Use of Hydrazine and Its Substitutes as Fuel
A.A.Boryaev
(Saint Petersburg State University of Architecture and Civil Engineering, 4 Vtoraja Krasnoarmejskaja St., Saint Petersburg 190005, Russia)
Abstract:Due to the properties and high reactivity of hydrazine, it is mainly used as rocket fuel not only in its pure form but also in combination with 1,1-dimethylhydrazine and oxidizers (nitrogen tetroxide or nitric acid) forming a self-igniting mixture with oxidizers. Aerozine 50 and UH 25 (a mixture of 75% UDMH (unsymmetrical dimethylhydrazine) and 25% hydrazine hydrate) are the best-known hydrazine mixtures with different hydrazine concentrations. The review addresses the use of hydrazine and its derivatives as fuel. Hydrazine is employed in fuel cells (with air oxygen as an oxidizer) to generate electrochemical energy for transport vehicles. Hydrazine is widely used as monopropellant to design low-thrust rocket engines for orientation and stabilization systems in space vehicles, as well as in energy units. The review also addresses such hydrazine derivatives as methylhydrazine, 1,1-dimethylhydrazine, hydrazine monoperchlorate, hydrazine diperchlorate, hydrazine diammonium tetraperchlorate, hydrazine mononitrate, hydrazine dinitrate, hydrazine nitroformate, hydrazine azides, tetrafluorohydrazine, etc. as well as composite propellants, and gel rocket propellants based on hydrazine. The materials in the review can be used as reference information on hydrazine fuels.
Keywords:liquid fuel; hydrazine; fuels based on hydrazine derivatives; properties and scope of application
Introduction
Hydrazine is a colorless liquid, fuming in the air. This liquid is highly hygroscopic and highly soluble in water, alcohols, amines, and polar solvents. It is an endothermic compound. Therefore, it is characterized by low stability, is highly flammable, and easily decomposes when heated and in the presence of catalysts to form ammonia, hydrogen, and nitrogen.
Hydrazine derivatives (alkyl homologues), or hydrazine fuels, include unsymmetrical dimethylhydrazine (UDMH) ((CH3)2N2H2), hydrazine hydrate (N2H4H2O), monomethyl hydrazine (NH2NHCH3), and ammonia (NH3). Besides, hydrazine is utilized in combination with methylhydrazine (or monomethylhydrazine), thus forming Aerozine 50 fuel widely used in the USA and Russia, or in combination with ammonia, as well as a part of bipropellants and hybrid propellants, and as a part of some liquid oxidizers.
Hydrazine and its derivatives are currently widely utilized. Liquid pure hydrazine is used as nitrogen-containing fuel in aerospace engineering, especially in liquid rocket engines (LREs), including low-thrust LREs (LTLREs), micro LREs (mLREs), expendable and reusable LREs (RLREs, RLTLREs, RmLREs), as well as in other science and industry fields[1-24].
UDMH is widely used in aviation as well as rocket and space equipment: in French Mirage III fighter aircraft; in Russian Kosmos, Cyclone, and Proton launch vehicles (LVs); in American Titan-series LVs; in French Ariane-series LVs; in Japanese N-series LVs; in Chinese Long March LVs; in propulsion systems of manned spacecraft and unmanned satellites, orbital and space stations, Buran (Russia) and Space Shuttle (USA) reusable spacecraft. Other hydrazine alkyl homologues are widely used in various combinations of rocket propellants and fuels. The scope of hydrazine application imposes particular limitations on the number of publications in this field.
All hydrazine fuels (and coolants) are toxic and hazardous to human health. However, despite this, due to their special technical properties, such fuels are still effectively used, first of all, in engines of spacecraft control systems and in engines of expendable and reusable spacecraft (air-based, space-based, airspace-based). When 1 mole of hydrazine decomposes, 46kJ of heat are released. Therefore, hydrazine is mainly used in LTLREs (including reusable ones) and gas generators (GGs) where the gases formed during decomposition reach a temperature of 1400K and a pressure of 1-2MPa. Currently, hydrazine is also widely used in mLREs and RmLREs to ensure efficient control over small satellites and nano-satellites in orbit[3].
Among non-metallic additives, advanced nano-additives in the form of pure dry fullerenes play a special part. Using those, it is possible to increase density and other thermal and physical properties of liquid fuels and coolants[8-31, 35-42]. Liquid fuel density increase is especially important for LREs, aircraft and spacecraft since it improves not only the efficiency of engines but also the efficiency of aircraft and spacecraft (by increasing the flight range, providing the possibility to increase the useful load when launching into orbit, etc.). Various researchers have studied the impact of fullerenes on increasing the efficiency of thermal and physical properties of liquid hydrocarbon fuels and coolants and developed new hydrocarbon fuels and propellants with introduced fullerenes.
Besides, hydrazine fuels (and coolants) and their derivatives, as well as their mixtures with other substances, have found use in terrestrial conditions[8-31]:
(1) in conversion LREs, LTLREs, and reusable power units used when: producing heavy bitumen oil; solving science and engineering problems in the development of existing and creation of new liquid and hybrid engines and their cooled and uncooled combustion chambers; creating emergency braking systems for large ground effect vehicles and their steering systems (when afloat); performing effective research on the properties of hot plasma; continuing and expanding scientific and academic work;
(2) in medicine: when developing new drugs;
(3) in agriculture and biology: when developing growth-regulating chemicals for plants and other chemicals;
(4) in small-scale power generation: when developing and operating low-temperature air/hydrazine fuel cells (batteries—electrochemical generators) of various sizes and capacities; when generating electric current and using it at various facilities and in various industries, including transportation;
(1) in large-scale power generation: during oxygen reduction in water used in boilers;
(2) in nuclear power generation: in nuclear fuel reprocessing (as reducing agents);
(3) in the chemical industry: when producing plastics, rubber, and explosives; in reduction of gold, silver, platinum-group metals, and copper from dilute saline solutions; in reduction of the carbonyl group of aldehydes and other substances; when cleaning industrial gases from CO2and mercaptans; when obtaining intermediate products and dyes;
(4) in mechanical engineering and other industries: in corrosion protection of water and steam circulation pipelines (steam generators, cooling and heating systems); when removing oxygen during water treatment; in preservation of decommissioned equipment; when providing fuel (hydrazine hydrate) for power units of deep-sea vehicles lowered to a depth of 6km and more (e.g., Russian Ocean (Okean) submersible); when obtaining a working medium at temperatures up to 650℃ (with hydrazine hydrate decomposition in a GG into nitrogen, hydrogen, ammonia, and water vapor), used as a heat carrier in the secondary circuit of Rankine closed-cycle turbines.
