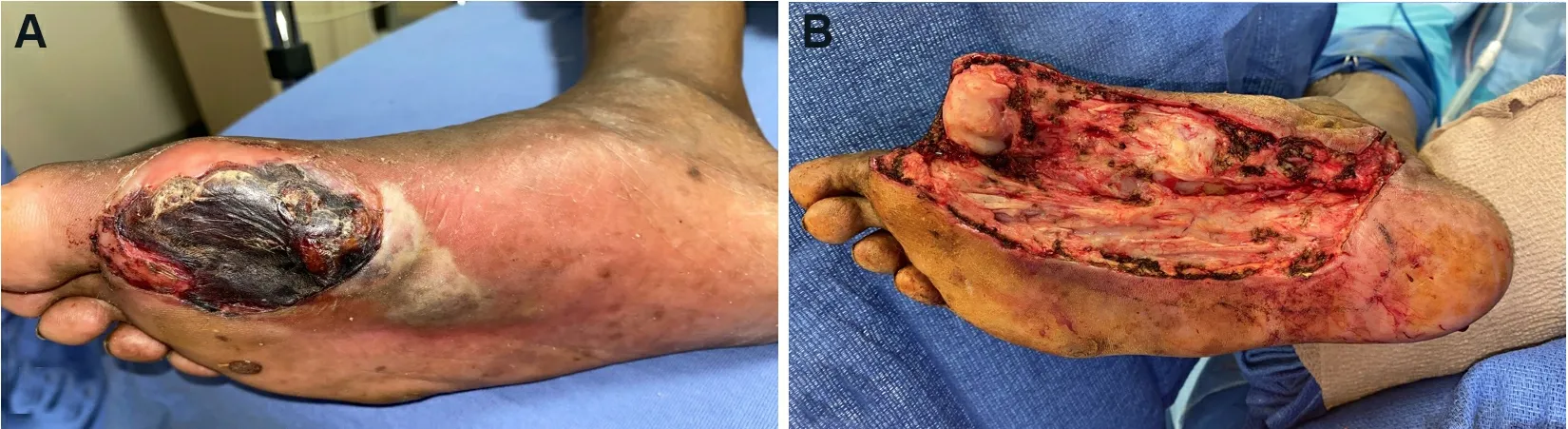
Figure 1.Wound bed preparation.(A) Pre-debridement photo: poorly-controlled diabetic male with systemic signs of sepsis and necrotizing soft tissue infection.Patient was taken urgently to the operating room for debridement.(B) Post-debridement photo: all necrotic debris was removed.The purulence tracked into the tarsal tunnel, which required incision and drainage.Patient underwent serial debridements until negative cultures, and ultimately required free tissue transfer for limb salvage.
Aerobic and anaerobic cultures are obtained deep to the wound surface before and after each debridement.Infectious disease (ID) specialists are consulted to assist in culture-driven antibiotic therapy.Antibiotics should be continued until cultures are negative; at this time, a patient is deemed ready for soft tissue reconstruction from an ID standpoint.
In addition, in our operating rooms, we use a two-table setup of sterile instruments in the operating room, including glove exchange to reduce instrument cross-contamination during excisional debridement and coverage or closure of infected wounds[28].Using a two-table setup, we found a 78% absolute risk reduction in cross-contamination[28].Therefore, it is critical to avoid recontamination of a surgically debrided wound as reinfection could lead to devastating sequelae.
Biomechanical principles
Prior to wound closure, patients should undergo biomechanical examination and gait analysis with podiatric and/or orthopedic surgery to identify mechanical factors that may contribute to wound development or recurrence if not addressed.Any foot or ankle deformity, in conjunction with diabetic neuropathy, can increase the risk of DFU development[29,30].Peripheral neuropathy is characterized by loss of protective sensation and proprioception, muscle weakness, and imbalance; all of these can contribute to increased plantar pressures during gait[31-34].Contracture of the Achilles tendon causes limited joint mobility at the ankle, defined as equinus deformity.This has been implicated as a major factor in the development of midfoot Charcot collapse and plantar ulcer formation[34-37].We routinely address equinus gait with Achilles tendon lengthening, which has been shown to reduce DFU recurrence by up to 95% in this population[38].Biomechanical foot and ankle stabilization may require surgical intervention, including tendon transfers, minor bony amputation, or Charcot realignment and stabilization.Calcaneal gait, characterized by increased ankle dorsiflexion during midstance, is another manifestation of Charcot foot[39].Calcaneal ulcers are difficult to heal and usually require surgery to correct the deformity causing the ulcer[40].
