The role of radiotherapy in metastatic bladder cancer
Jananie Perera, Peter Hoskin,2
1Mount Vernon Cancer Centre, Rickmansworth Road, Northwood, Middlesex HA6 2RN, UK.
2Division of Cancer Sciences, University of Manchester, Manchester M20 4GJ, UK.
Abstract The management of metastatic bladder cancer is palliative.Best outcomes are achieved in those who are fit enough for systemic therapies.The place of radiotherapy in these patients is mainly for symptom control, in particular haematuria.However, a small proportion, especially those with oligometastases, will benefit from more radical treatment.In this review, we look at the evidence currently available for radiotherapy in this setting.
Keywords: Bladder cancer, radiotherapy, oligo metastases, haematuria, bone metastases, brain metastases
INTRODUCTION
Bladder cancer is the ninth most common cancer in the world[1,2].The main histological subtype is urothelial carcinoma.It is a disease of the elderly and tobacco smokers with both of these impacting the ultimate prognosis.Around one-fifth will present with metastatic disease, and only about one-third of these will survive over a year[3].In addition, half of those with muscle invasive disease treated with curative intent eventually relapse with nearly 50% of them developing distant metastasis[4,5].
The management of metastatic bladder cancer is palliative.Prognosis is poor even in those fit enough for treatment.The treatment of choice remains platinum-based chemotherapy with a weak recommendationfor first line immunotherapy for those not fit enough for cytotoxic chemotherapy[6].Cisplatin-based chemotherapy achieves a median overall survival (OS) of up to 15 months[7].The addition of maintenance immunotherapy has the potential to increase the median overall survival to 21.4 months[8,9].In the UK, some form of radiotherapy is used in almost 30% of those with metastatic disease within 12 months of diagnosis[3].
Long-term follow-up data from palliative chemotherapy trials have shown a 10%-20% 5 year survival suggesting the existence of a subgroup with a good prognosis[7,10].In addition, several surgical series have shown survival benefit with metastasectomy in both synchronous and metachronous settings[11-16].There is also limited evidence of the benefit with ablative radiotherapy in selected patients with oligometastases.
The role of radiotherapy remains mainly palliative.There is limited evidence for its use in consolidative therapy post-chemotherapy and more radical ablative treatments for oligometastatic disease.
Palliative bladder radiotherapy
Radiotherapy is commonly used to control haematuria as well as other bladder symptoms like dysuria,frequency, and pain.A survey conducted by the Royal College of Radiologists in the UK in the early 1990s found a multitude of dose fractionation regimens being used with 30 Gy in 10 fractions being the most common[17].A more recent review of palliative practice in two leading cancer centres in the UK has shown a similar spread of dose fractionation schedules still being used[18].Several studies have looked at different dose fractionation schedules in this setting and found them essentially similar in effectiveness and toxicity[Table 1].Most of the trials included heterogeneous populations of patients unsuitable for radical treatments and were for both advanced symptomatic local disease as well as metastatic disease.This needs to be considered when interpreting symptom-free survival benefit.One consistent finding, however, is the efficacy in achieving haemostasis in patients presenting with haematuria.