1 Fuels based on hydrazine
Below we present a classification of hydrazine rocket propellants based on blend and chemical composition:

Such an approach makes it possible to better reflect the energetic, physical and chemical, as well as performance properties of rocket propellants depending on their chemical nature. The following requirements are imposed on hydrazine rocket propellants[43]:
(1) Available sources of raw materials and production capacities, low production cost.
(2) Low pour point (below -60℃), high boiling point (above 80℃), and low viscosity; physical and chemical stability on long storage under normal conditions; compatibility with structural materials, no corrosion effect; non-explosive and fire-proof; no toxic effect on operating personnel.
(3) Easy and effective fuel flammability in the combustion chamber; short ignition delay period and wide flammability limits; high flame propagation rate; low surface tension and viscosity; low pressure of saturated vapors, high heat capacity, thermal conductivity, and heat of evaporation of coolants; high physical and chemical stability in a wide range of temperatures and pressures.
(4) Generation of the maximum specific thrust impulse upon the minimum possible engine mass. Propellant shall have high calorific power and high density, and combustion products shall have optimal physical characteristics (molecular mass, heat capacity, etc.).
Hydrazine bipropellants using liquid oxygen and fluorine-containing reagents (OF2, F2, N2F4) as oxidizers suit the listed requirements the most (Table 1, Fig.1)[43]. Tripropellants with the same oxidizers are also characterized by high specific thrust impulse (Table 2)[45]. It should be noted that numerous requirements are imposed on the components of hydrazine rocket propellants. Critical characteristics determining the fuel and oxidizer choice may be different in each individual case.

Table 1 Energetic properties of hydrazine rocket bipropellants
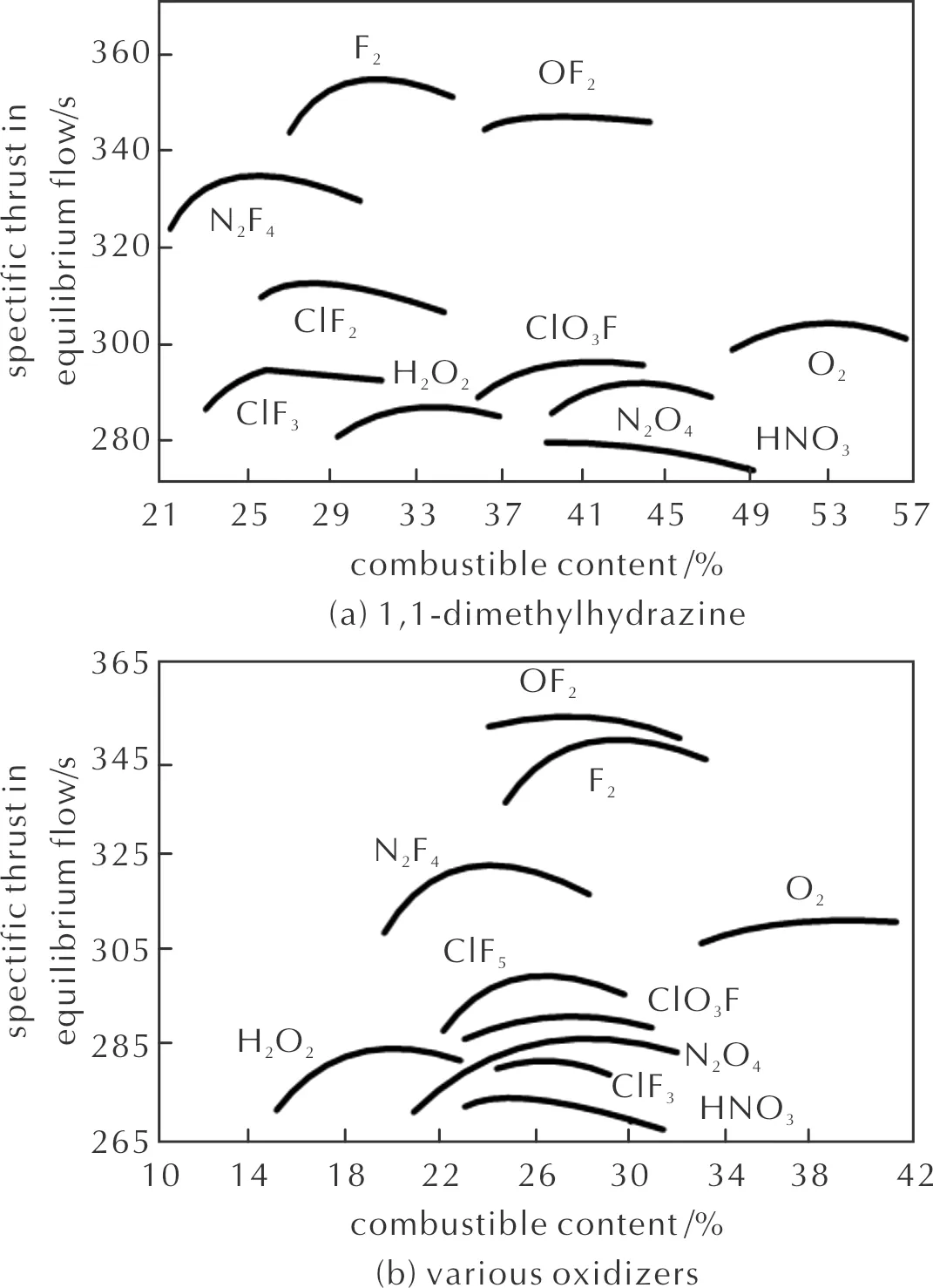
Fig.1 Characteristics of rocket propellants based on hydrazine[47]
One of the ways to improve the efficiency of LREs and LTLREs powered by hydrazine is to improve the efficiency of liquid fuels and coolants[1-7, 24-42]. It is possible to improve the efficiency of pure liquid hydrazine (and its derivatives) by the following methods:
(1) introducing various metallic and non-metallic additives (after engine start and during its operation);
(2) mixing it with other existing advanced energy-intensive liquid fuels and coolants (before and during flight);
(3) using pure liquid hydrazine with a pressure in the range of critical pressures (to increase the heat transfer coefficient by 2-3 times due to particular thermal and physical properties, which is essential for engine cooling systems);
(4) applying electrostatic fields (to increase the heat transfer coefficient for complete pre-fuel treatment, fuel electrospraying in case the pumping system is out of order), etc.[31-42].