Vascular workup
The prevalence of DM and peripheral vascular disease (PVD) is high in patients that suffer from chronic LE wounds[41-43].Decreased perfusion in the LE results in impaired wound healing, which can lead to repeat ulceration, infection, and necrosis[41,44].The same pathology that impairs perfusion needed for wound healing also creates barriers for successful FTT, as hypoperfusion is the primary predictor of flap failure[41,45].Venous reflux causing congestion and delayed venous thrombosis is another leading cause of flap failure[46,47].Therefore, it is imperative that vascular studies to assess the arterial and venous systems are obtained prior to free flap planning to mitigate any inflow or outflow issues that could arise.
In our practice, preoperative vascular workup includes an angiogram and venous duplex ultrasound.Angiogram is performed to evaluate the arterial system of the affected limb as well as to optimize recipient vessel selection for the free flap[41].This allows for the identification of any diseased vessels as well as a direct intervention using balloon angioplasty or stenting.Angiogram is preferred over computed tomography angiography because the dye load is much less, and thus, there is a lower risk for acute kidney injury.Janhoferet al.[41]evaluated routine preoperative angiograms completed on patients undergoing LE FTT and found abnormalities in 67.8% of patients, which included vessel stenosis, occlusion, and non-visualization; 27.5% of patients required some form of vascular intervention, and of these patients, 15.3% were newly diagnosed with PVD.Venous duplex can help identify venous insufficiency and pre-existing deep vein thrombosis (DVT).The ideal recipient vein can be determined preoperatively based on reflux studies and high venous pressure.If high venous reflux is detected, a different recipient venous system with less disease burden can be chosen[46].Our group has previously reported that venous insufficiency (defined as < 0.5 s of reflux) was detected in 39% of patients, and DVT was found in 6.78% of patients undergoing FTT[46].Identification of both arterial and venous disease prior to FTT allows for optimization of recipient arterial and venous selection to yield free flap success.
Angiosomes of the foot and ankle
Taylor and Palmer[48]introduced the angiosome concept, separating the body into distinct threedimensional blocks of tissue and overlying skin fed by “source” arteries.The senior authors (Attinger CE and Evans KK) described six angiosomes of the foot and ankle that originate from three main arteries[49][Figure 2].Blood flow to the foot and ankle is redundant because of the multiple arterial-arterial connections between the three main arteries[49].
Application of the angiosome theory is important in reconstructive success of the foot and ankle[50].Knowledge of the vascular anatomy of the foot and ankle can guide the effective revascularization of occluded arteries[49,51].Incisions in the foot and ankle should be made between angiosome boundaries to limit perfusion compromise.Detailed descriptions of the vascular anatomy and angiosomes of the lower leg, foot, and ankle have been explained elsewhere[50,52-54].
Local flaps for LE reconstruction
In general, flap coverage is the reconstructive choice for wounds with exposed tendon, joint, or bone[55].The primary goal of the lower leg, foot, and ankle reconstruction is to preserve function, centered on ambulation.Local flaps can improve blood flow to the defect and provide a surface for subsequent skin grafting[50].Historically, the gastrocnemius and soleus muscle flaps have provided dependable coverage of lower limb defects[56,57].The gastrocnemius local flap with skin graft has been widely used for reconstruction of knee and proximal leg defects [Figure 3].The soleus muscle flap can provide reliable soft-tissue coverage of middle and lower leg defects[57,58].In select patients, the medial sural artery perforator (MSAP) flap is useful as a pedicled or free flap to cover knee or leg defects.It is based on perforators from the medial sural artery, which are dissected through the gastrocnemius muscle[59][Figure 4].