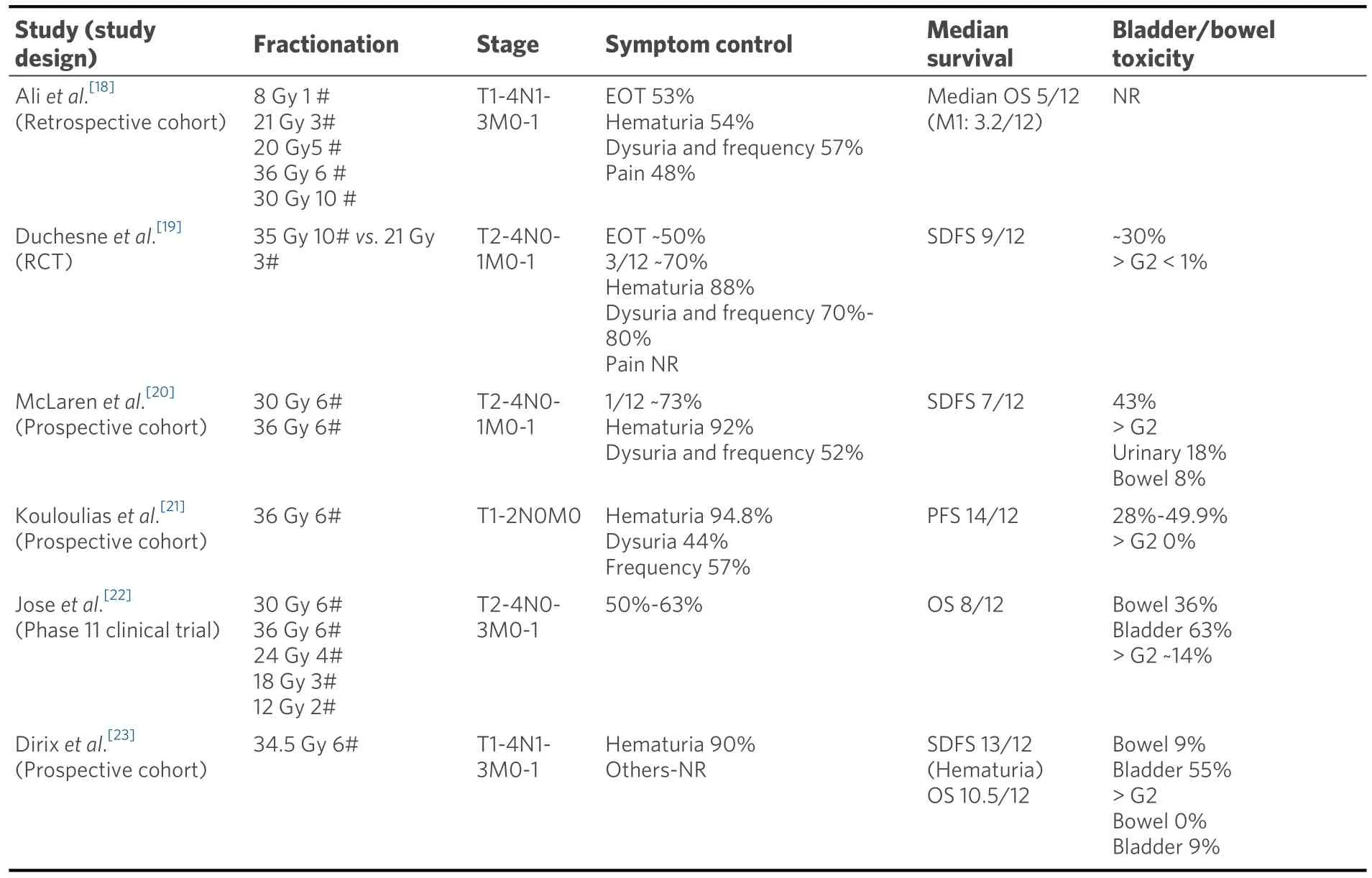
Table 1.Summary of large studies on palliative bladder radiotherapy
The largest and the only randomized controlled trial on the subject was Medical Research Council (MRC)BA09 (19).It randomized 500 muscle invasive bladder cancer patients, either unfit or too advanced for radical treatment between the then standard of care of 35 Gy 10# and 21 Gy 3#.Only 40 patients with confirmed metastatic disease were included.The benefit was defined by an improvement in at least 1 symptom without deterioration of any others.No statistically significant difference was seen at the end of the treatment or after 3 months for efficacy or toxicity between the two fractionation schedules.Over onefifth of the cohort had died by the 3-month assessment, and there was no improvement in quality of life for almost two-thirds of the survivors.At the end of treatment, a significant proportion had worse symptoms which improved with time[19].Similar findings have been seen with other studies evaluating hypofractionation schedules with reported acute toxicity being in the range of 20%-50%[20-23].