Monopropellants have got widespread use in small rocket engines employed for spacecraft orientation and stabilization or flight trajectory changing, in individual engines for astronaut movement, etc. Unitary propellants have significantly lower specific thrust impulse as compared with bipropellants (Table 3)[43, 45]. The combustion temperature of unitary propellants is relatively low. Low specific thrust impulse is, to a certain extent, compensated by the simple design of the equipment used.

Table 2 Hydrazine tripropellants

Table 3 Energetic properties of hydrazine rocket monopropellants
Hydrazine LREs are safe and reliable. They also have a long lifetime and can be easily restarted (can operate in pulse mode).
Hydrazine is a colorless and rather viscous liquid, fuming in the air, with the basic substance content of 95%. Commercial-grade hydrazine (primary product obtained with traditional production technologies) contains water, carbon dioxide, hexane, toluene, hydrazine carboxylic acid, 1,1-dimethylhydrazine, and aniline as contaminants. The most effective method of hydrazine purification is cyclic freezing, which includes several stages: crystallization (most contaminants remain in the liquid phase), liquid draining, and hydrazine melting. In 3-4 cycles, high purification degree can be ensured. To prevent hydrazine from being contaminated with water and carbon dioxide from the air, it is recommended to purify hydrazine in a dry nitrogen environment.
The low-temperature properties of hydrazine can be improved by adding 3%—18% hydrochloric acid and 3%—30% glycols. Such rocket fuel has a crystallization point of up to -49℃. To improve the low-temperature properties and increase the density of hydrazine (rocket fuel), it is recommended to introduce hydrazine alkanes into its composition. For instance, 1,2-dihydrazine ethane in an amount of 5% to 95% shall be mixed with anhydrous hydrazine. Hydrazine with1,2-dihydrazine ethane in an amount of 60% forms a system with a setting point of approx. -33℃[43].
In order to obtain liquid rocket propellant based on hydrazine with improved low-temperature properties (freezing point from -40 to -60℃), it is recommended to introduce 30%—35% methoxylamine hydrochloride[46], 10%—25% methoxylamine nitrate or 5%—35% methoxylamine nitrate and up to 20% water[44], 15%—25% methoxylamine perchlorate or 20% methoxylamine and 14% water[5]. Rocket propellant containing 24% hydrazine, 45% methylhydrazine, and 31% hydrazine nitrate freezes at -44℃. The freezing point of hydrazine can also be lowered by adding lithium bromine hydride, cyanohydride, and ammonium thiocyanide[43].
It should be noted that in terms of low-temperature properties hydrazine hydrate significantly exceeds hydrazine. The crystallization point of hydrazine hydrate can be lowered by introducing hydrazine azide[48]. The melting point of ternary eutectics of a binary mixture is in the range from -15 to -28℃, which makes it possible to use hydrazine azide to lower the freezing point of hydrazine without deteriorating its properties as rocket propellant.
The use of hydrazine as rocket propellant was preceded by studies of its decomposition under the influence of various factors. Thermal decomposition of hydrazine occurs at 250-310℃. The mechanisms of thermal decomposition and decomposition with explosion include reactions involving free radicals[43, 45]:
N2H2→2NH2
NH2+N2H4→NH3+N2H3
2N2H3→N2+2NH3
NH2+N2H3→N2+H3+NH3
2NH2→N2+2H2
2NH2+N2H4→2N2+4H2
Photochemical (λ<2400Å) hydrazine decomposition can be described by the following equation:
N2H4→N2+2H2
In this case, only a small amount of ammonia is generated. If we present the reaction in general form[49]:
3N2H4→4(1-x)NH3+(1+2x)N2+6xH2
then, during thermal decomposition, the value ofxwill be 0.06, and during photochemical decomposition, it will vary in the range of 0.93—0.96[45]:

Thermal, 250—310℃0.06On a wire platinum, 205—530℃0.17—0.44tungsten, 360—500℃0.18—0.30Decomposition with spark-ignited explosion, 100℃0.38Photosensitized, 2537A0.25Photochemical<1990Å0.43—0.76<2400Å0.93—0.96
Researchers have studied hydrazine decomposition in the presence of various catalysts containing iridium, nickel, ruthenium, rhodium, platinum, palladium, osmium, or their mixtures. Since hydrazine is highly associated at high concentrations and ordinary temperatures, the following mechanism seems possible for catalytic hydrazine decomposition on heavy metals:
2N2H4→2NH3+N2H2
N2H2→N2+H2
For reactions of hydrazine decomposition on a platinum catalyst, thexvalue is 0.25. As a result of hydrazine decomposition in contact with nickel (x=0.5), a large amount of gases is generated, according to the following equation:
3N2H4→2NH3+2N2+3H2
The mechanism of this process involves hydrazine trimer formation. Not only free radical reactions but also surface reactions are possible[45]. At temperatures up to 50℃, the rate of hydrazine decomposition is low: at the boiling point, it is 0.01%—0.1% per day, and at 250℃, it reaches 10% per minute. Iron, copper, molybdenum, and chromium ions accelerate hydrazine decomposition. Oxides of these metals catalyze hydrazine decomposition more actively than their ions[43].