Fasciocutaneous flaps can also be used for coverage of the knee, leg, and posterior heel.The reverse sural artery flap offers coverage of distal leg and heel defects when microsurgery is not feasible; however, venous congestion and distal flap loss are common complications[60][Figure 5].Perforator-based local flaps, such as propeller or keystone flaps, have expanded the repertoire for lower limb reconstruction.Figure 6 depicts a saphenous artery perforator-based rotation advancement flap.Moreover, the perforator-plus flap, a modification of the classic perforator-based fasciocutaneous flap, offers dual blood supply to the flap from the dissected perforator plus the retained cutaneous base[61].By keeping the base of the flap attached, the subdermal plexus augments arterial inflow, improves venous outflow, and shortens the arc of flap rotation[62].AlMugarenet al.[56]recently published an algorithm for the reconstruction of LE defects.Disadvantages of local flaps include limited bulk and reach[55].
Local flaps for foot reconstruction
Local muscle flaps can be used for coverage of small foot defects (3 cm × 6 cm or less) that are within reach of the local flap[55].At our center, we primarily use three intrinsic foot muscles for coverage of diabetic foot wounds: (1) abductor hallucis (AH); (2) abductor digiti minimi (ADM); and (3) flexor digitorum brevis (FDB) [Figures 7-9].To harvest each of these flaps, the incisions are made along the border of adjacent angiosomes.Thus, the soft tissue on either side of the donor site incision should be well-vascularized to allow the wound edges at the donor site to heal primarily[55].We use the AH flap to cover wounds on the medial midfoot, ankle, and heel, and the ADM flap to cover lateral ankle and foot[55].The FDB flap can be used for calcaneal defects.Limitations of local pedicled flaps include limited bulk and reach.Preoperative planning should ensure that the rotated muscle will sufficiently definitively cover exposed joint, tendon, or bone; any uncovered granulating tissue can be covered by skin grafts or biologic wound matrices[55].
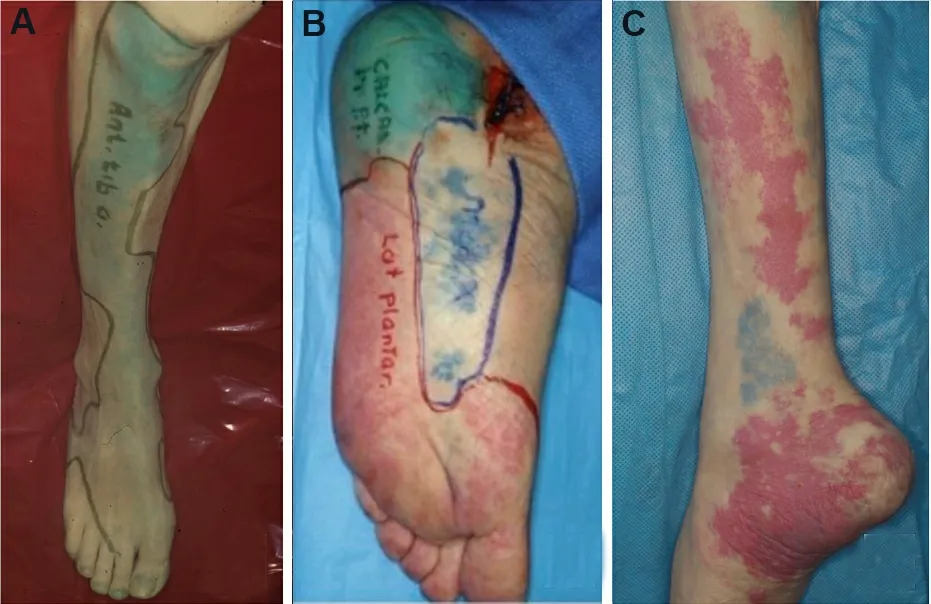
Figure 2.Angiosomes of the foot and ankle.(A) Anterior tibial angiosome: supplies the anterior ankle and continues as the dorsalis pedis artery to supply the dorsal foot.(B) Posterior tibial angiosome: supplies the plantar foot via 3 branches: calcaneal, medial plantar, and lateral plantar arteries.(C) Peroneal angiosome supplies the anterolateral portion of the ankle and hindfoot via 2 branches: lateral calcaneal and anterior perforator arteries.Figure adapted with permission from Ref.[49].