Despite the findings of the MRC study the most common schedule in use is 30 Gy in 10#, possibly reflecting uncertainty around the use of fraction sizes of 7 Gy in the pelvis.In a similar population of patients to those in the MRC study, only haematuria and incontinence showed improvement in those surviving at 3 months with this schedule.This was presumed to be due to the low radiobiological dose delivered with this fractionation schedule[24].
Since the full benefit of treatment over acute radiation toxicity may take some months to emerge, the most important focus is to identify those who will actually benefit from palliative radiotherapy with a life expectancy of more than three months.
Bone metastases
Bone metastases are seen in up to 50% of those with metastatic urothelial carcinoma.However, it is limited to the bone in less than 10%[25-27].Bone metastases have the potential to cause pain, spinal cord compression and fractures, and radiotherapy is commonly used as a palliative and preventive measure.
In metastatic bladder cancer, current accepted palliative radiotherapy practices are applicable.Both single and multiple fractionation schedules are in use.Several large systematic reviews evaluating the evidence for radiotherapy in bone metastases are in existence.Although the latest evidence suggests there is a slightly lower overall response rate [72%vs.75%, OR = 0.96, (95%CI: 0.93-0.99)] and an increased need for retreatment [OR = 2.38, (95%CI: 1.84-3.08)] with 8 Gy single fraction compared to multi-fraction treatments, the clinical significance of this is questionable for patients with metastatic urothelial cancers in view of their poor prognosis[28,29].
In a recent review, 10 Gy single dose treatment had the best pain response and the need for re-treatment,incidence of pathological fractures and spinal cord compression were reduced with the increase in dose per fraction.However, the majority of data is for 8 Gy single dose treatment and demonstrates high efficacy despite the retreatment rate of 20%-30%, with low rates of other complications such as pathological fracture(4%) and spinal cord compression (3%)[30].Amongst the many multiple fraction schedules commonly used,22.5 Gy in 5 fractions was seen to have the highest overall response (ORR) rate, 30 Gy in 15 fractions, the best complete response (CRR) rate and 20 Gy in 2 fractions, the best partial response rate (PRR) in a systematic review.Interestingly, the commonly used palliative schedules, 20 Gy in 5 fractions and 30 Gy in 10 fractions were found to be only moderately effective with ORR of 60% and 51%.Their CRR was 24% and14%, while retreatment was needed in 16% and 11%, respectively[31].The number of bladder cancer cases in the published meta-analyses is small with only 4 studies specifying it as a significant contributor as seen in Table 2.