Catalysts facilitating hydrazine decomposition into ammonia, nitrogen, and hydrogen require elevated temperatures. Typically, alumina is used as a carrier for hydrazine decomposition catalysts. Iridium catalysts based on alumina containing 5% BaO and 6% SiO2were tested during cold start of LREs. Good properties were obtained in hydrazine decomposition with the use of carriers treated with water. The catalytic activity depends on the amount of liquid hydrazine (excess of hydrazine reduces catalysis efficiency) as well as the amount of oxygen adsorbed on the catalyst surface. The composition of products of catalytic hydrazine decomposition depends on the time of contact with the catalyst, pressure, and mass flow rate per unit area of the catalyst surface[43]. Hydrazine usually decomposes with the formation of ammonia and nitrogen, and then 30%—40% of ammonia decompose into nitrogen and hydrogen[50]:
3N2H4→4NH3+N3
4NH3→2N2+6H2
Depending on the process conditions, ammonia dissociation may be incomplete, and then decomposition products will be contaminated with ammonia, which will affect theTandRTparameters of decomposition products (Fig.2)[51].

Fig.2 Relationship between the T (1) and RT (2) parameters of hydrazine decomposition products and the mole fraction of ammonia generated
Hydrazine and its mixture with hydrazine hydrate, intended to be used as unitary propellant, decompose immediately in contact with catalysts consisting of rhenium, molybdenum, iron, nickel, copper, silver, gold, iridium, or ruthenium deposited on highly porous carriers of aluminum, thorium, or zinc oxides[52].
A propellant composition with a flash point below 25℃, consisting of N2H4(24.41%—43.66%), CH3NHNH2(4.38%—35.04%), and C2H5OH (25.77%—68.02%), was developed to be used in LREs with a catalytic decomposition chamber[1]. Charcoal, silica gel, and activated alumina, impregnated with oxidizers (N2O4, Cl2, or POCl3) in an amount of approx. 30%, can serve as catalysts for hydrazine, methylhydrazine, and 1,1-dimethylhydrazine decomposition. Adsorbent heating caused by the exothermic reaction ensures continuous propellant decomposition even after the oxidizer has been used up[53].
2 Fuels based on hydrazine derivatives
Methylhydrazine.A colorless liquid with an odor characteristic of alkylamines with a short aliphatic chain. It is miscible with water, hydrazine, low-molecular monohydric alcohols, and hydrocarbons in all proportions. Methylhydrazine has weak alkaline properties and is a good reducing agent. It self-ignites in contact with strong oxidizers (fluorine, chlorine trifluoride, nitrogen tetroxide, hydrogen peroxide, and fuming nitric acid) and is insensitive to impact and friction. In terms of performance properties, it occupies an intermediate position between hydrazine and 1,1-dimethylhydrazine.
1,1-dimethylhydrazine.A colorless liquid with a strong unpleasant odor. It mixes well with water, petroleum products, alcohols, and most organic solvents. 1,1-dimethylhydrazine has good low-temperature properties and is used as unitary rocket fuel. When it is heated in small amounts in glass capillaries, no decomposition is observed at temperatures of 220 and 228℃ (within 30 minutes). Thermal decomposition of 1,1-dimethylhydrazine in the temperature range of 371—427℃ can be represented by the following reactions[43, 45]:

Thermal decomposition of 1,1-dimethylhydrazine is accompanied by the formation of resins; during decomposition of methylhydrazines, highly toxic hydrogen cyanide can be formed[45]. Researchers designed a process of 1,1-dimethylhydrazine decomposition in the gas phase on platinum catalysts deposited on asbestos. The initial decomposition temperature is 100—150℃. Besides asbestos, it is recommended to use titanium, zirconium, aluminum, silicon, and magnesium oxides as catalyst carriers. During 1,1-dimethylhydrazine decomposition, the following reactions are also possible:

Among rocket fuels, hydrazine fuels are characterized by the lowest chemical stability when stored in tanks with air access. When methylhydrazines are oxidized with atmospheric oxygen, dimethylamine,water,tetramethyltetrazene(CH3)2NNNN(CH3)2, dimethylnitrosamine, polymethylenes, and resins are formed. The characteristics of resins (1,1-dimethylhydrazine oxidation products) show that they are mainly represented by dimers of primary fuel oxidation products. The rate and extent of 1,1-dimethylhydrazine oxidation depend on oxygen concentration, temperature, oxidation duration, and catalysts[43].