Free flaps for LE reconstruction
Because of continued advances in microsurgery, FTT has become a reliable reconstructive solution, with high rates of flap success and limb salvage[63,64].Large wounds in the distal third of the leg, ankle, and foot often require free tissue reconstruction[65].There are three main considerations to guide the choice of optimal free flap for LE defect: (1) flap composition; (2) functional and aesthetic outcomes; and (3) donor site morbidity.There are four flap compositions that can be used for most LE reconstructions: fasciocutaneous, muscle only, musculocutaneous, or chimeric[66].Hollenbecket al.[67]described the subunit principle of the foot, which provides a framework to determine which free flap will lend the best functional and aesthetic outcome in a given area of the foot or ankle.
At our institution, we most commonly use flaps from the descending branch of the lateral circumflex artery [i.e., the anterolateral thigh (ALT) or vastus lateralis (VL) flaps] for LE reconstruction[50][Figure 10].These workhorse flaps for LE reconstruction offer low donor site morbidity and long pedicles that are large in diameter[50,68].However, the bulk offered by these flaps is not ideal for the dorsal surface of the foot, which requires coverage with thinner tissue so the foot can still fit into a shoe.In these cases, flaps with thinner paddles are preferred: the radial forearm flap, MSAP flap, or superior circumflex iliac artery perforator (SCIP) flap are reasonable options[50,59][Figure 11].Limitations of the SCIP flap include a short, small caliber pedicle, while the MSAP flap requires tedious muscle dissection and susceptibility to vein damage given the larger caliber of tributaries[65,69].The gracilis flap is another thigh-based option, but its short pedicle length often limits its usefulness [Figure 12].
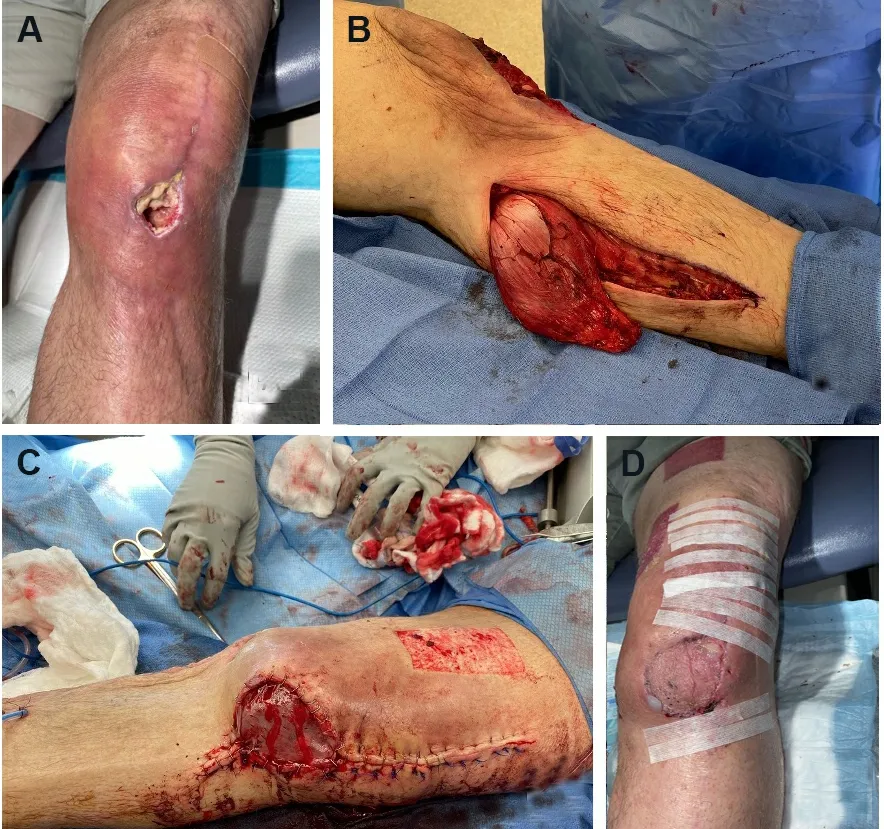
Figure 3.Gastrocnemius flap.(A) Preoperative photo of a diabetic male who was four weeks s/p total knee arthroplasty with dehiscence and drainage at the left knee incision.(B) The medial gastrocnemius flap was harvested and rotated to cover the knee defect.(C) Flap inset into the knee defect.A split-thickness skin graft (STSG) was harvested from the medial thigh and placed over the flap.(D) Four weeks after flap with STSG.