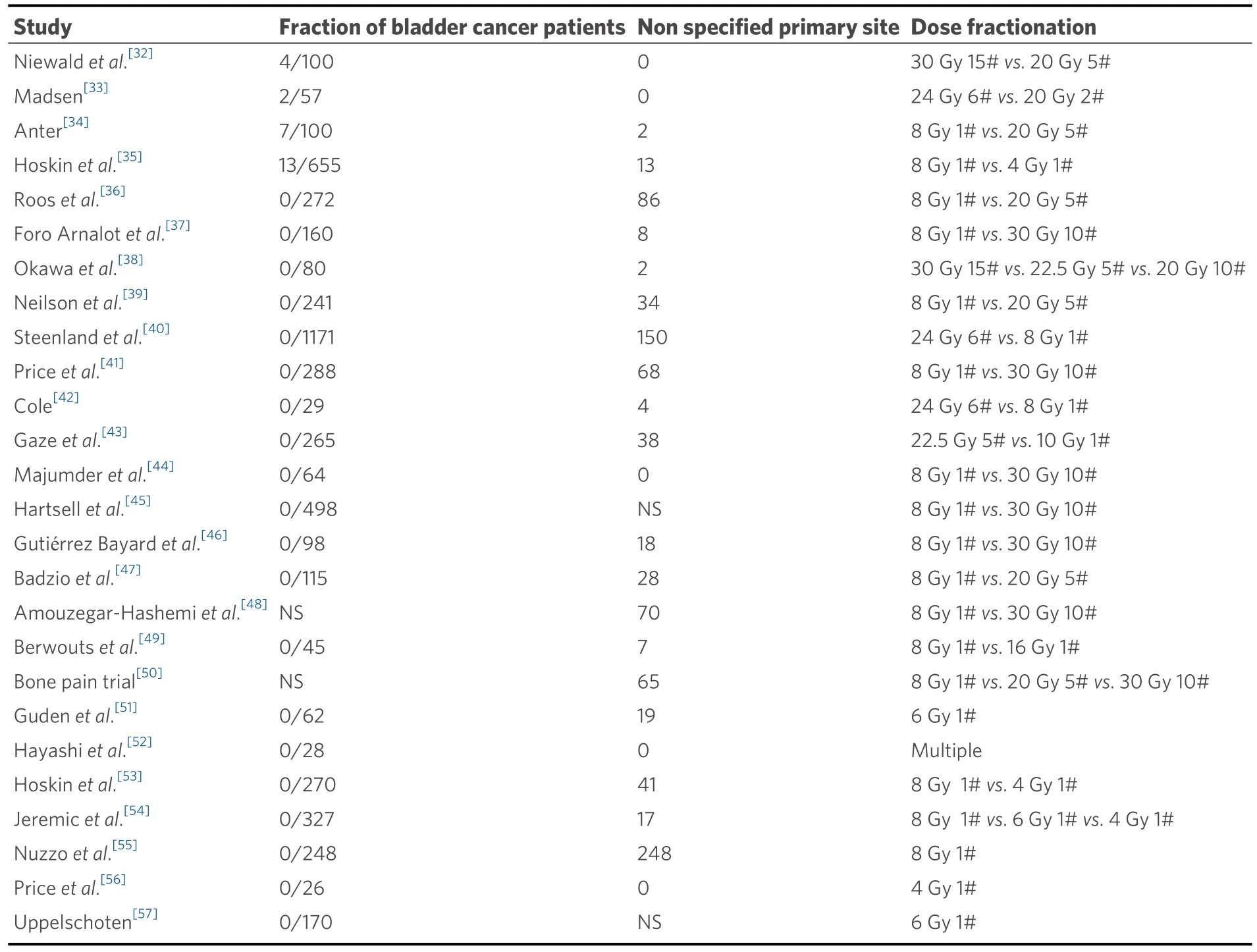
Table 2.Studies with bladder cancer patients included in the 2 meta-analyses[30,31]
Brain metastases
Brain metastases are rare in urothelial cancers affecting only about 5% of patients at presentation.They are commonly found together with thoracic and bone metastases[25].The management remains similar to brain metastases from other common primary carcinomas.Radiotherapy may be used adjuvant to surgical resection or as a primary modality with stereotactic radiosurgery (SRS) for localised metastases and whole brain radiotherapy for multiple metastases.
Most of the data on the effectiveness of these treatments in urothelial cancer patients comes from single institutional reviews.An exploratory patient-point meta-analysis based on a comprehensive literature review including 85 publications found the median residual survival from time of diagnosis of brain metastases to be around 3 months (95%CI: 2.9-3.7).Longer survival was only significantly associated with metastasectomy in those with single brain metastasis (9 months, 95%CI: 6.1-11.9vs.2 months without resection, 95%CI: 1.1-2.9,P< 0.001).This benefit with surgery was not seen in those with multiple deposits.No survival benefit was seen with radiotherapy.There was no analysis of symptom relief or quality of life(QoL)[58].
Adjuvant whole brain or stereotactic radiotherapy is known to reduce local recurrence and death from neurological causes[59-65].However, there was minimal contribution from urothelial carcinoma patients in the published studies due to its rarity.In one of the larger series of 62 bladder cancer patients with brain metastases, there was no survival benefit with surgery plus radiotherapy over radiotherapy alone[66].
Whole brain radiotherapy (WBRT) is conventionally used for palliation in those ineligible for surgery or SRS.Most bladder cancer patients will fall into this category due to age, comorbidities, and the presence of extracranial metastases.No fractionation schedule has been shown to be superior to others[67]and in the Radiation Therapy Oncology Group studies from the 1970s, over 60% symptom improvement was reported.A large randomised trial in non-small cell lung cancer, the Quality of Life after Treatment for Brain Metastases, failed to find a benefit for WBRT over best supportive care, with regards to OS and QoL[68,69].Any benefit from radiotherapy for multiple brain metastases may be limited to young patients with very good performance status.