Various hydrazine substitutes are recommended as rocket fuel: alkylene hydrazines (C1—C6), glycerol hydrazine, dihydrazinoacetonehydrazone, 3-hydrazino-2-hydroxypropylaldehyde-hydrazone, and hydrazinoacetaldehydrazone. To improve the energetic characteristics of propellants, 12%—34% Be, BeH2, AlH3are added. The suggested types of hydrazine fuel are characterized by good technological properties of the uncured mass, high energetic properties, mechanical strength, and elastic charges. To improve viscous as well as physical and mechanical properties and increase specific impulse, plasticizers are introduced into fuel: hydrophilic polymers (polyacrylamide, polyvinylstyrene, cellulose acetate or aceto-butyrate, polyvinylformal, polyethyleneimine, polyethylene-glycerin, ethylenepolyamine, methyl cellulose, oxyethylene cellulose, and oxycellulose, polyoxyethylene, polyoxypropylene, polyacrylonitrile, modified polyurethane, soluble starch and cellulose). The fuel/plasticizer weight ratio ranges from 15∶1 to 1∶3[50, 52, 53, 54].
High-energy fuels (2-propenyl, 1,1-di-(2-propenyl)- and 1-ethyl-1-(2-propenyl) hydrazines) were synthesized, which ensure high specific thrust impulse when HNO3, H2O2, O2, and N2O4are added as oxidizers. These fuels are characterized by a short ignition delay period and a low crystallization point (from -45 to -60℃) and are 1.8—2.3 times superior to hydrazine in terms of heat of combustion. These fuels can be used in mixtures with aniline and aliphatic hydrocarbons. Dihydrazine hydrocarbons (e.g., 1,2-dihydrazine ethane), nitrohydrazines, and products of interaction between alkylhydrazines with trimethylaluminum can be used as high-energy rocket fuels[43].
Unitary fuel is stable during storage. It represents a mixture of hydrazines RR′NNH2and hydrazine polyhydrodecarborates (RR′NNH3)2B12H12-nXn, where R and R′—H or lower alkyls, X—OH or NH2,n=0÷4[55]. When heated for several hours at a temperature of 95—100℃, such fuel does not decompose. It can be stored in tanks designed for hydrazine storage and transportation. Such fuel is characterized by high thermal stability and high energetic properties and can be used in rocket engines with small amounts of oxidizers (nitrogen tetroxide, fluorine oxide, etc.) added. To improve fuel performance properties, depressants (hydrosulfuric, cyanic, and other acids) can be added.
A mixture of hydrazine polyhydroborates ((RR′NNH3)2B12H12and (RR′NNH3)2B10H10, where R and R′—hydrogen or lower alkyls, and the boron content is 25%—90% of the total mass of the boron-containing compound) was also suggested as high-energy rocket fuel. This fuel can be used in combination with oxidizers such as nitric acid, nitrogen tetroxide, fluorine oxide, etc. The bipropellant is characterized by high specific impulse and heat of combustion, good ignition characteristics, and sufficient stability in storage. It can be used in LREs with 58%—85% hydrazine added or as a high-energy component of solid rocket propellants[43].
Tetradecahydroundecaborates mixed with hydrazine salts are used as monopropellants characterized by high thermal and oxidation stability in storage and use[56]. bis-(hydrazine)- and bis-(methylhydrazine) nonaboranes-13 were suggested as high-energy combustible components of liquid and solid rocket propellants. They are characterized by high combustion temperature and are relatively safe in use[57]. bis-(hydrazine) decaboranes-12 of the general formula (N2HxR4-x)2B10H12(where R—H or lower alkyl,x=0÷4) were also recommended as high-performance rocket fuel[58].
Tetraalkyltetrazenes, bis-tetraformaltriazines, 2,5,8-trihydrazino-tri-(s-triazines), and piperazine alkyl derivatives were suggested as rocket fuels for LREs with advanced performance properties[43]. Polyethylene hydrazine is introduced into solid rocket propellants to increase the combustion rate. Together with polyethylene hydrazine, fine powders of aluminum, manganese, zinc, hafnium, beryllium, boron, and titanium are added[59]. By mixing polyethylene hydrazine with triaminoguanidine borohydrazide-14, dihydrazine perhydrodecaborate, monohydrazinate, or dihydrazinate, solid propellants are obtained that ensure ignition and complete combustion under conditions of deep vacuum characteristic of space flights.
3 Gel fuels based on hydrazine and its derivatives
In terms of state of matter, gel rocket propellants are in between liquid and solid propellants and can have various viscosities. What they have in common is the gel base, which consists of low-molecular polymers, heavy hydrocarbons, alcohols, or structured colloidal dispersions of a gelling agent in a liquid dispersion medium. The gel base can be used both in free form to improve the performance characteristics of liquid propellants and as a carrier of a powdered component (high-energy metals and their hydrides, inorganic oxidizers, catalysts, process additives)[61]. Compared to liquid and solid propellants, gel rocket propellants are less toxic and less flammable and are characterized by less corrosion aggressiveness, which facilitates their storage and transportation.
Gel propellants based on hydrazine and its substitutes differ in thickeners and emulsifiers. One of the issues associated with the development of heterogeneous propellants based on hydrazine is the mechanical stabilization of the suspended phase and its chemical compatibility with the liquid carrier. Besides, hydrazine components are thermodynamically unstable and prone to decomposition with the formation of gaseous products. Usually, low hydrazine decomposition rates do not result in significant propellant losses. However, they change the specific volume and rheological properties of the heterogeneous system.
The chemical stability of heterogeneous gels based on hydrazine, containing beryllium and AlH3, is most effectively ensured by filler particles′ passivation. By dispersing beryllium particles in a mixture of a saturated solution of K2Cr2O7and 85% H3PO4or an aqueous solution of H3PO4and CrO3, chromium-containing coatings with high corrosion resistance can be obtained. To obtain a homogeneous thixotropic suspension of AlH3in hydrazine and prevent catalytic hydrazine decomposition with the release of gaseous products during storage, it was suggested to cover AlH3particles with a polyurethane film. Such propellant remains stable for 93 days[62]. To increase the stability of hydrazine, methylhydrazine, and 1,1-dimethylhydrazine gels and their mixtures under high-g conditions as well as high vibrations or temperatures, oxycellulose, ethyl cellulose, or acetyl cellulose are used. Researchers synthesized gel propellant by mixing hydrazine and its alkyl derivatives with gelling additives (0.25%—3%) of hydrophilic compounds (gum arabic, tragacanth, Irish moss extract, karaya gum, black locust seed oil, methyl cellulose, or polymers of galactose) at room temperature. Emulsified fuel was suggested that consists of methyl-, ethyl- and 1,1-dimethylhydrazine in the amount of 85%—95% as the main components as well as paraffinic, naphthenic, and aromatic hydrocarbons. Oxyalkylated alcohols, phenols, acids, ethers and esters (0.05—5 vol.%) are used as emulsifiers. Such propellant remains stable at a temperature of 10—15℃ for several weeks[43, 47, 49, 50].