Figure 4.Medial sural artery perforator (MSAP) flap.(A) The MSAP flap spares the gastrocnemius muscle and can be used as a pedicled or free flap.(B) The MSAP flap dissected out.

Figure 5.Reversed sural artery flap.(A) Initial presentation of calcaneal wound, flap was designed based on sural artery on posterior leg.(B) Dissection and elevation of the flap.(C) Rotation of the flap towards the calcaneal wound.(D) Healed flap; superior and inferior aspects of the donor site were covered with split-thickness skin graft, and the remainder was closed primarily.
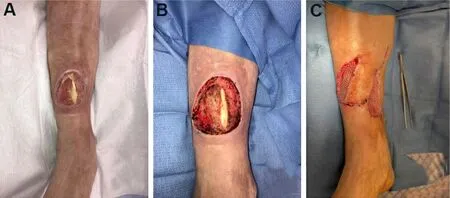
Figure 6.Saphenous artery perforator propeller flap.(A) Initial presentation of circular wound over distal anterior leg with exposed anterior tibial tendon.(B) Wound after excisional debridement.(C) Flap was inset, and a split-thickness skin graft was placed over the lateral portion of the wound where tendon was not exposed and the medial defect where the flap was harvested.
Lastly, donor site morbidity is an important aspect to consider during free flap planning.Our group has found that donor site morbidity is lowest for ALT and VL flaps, especially when utilizing partial harvest of the VL tailored to the defect[9].Alternatives such as the latissimus dorsi or free rectus flap are less preferred as they maintain upper body strength, should future amputation be necessary.Purnellet al.[70]retrospectively reviewed 352 thigh-based flap donor sites and found similar donor site complications in both lateral and medial thigh-based flap harvest.Furthermore, they suggested that the inclusion of muscle does not increase complications, but the inclusion of a skin paddle with gracilis muscle or skin grafted lateral thigh donor site resulted in increased wound healing complications[70].
Microvascular anastomotic techniques
A reliable anastomotic technique during microsurgical reconstruction is vital for free flap success.Patients with LE wounds are often vasculopaths with fragile, fibrotic, and stenotic arteries.End-to-side (ETS) anastomosis is important in this patient population to preserve distal flow to the extremity [Figure 13].Our preferred approach is a longitudinal slit arteriotomy ETS microvascular anastomosis[43].ETS anastomosis ensures laminar flow and may even have higher flow rates than end-to-end anastomosis[43,71,72].The longitudinal slit technique allows for minimal intimal disruption and controls vessel mismatch[43,71,73].After flap harvest, a 70-degree bevel is created on the flap artery to obtain a final 20-degree resting angle.An ophthalmic blade can be used.The recipient vessel is examined carefully for an area of least calcification, and proximal and distal control is obtained.A single longitudinal straight-line incision is made to the length of the beveled flap artery.It is critical to make the slit arteriotomy in a smooth fashion to prevent intimal flap formation.The vessels are then hand-sewn ETS using 8-0 or 9-0 nylon suture from outside-to-inside fashion on the flap vessel and an inside-to-outside fashion on the recipient vessel to allow for intimal tacking.Our center showed 93% flap success and a limb salvage rate of 83.5% with longitudinal slit arteriotomy ETS anastomosis[43].When feasible, dual venous anastomoses to the deep or superficial venous system are performed with a GEM microvascular coupler (Synovis Micro Companies, Alliance, Inc., Birmingham, AL).In the absence of high-quality deep-system veins (i.e., pre-existing thrombosis, inadequate caliber, and/or scarring), the saphenous vein can be used.Intraoperatively, an implantable Cook Doppler probe (Cook Medical, Bloomington, IN) can be placed on the venous pedicle to monitor the patency of the anastomosis[74].