Consolidation radiotherapy
Primary tumour
The potential survival benefit in patients with metastatic disease of controlling the initial disease burden by localized therapies alongside systemic therapy has been explored in a few studies with interesting results.
One retrospective analysis amongst patients receiving systemic therapy for metastatic disease found a significantly longer median overall survival (14.9 monthsvs.9.9 months) when radical cystectomy or radical radiotherapy (> 50 Gy) to the bladder was also used compared to those who received conservative palliative local treatment only.In addition, those who received radiotherapy to consolidate a response to chemotherapy did better than those selected for a cytoreductive approach with local treatment before chemotherapy (median OS 17.7 monthsvs.12.4 months)[70].This is supported by a population-based study from the United States, which showed improved OS and cause-specific survival with radical cystectomy in patients with solitary metastases[71].
Lymph nodes
Lymph node only metastasis carries a better prognosis than other metastatic sites[71,72].Treatments strategies include chemotherapy and chemo-radiotherapy.The Intensity-Modulated Pelvic Node and Bladder Radiotherapy trial in bladder cancer has shown the safety and long-term disease control benefits of simultaneously boosting involved pelvic lymph nodes to 60 Gy in 32 fractions with low late toxicity rates of only 5%[73].The safety of radical pelvic lymph node treatments is also demonstrated in other studies[74,75].
Distant metastasis-directed therapy
Cancer-specific and overall survival in patients with metastatic urothelial carcinoma depends on the sites of visceral involvement and the number of deposits.Those with a single metastatic deposit in the lung or bone have better survival than those with liver involvement.Distal lymph node only metastasis have significantly better survival than involvement of other sites as shown in Figure 1[71].This has been reported in other series also and has led to defining the M1a sub staging of distal lymph node only metastasis in the AJCC 8th edition[7,76,77].
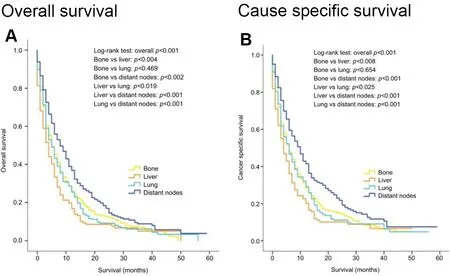
Figure 1.Kaplan-Meier curves of overall survival (A) and cancer-specific survival (B) according to the sites of metastases in patients with single metastatic site (Reproduced from Ref.[71]).
Several surgical series have looked at the potential benefits of metastasectomy in this setting.A multiinstitutional retrospective review from Japan showed a significantly longer median survival for solitary lymph node or lung metastasis than for other sites (81 monthsvs.19 months)[11].Metastasectomy, especiallywhen the disease is limited to the lung, is supported by several studies with some showing median overall survival in excess of 2 years following complete resection[12-16].Based on findings such as these the latest European Association of Urology, European Association of Medical Oncology guideline on advanced urothelial carcinoma recommends consideration of radical treatment in the presence of a single metastatic site as it is potentially curable[6].
Although there is a growing evidence base for ablation of metastatic deposits using radiotherapy, data specific for urothelial cancers are scarce.Recently presented long-term results of the SABR-COMET study comparing stereotactic radiotherapy (SBRT) with palliative treatment in those with a controlled primary and 1-5 metastatic deposits shows improved median OS of 50 monthsvs.28 months (P= 0.006) along with improved PFS and local site control.However, the proportion of bladder cancer patients included in this study is not stated[78,79].Others also have seen similar survival benefit with SBRT in those with oligometastatic disease, but again the number of patients included with urothelial cancer is either small or not stated[80-83].There are few published SBRT studies that are specific to bladder cancer.An Italian single institution retrospective review of stereotactic irradiation of synchronous lymph node only metastases from urothelial carcinoma reported a local control rate of 100% at 2 months with 6 out of 14 target lesions showing partial response and 8 remaining stable.The median treated lymph node PFS was 11.4 months(95%CI: 3.4-19.4), but overall PFS was 2.9 months (95%CI: 2.6-3.1) and OS was 14.9 months (95%CI: 12.3-17.5)[84].SBRT using Cyberknife stereotactic radiotherapy to bone, lung and lymph node metastases in urothelial cancer has been reported with one-third remaining disease-free at 30 months with no major toxicity[85].