Gels based on thickened hydrazine and its substitutes are used as high-boiling components of rocket propellants. By adding beryllium, boron, aluminum, or their hydrides, it is possible to significantly increase the density and theoretical specific impulse of heterogeneous fuels based on hydrazine. For instance, when beryllium and aluminum are added, the specific impulse of N2H4-N2O4propellant composition increases by approx. 12% and 4%, respectively. An increase in specific thrust impulse was also noted for other systems (Table 4)[63].

Table 4 Theoretical specific impulse of heterogeneous fuels based on hydrazine
4 Heterogeneous propellants based on hydrazine
One of the first heterogeneous propellants based on hydrazine is aluminizine, containing 66.5% hydrazine, 33% aluminum, and 0.5% modified polyacrylic acid (a gelling additive intended to ensure uniform aluminum content throughout propellant during storage, preventing metal deposition) (Table 5)[63]. Aluminizine burns steadily in commercial LREs. No metal deposition is observed during propellant discharge at normal temperature and acceleration of 3—6g. However, when other gelling agents are used, an increase in temperature above 43.3℃ results in gel liquefaction, metal deposition, and, in some cases, chemical reactions in propellant.

Table 5 Properties of anhydrous hydrazine and aluminizine
Aluminizine remains stable for five years. Its disadvantage is its sensitivity to carbon dioxide, which makes it difficult to use the propellant
To thicken hydrazine propellants, fatty acid esters (e.g., sorbitan mono-, di- and trioleates, vinyl esters of succinic and sebacic acids), cellulose derivatives (carboxymethyl cellulose, oxycellulose, and ethyl cellulose), polyacrylic acids, polyacrylamide, and colloidal SiO2are used (Table 6). Acetylene black and rubbers are also used as gelling additives. It should be noted that hydrazine gels formed by modified acrylic acid during hydrazine thickening are very sensitive to impurities. For instance, the contact of propellant with carbon dioxide from the air results in gel destruction[63].

Table 6 Compositions of gel propellants based on hydrazine and its substitutes
At certain ratios between hydrazine, sodium carboxymethyl cellulose, and antimony potassium tartrate, transition from hydrazine gels to solid rocket propellant compositions is possible[64]. For instance, to obtain solid rocket propellant, 85%—90% hydrazine, 9%—12% sodium carboxymethyl cellulose, and 1%—4% antimony potassium tartrate are mixed, and the homogeneous mixture is cured at about 20℃ for 24h. The developed compositions are effective as fuel for hybrid rocket engines. Quaternary ammonium salts of polyacrylic acid (tetramethyl-, tetraethyl-, tetrapropyl- or tetrabutyl ammonium polyacrylate) serve as effective gelling agents for hydrazine propellants. Compositions containing 50%—75% oxidizer (ammonium perchlorate, ammonium nitrate, and hydrazine nitrate), approx. 15% high-energy additives (Al, Mg, Be, B, Li, and their mixtures), up to 5% thickener (salts of high-molecular acids or SiO2), surfactant additives, and combustion catalysts are introduced in heterogeneous gel monopropellants.

Continued
Gel propellants with liquid oxygen or fluorine as an oxidizer include 10%—60% combustible binders with a melting point of 30—125℃ (paraffins, naphthalenes, wax, polyethylene with a molecular mass of 4000—8000, halogenated and nitrated hydrocarbons, stearic acid, etc.). Besides, 40%—90% of high-energy additives (Mg, Ti, Li, Al, B, Be, or their carbides and some hydrides), 0%—20% of a high-energy oxidizer (trinitrotrimethylene triamine, trinitrotoluene, ammonium nitrites, hydrazine dinitrate, picric acid, nitroglycerin, etc.) are also introduced into the composition of such propellant[63].
Unitary gel propellant consisting of 77%—95% oxidizer, 5%—23% solid and liquid fuel, and approx. 1% gelling agent and surfactants is recommended for use. Ammonium or hydrazine perchlorates, hydrazine diperchlorate, dihydrazine nitroform, and their mixtures are used as oxidizers, aluminum, beryllium, boron, their hydrides and mixtures are used as solid fuels. Hydrocarbons (nonane or 2,2,8-trimethylhexane) are used as the dispersion medium, and tri-(monobutyl-2-thiadodecine) ammonium phosphate is used as a thickener. Derivatives of sorbic and oleic acids are used as surfactants. To increase specific thrust impulse, NH4ClO3or NH4NO3are dissolved in hydrazine with the addition of 2%—3% gelling agent and 10%—25% powdered aluminum. A similar monopropellant composition includes hydrazine, hydrazine nitrate, a thickener (which forms a stable hydrazine gel in the presence of hydrazine nitrate), and finely dispersed beryllium, aluminum, magnesium, zirconium, hafnium, their alloys, mixtures, and hydrides[63]. Heterogeneous gel monopropellants have a higher specific impulse than unitary liquid propellants. They have the following advantages over solid propellants: the possibility of fuel filling during launching and relatively simple thrust module control. The development of new gel propellants and their combination with modern oxidizers make it possible to reduce the size of large launch vehicles by almost 60%[65].