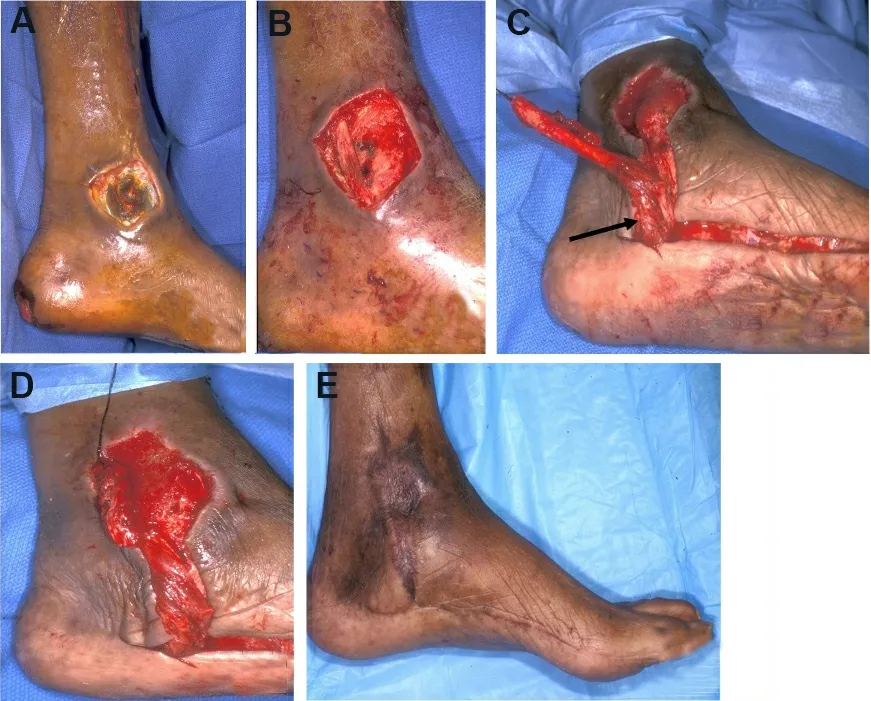
Figure 7.Abductor hallucis (AH) local flap.(A) Initial presentation of a medial ankle wound; depth is down to bone.(B) Wound after debridement.(C) After dissection of the AH muscle, it was released from its insertion point distally; arrow points to the dominant vascular pedicle (medial plantar artery).(D) Flap inset.(E) Results at follow-up.The skin graft and donor site incisions were wellhealed.The muscle flap provided adequate bulk over the bony heel defect.

Figure 8.Abductor digiti minimi (ADM) local flap.(A) Initial presentation of osteomyelitis of the lateral calcaneus; purple line marks where incision was performed.(B) After dissection of the ADM muscle, it was released from its insertion point distally; arrow points to the dominant vascular pedicle (lateral plantar artery).(C) Flap inset.(D) The wound and donor site incisions were closed primarily and healed well.

Figure 9.Flexor digitorum brevis (FDM) local flap.(A) Initial presentation of plantar wound.(B) Dissection of the FDM muscle; arrow points to the dominant vascular pedicle (lateral plantar artery).(C) Flap inset.(D) The wound and donor site incisions were closed primarily and healed well.
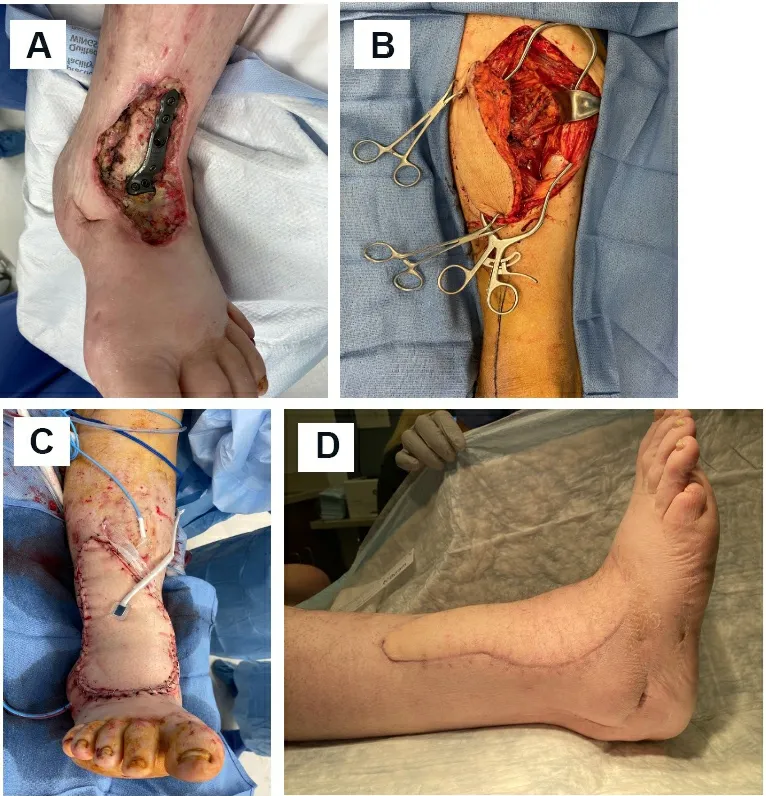
Figure 10.Anterolateral thigh (ALT) free flap.(A) large non-healing wound with exposed hardware and bone over anterior ankle.(B) Harvest of ALT flap from the ipsilateral thigh.(C) Flap inset over the wound.(D) Healed flap.