In another study, following partial response to cisplatin-based chemotherapy for nodal or distal metastases consolidation radiotherapy to residual sites of disease resulted in a median RFS of 19 months and OS of 49 months for the whole cohort of 22 patients with 2 patients having single lung and mediastinal deposits surviving over 6 years[86].A similar OS benefit of 43% at 3 years with consolidation radiotherapy to metastasis following cisplatin-based chemotherapy has been reported; however, when controlled for sex,performance status, haemoglobin level and number of organs with metastasis, this was not associated with longer survival (HR = 0.666,P= 0.0966).Metastasectomy however, remained significantly associated withimproved OS (HR = 0.358,P= 0.0006)[87].In another larger Italian retrospective series in patient with controlled primary disease, the 2-year local control was 88.9% (95%CI: 76.1%-95.1%) for those with 1 to 5 metastases.The 2-year distant and overall PFS for the entire cohort was around 40% with a median 12-month freedom from start or change of systemic therapy.Inferior local control was associated with the number of prior lines of systemic therapies, and PFS was associated with the number of metastases.The reported median OS was 25.6 months which correlated with total dose and biologically equivalent dose(BED10) in univariate analysis[88].
Clearly, further studies are needed to identify metastatic bladder cancer patients who may benefit from these radical ablative treatments alongside systemic therapy.
Radiotherapy and immunotherapy combination
There is a growing pool of preclinical and early phase clinical trial data that suggests radiotherapy and immunotherapy act synergistically in cancer treatments.The effect is thought to be mediated by radiotherapy reducing tumour growth allowing time for immunotherapy to act, altering the tumour immune microenvironment to increase susceptibility to immunotherapy and through the abscopal effect[89].A phase 1b study has shown the combination of durvalumab with radiotherapy for locally advanced unresectable non-metastatic bladder cancers to be safe with a 70% complete clinical response rate[90,91].A further phase II clinical trial, NCT03115801 is investigating the place of immunomodulation using nivolumab, atezolizumab or pembrolizumab alonevs.in combination with radiotherapy 30 Gy in 10 fractions for metastatic urothelial and renal cancers[92].This is likely to be an area of considerable interest and trial activity in the forthcoming years.
CONCLUSION
The conventional and historical place of radiotherapy in metastatic bladder cancer is in palliation, where it offers effective symptom control in haematuria and symptomatic bone and brain metastases.However, in those with limited metastatic disease, fit for systemic therapy, consolidation for those responding to systemic agents with surgery or radiotherapy may be beneficial.Given the poor overall survival in these patients, selection of patients for either treatment needs to be streamlined to ensure they are offered only to those who will live long enough to benefit.Further bladder cancer-specific data is needed to help guide clinicians in individualizing these treatments.
DECLARATIONS
Acknowledgments
Peter Hoskin is supported by the NIHR Manchester Biomedical Research Centre.
Author’s contributions
Design, review of evidence and interpretation of the data presented: Perera J, Hoskin P
Availability of data and materials
All data referred to in this paper is available in the referenced publications.
Financial support and sponsorship
None.
Conflicts of interests
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
 Journal of Cancer Metastasis and Treatment2022年1期
Journal of Cancer Metastasis and Treatment2022年1期
- Journal of Cancer Metastasis and Treatment的其它文章
- New developments in management of metastatic thyroid cancer
- AUTHOR INSTRUCTIONS
- Biomarkers for therapy selection in metastatic urothelial cancer
- Serum squamous cell carcinoma antigen is a predictive factor of outcomes in patients with locally advanced unresectable esophageal squamous cell carcinoma treated by definitive chemoradiotherapy
- The role of MT1-MMP in the progression and metastasis of osteosarcoma