5 Hydrazine-containing oxidizers of rocket propellants
Below, we describe some properties of hydrazine-containing compounds used as oxidizers in rocket propellants (Table 7)[45].

Table 7 Properties of hydrazine-containing oxidizers for rocket propellants
Hydrazine monoperchlorate (N2H4·HClO4) was first obtained by adding 48% solution of HClO4to hydrazine hydrate to reach pH 3.2. Hydrazine monoperchlorate can form N2H4·HClO4·1/2H2O semihydrate, which is stable up to a temperature of 60.5℃. When it is dried under vacuum at 70—80℃, anhydrous salt with a melting point of 140—143℃ can be obtained[66]. Above the melting point, hydrazine monoperchlorate decomposes according to the following overall equation[67]:
8N2H4·HClO4→7NH4ClO4+NH4Cl+4N2+4H2O
The first stage of decomposition is hydrazine monoperchlorate dissociation with the formation of free perchloric acid and hydrazine:
N2H4·HClO4→N2H4+HClO4
The next HClO4decomposition stage in the vapor phase determines the rate of the overall reaction. Intermediate products HO and ClO3, formed during HClO4decomposition, react with hydrazine monoperchlorate:
HO+N2H5ClO4→H2O+NH4ClO4+0.5N2
ClO3+7N2H5ClO4→3H2O+6NH4ClO4+1,5N2+NH4Cl+HClO4
The process of hydrazine monoperchlorate decomposition can be described by the following kinetic relationship[67]:
which shows that the rate of gas release increases until the concentrations of all oxidizing products reach steady-state values. At the end of this time (ti), the concentration of intermediate products remains constant and depends on the pressure of hydrazine monoperchlorate, determined by the temperature of the experiment. The rate is kept constant until all hydrazine monoperchlorate decomposes.
Hydrazine monoperchlorate may detonate from friction or impact. In terms of impact sensitivity, it is comparable to initiating explosives; for the explosion of finely powdered hydrazine monoperchlorate, an impact of less than 2.5kgf/cm2is sufficient. It quickly ignites using an electric igniter made of nichrome wire[45]. To reduce sensitivity to mechanical impacts in the production of solid rocket propellants, it is recommended to prepare eutectic mixtures of hydrazine monoperchlorate with lithium perchlorate in a ratio from 1∶1 to 1∶2 in an inert solvent[68-69].
Hydrazinediperchlorate(N2H4·2HClO4) is a white crystalline powder with the basic substance content of over 99%. It contains 0.15%—0.42% monoperchlorate and 0.04%—0.22% free perchloric acid as primary impurities. Hydrazine diperchlorate is sensitive to impact and heat. It ignites when heated to temperatures above 100℃ at various intervals depending on the particle size and purity degree[66].
The thermal decomposition of hydrazine diperchlorate in an isolated system is characterized by an induction period, at the end of which the reaction accelerates sharply and the substance decomposes completely[67]:
12N2H4·2HClO4→4NH4ClO4+12HClO4+22H2O+10N2+5O2+4Cl2
At the first stage of decomposition, hydrazine monoperchlorate and perchloric acid are formed:
N2H4·2HClO4→N2H4·HClO4+HClO4
The next (autocatalytic) reaction of perchloric acid decomposition determines the rate of the overall process. The activation energy of the thermal decomposition of hydrazine diperchlorate at 100—150℃ is 23.5kcal/mol. During the thermal decomposition of hydrazine diperchlorate, ClO2, ClO4, Cl2O6, and Cl2O7can form, which oxidize the hydrazine component to ammonia with the release of perchloric acid. Intermediate products, HO and ClO3, formed during thermal decomposition, can interact with the initial hydrazine diperchlorate[67]:
HO+N2H5ClO4·HClO4→HO+0.5N2+NH4ClO4+HClO4
ClO3+6N2H5ClO4·HClO4→3H2O+3N2+6NH4ClO4+0.5Cl2+6HClO4
To improve performance properties (reduce sensitivity to mechanical and thermal effects), hydrazine diperchlorate is co-crystallized with ethylenediamine tetraacetic acid[70].
Hydrazinediammoniumtetraperchlorate(NH4)2N2H6(ClO4)4is a highly effective oxidizer ensuring high stability of solid rocket propellant characteristics. It is less hygroscopic than other hydrazine perchlorates. Solid rocket propellant containing 67% hydrazine diammonium tetraperchlorate, 16% hydrocarbon binder, and 16%—20% aluminum has high performance properties[70].
Hydrazinemononitrate(N2H4·HNO3) exists in two crystalline forms. Upon crystallization of hydrazine mononitrate from aqueous solutions at room temperature, a stableα-form is formed with a melting point of 70.7℃. At a temperature of 140℃, theα-form evaporates without decomposition; when heated under vacuum, it decomposes at 200℃. Theβ-form is inherent in a cooled strong solution of hydrazine mononitrate and represents silky needle crystals with a melting point of 62℃. At room temperature, theβ-form changes into a more stableα-form.
The decomposition of hydrazine mononitrate can be described by the following equation[45]:
4N2H4·HNO3→5N2+2NO+10H2O
The rate of decomposition in the air in the temperature range of 188.7—220.4℃ at atmospheric pressure can be described either by the Arrhenius equation:
k=1012.17·e-38,100RT
or the Eyring equation:
k=109.949·e-38,100RT
In the temperature range from 0 to 211℃ (if the difference between the melting heat of NH4NO3and the melting heat of N2H5NO3is neglected), the equation for the thermal decomposition of hydrazine mononitrate takes the following form[71]:
N2H5NO3→0.75nh4NO3+0.25N2O+0.33N2+0.33NH3+0.5H2O+Q
It includes several stages:
N2H5NO3(l)→N2H4(l)+HNO3(l)
3N2H4(l)→4NH3+N2
2HNO3(l)→2NO2+0.5O2+H2O
NH3+N2H5NO3→NH4NO3+N2H4
N2H4+NO2+O2→N2+H2O+N2O
The first stage is equilibrium dissociation characteristic of the thermal decomposition of ammonium salts. The released hydrazine decomposes into ammonia and nitrogen, and nitric acid decomposes into nitrogen dioxide, water, and oxygen. The rate of the overall thermal decomposition of hydrazine mononitrate is determined by the overall reaction of the decomposition of hydrazine and nitric acid.