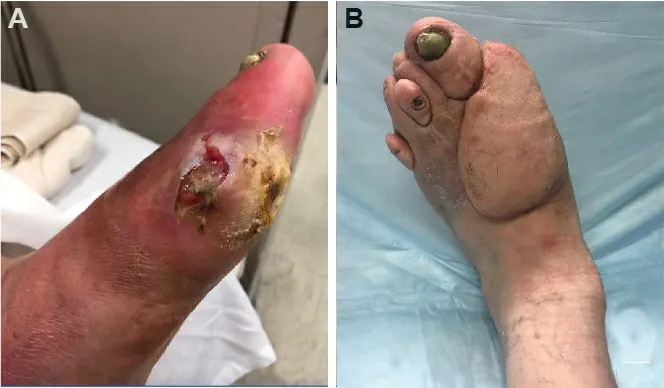
Figure 11.Superior circumflex iliac artery perforator (SCIP) free flap.(A) Diabetic foot ulcer of the left hallux with exposed first metatarsal joint.(B) Healed SCIP flap several months after surgery.

Figure 12.Gracilis free flap.(A) Initial presentation of medial ankle wound.(B) Gracilis free flap was marked out preoperatively.(C) Flap inset with an overlying split-thickness skin graft.(D) Healed flap one year after surgery.
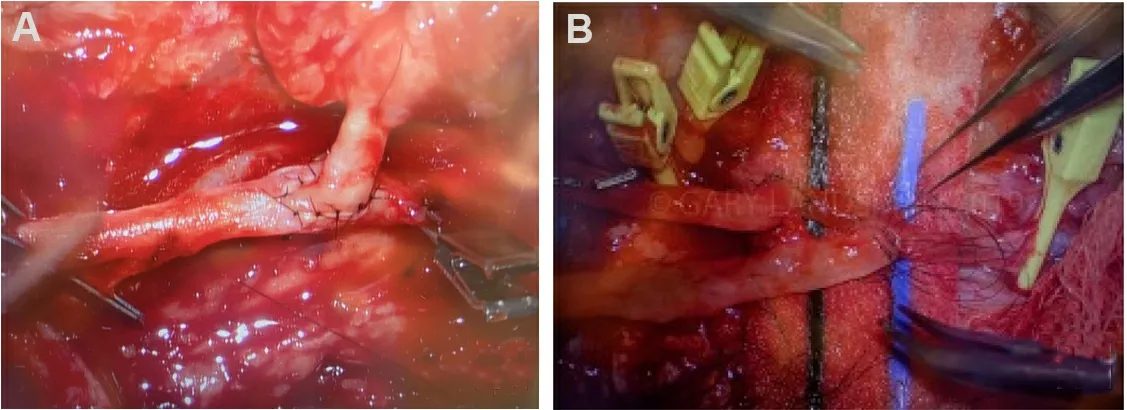
Figure 13.End-to-side arterial anastomosis.(A, B) end-to-side microvascular arterial anastomosis.
Free flap LE reconstruction in comorbid patients
Many patients who present with chronic LE wounds have underlying comorbidities that affect their vasculature, such as DM and PVD.DM was previously regarded as a relative contraindication to LE free flap reconstruction; however, multiple studies have shown successful microvascular FTT in diabetic patients[5,10,63,75,76].Nevertheless, these patients present a unique challenge for free flap reconstruction because the vessels available for anastomosis are often fragile, fibrotic, and stenotic as a result of infection, severe calcification, and comorbid atherosclerosis[50,77].The calcified arteries increase the risk of intimal dissection during microsurgical anastomosis, which can lead to vessel thrombosis and other complications[78].
Additionally, diabetic patients often have concomitant PVD, which may hinder the number of recipient vessels available for anastomosis.FTT should be considered for LE defects located in ischemic angiosome regions with minimal in-line blood flow.In these instances, performing ETS anastomosis during FTT can serve as a vascular bypass and provide “indirect extremity revascularization”[79].In instances when both the flap and recipient arteries are calcified, the senior author (Evans KK) performs ETS saphenous interposition vein graft, which decreases the risk of intimal disruption[43,50,80].Vascular instruments, such as Debakey or Satinsky clamps, are sometimes used to attain proximal and distal control in these vessels as the usual microvascular bulldog clamps are not strong enough to occlude the vessels if there is hardened calcium present.A specialized hardened cardiac needle is also needed to penetrate the calcium to perform microanastomosis [Figure 14].
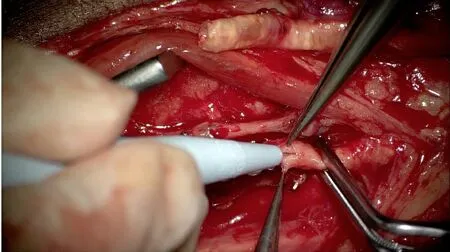
Figure 14.Calcified flap and recipient arteries.Calcified descending branch of the lateral circumflex artery (top of photo) and posterior tibial artery.Satinsky clamps were used to attain proximal and distal control on the artery.Calcifications are seen in the lumen of both arteries.
Postoperative flap care
After minimal tension closure with nonabsorbable suture and staples, a nonadherent dressing is applied to the flap.A bulky padded dressing is applied to minimize pressure and shear on the flap.An external fixator can be applied for the purpose of postoperative offloading.All flaps should be carefully monitored after surgery.The flap should be routinely examined for color or temperature changes and signs of infection.Darkening of the flap may indicate venous congestion, whereas pale color or coolness to the touch may signal a decrease in arterial flow to the flap.A handheld Doppler is useful for examining possible arterial compromise.In the setting of free flaps, a high index of suspicion for possible flap compromise is critical, as a prompt surgical intervention to interrogate the anastomosis can salvage the flap.Hyperbaric oxygen therapy is a useful adjunct in settings of compromised flap vascularity.It is critical that patients remain nonweight bearing (NWB) on the operative limb.NWB is usually recommended for 4-6 weeks following surgery, and gradual weight bearing begins after the incisions have healed.Physical therapy is required to improve strength and conditioning.
CONCLUSION
Soft tissue reconstruction of LE defects should be centered on attaining optimal functional outcomes.Flap coverage is the reconstructive modality of choice for defects with exposed tendons, joints, or bones.Local flaps are viable options for small defects that are within reach.Free flaps have become a mainstay in LE reconstruction with high rates of flap success and long-term limb salvage.Preoperative optimization, a multidisciplinary team approach, and adequate postoperative care are crucial.FTT reconstruction is not “one size fits all”; careful consideration of patient comorbidities, donor site morbidity, and functional and aesthetic outcome are of utmost importance when choosing flap type.
DECLARATIONS
Authors’ contributions
Designed and wrote majority of the manuscript: Deldar R Contributed to writing manuscript: Merle C
Edited the manuscript: Attinger CE Provided main conceptual design of manuscript and edited the manuscript: Evans KK
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was approved by institutional review board.IRB Study 0007611 entitled “MedStar Plastic Reconstructive Surgery Outcomes Registry” was approved by MedStar Health Research Institute.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
 Plastic and Aesthetic Research2022年2期
Plastic and Aesthetic Research2022年2期
- Plastic and Aesthetic Research的其它文章
- Experimental assessment of regenerative properties of platelet rich plasma on the human skin - a review
- AUTHOR INSTRUCTIONS
- Management of urethral strictures after masculinizing genital surgery in transgender men
- Epispadias: recent techniques
- Partial auricular reconstruction surgery for facial skin cancer
- Parastomal hernia repair