Hydrazine mononitrate burns in the air and explodes in an enclosed space. In concentrated sulfuric acid, it decomposes to form nitrogen oxides. When it is heated with diluted sulfuric acid, hydronitric acid is formed. Hydrazine mononitrate ignites in contact with such oxidizers as permanganates, chromates, and peroxides. In molten form, it reacts violently with finely powdered copper and zinc, decomposing to produce a flame. Hydrazine mononitrate is resistant to friction; its impact sensitivity is 40—50kgf/cm2.
Researchers studied the effect of pressure (20—130atm) and initial temperature (20—65℃) on the combustion rate of hydrazine mononitrate and temperature distribution in the combustion zone[72]. Such additives as KNO3, NaNO3, LiNO3, and KCl increase the combustion rate significantly but have almost no effect on the lower limit of steady-state combustion. At low pressures, the difference in the accelerating effect of such additives as nitrates and chlorides of alkali metals is less noticeable than at high ones. In their studies, researchers also address the mechanisms of combustion and the effect of additives.
Hydrazine mononitrate is introduced into the composition of liquid rocket propellants based on hydrazine and its methyl-substitutes to reduce the pour point of the propellant. It binds free water and, at the same time, increases specific thrust. The combustion products are characterized by a low molecular mass. Optimal viscous and energetic properties as well as optimal low-temperature behavior are ensured by compositions containing up to 23% hydrazine, 45% methyl-, 1,2-dimethyl- and 1,1-dimethylhydrazine, where the rest is N2H5NO3, NH4NO3, NH4C1O4, and C(NO2)4[73].
Hydrazinedinitrate(N2H4·2HNO3) melts upon rapid heating at a temperature of 103—104℃. It is less stable than mononitrate and decomposes slowly, bypassing the melting stage with the formation of hydronitric acid as one of the reaction products[45].
HydrazinenitroformateN2H4·HC (NO2)3is an orange-yellow solid matter; its physical properties are described in Table 6. Its self-ignition temperature (in 5s) is 165℃, its impact sensitivity is 35—45kgf/cm2. Hydrazine nitroformate is stable under vacuum up to 75℃ for 72 hours; partial decomposition occurs at temperatures above 75℃. Researchers suggested rocket propellant consisting of 50%—70% hydrazine nitroformate as an oxidizer, 5%—25% binder (polybutylenes, hydroxyl-terminated hydrogenated polybutadienes or hydrogenated polyisoprenes), 1.5%—20% Al, and a curing agent (polyisocyanate, polymethylene diisocyanate with an NCO/OH ratio of approx. 0.95—1.3)[74]. Solid rocket propellant including hydrazine nitroformate in combination with hydroxyl-terminated polybutadiene, retains its elasticity for a long time when nitroguanidine is added[75]. High-impulse solid rocket propellant, chemically stable during production and storage, cured at 20℃, can be obtained by mixing 20%—38% hydrazine nitroformate, 5%—35% AlH3, and 30%—60% plasticized nitrocellulose[76].
Hydrazineazidesare suggested to be used as oxidizers for new high-energy solid rocket propellants containing boron, solid borohydrides, borohydride complexes, and ammonium salts of borohydrides as a combustible component[77].
Tetrafluorohydrazine(N2F4) is colorless in the gaseous state but white in the solid state. It has an unpleasant smell. The physical properties of N2F4are given in Table 7. When ignited with a hot filament, N2F4dissociates completely into NF3and N2[45]. At room temperature, it slowly reacts with air and water but does not interact with alkali solutions; at a temperature of 133℃, it is quickly destroyed, being in contact with acidic, neutral, and basic solutions.
During N2F4alkaline hydrolysis, nitrous oxide is mainly produced[78]:
N2F4+4OH-→2NO+4F-+2H2O
4NO+2OH-→N2O+2NO2+H2O
When N2F4interacts with water or aqueous solutions of HCl, a significant amount of nitrogen is released in addition to nitric oxide. Studies of the N2F4-H2O system kinetics indicate a complex hydrolysis mechanism. In this case, the following reactions are possible:
Tetrafluorohydrazine easily reacts with nitrogen dioxide to form NOF, which rapidly hydrolyzes or reacts with glass, thus regenerating nitrogen dioxide. An increase in acidity results in higher concentrations of free nitric oxide by shifting the equilibrium to the left[78].
6 Conclusions
Due to their properties and high reactivity, hydrazine and its derivatives are widely used in various fields of science and technology. In the literature available, considerable attention is given to the use of hydrazines in rocket engineering, fuel cells, etc. The review addressed the properties of such hydrazine fuels as methylhydrazine, 1,1-dimethylhydrazine, hydrazine monoperchlorate, hydrazine diperchlorate, hydrazine diammonium tetraperchlorate, hydrazine mononitrate, hydrazine dinitrate, hydrazine nitroformate, hydrazine azides, tetrafluorohydrazine, etc. as well as composite propellants, and gel propellants based on hydrazine. Depending on tasks to be performed, pure hydrazine or its derivatives can be employed following the information provided in the review. The materials in the review can be used as reference information on hydrazine fuels.
7 Conflict of interest
The author confirms that the provided information contains no conflicts of interest.

