Optimization of Supercritical CO2 Extraction of Curcumin by Response Surface Methodology and Its Antioxidant Activity
Lei WANG Lijun FU Yongcun WANG Xiangdong WANG Shangqing ZHANG Yuxiang LU Liang ZHANG Hefeng ZHANG




Abstract [Objectives] This study was conducted to establish a mathematical model for supercritical CO2 extraction of curcumin.
[Methods] With ginger as the experimental raw material, a quadratic polynomial mathematical model of the yield of curcumin extracted by supercritical CO2 was established by response surface methodology (RSM). The validity of the mathematical model was verified, and the effects of extraction temperature (60-70 ℃), pressure (30-50 MPa) and time (70-90 min) on curcumin yield were analyzed.
[Results] According to the model, the process parameters were optimized. Taking curcumin yield as the index, the optimal process conditions for supercritical CO2 extraction obtained were: temperature 62.6 ℃, pressure 37.7 MPa, time 82.9 min, under which the yield of curcumin was as high as 7.34%. Under the optimal extraction conditions, curcumin had a certain reducing capacity, and showed strong scavenging capacities to ·OH, O -2· and DPPH, and its IC 50 values were 9.40, 9.03 and 8.04 mg/ml, respectively. Therefore, it is feasible to extract curcumin from ginger using supercritical CO2.
[Conclusions] This study provides a theoretical basis for the development and utilization of curcumin.
Key words Ginger; curcumin; Supercritical CO2; Response surface methodology; Antioxidant activity
Ginger refers to the tuberous rhizomes of plants in Zingiber, which are warm in nature and can effectively treat abdominal distension, abdominal pain, diarrhea, vomiting, etc. , caused by eating too much cold food. After eating ginger, people will feel hot in the body, because it can dilate blood vessels, speed up blood circulation, and promote the opening of pores on the body, which will not only remove excessive heat, but also bring out germs and cold in the body. When people eat cold things, get drenched in the rain or stay in an air-conditioned room for a long time, eating ginger can eliminate all kinds of discomfort caused by cold in the body in time [1] .
At present, there are many research reports on curcumin, and the conventional methods for extracting curcumin mainly include solvent extraction, biological enzyme extraction, acid-base extraction, etc. However, these extraction methods all have certain defects. The supercritical CO2 extraction technology [2-4] is a new type of extraction technology developed in the past ten years. It is known as a "green separation technology" because of its advantages of non-toxicity, no solvent residue, low treatment temperature, strong selectivity, non-flammability and safety.
Response surface methodology (RSM) [5-8] is a statistical method that solves multivariate problems by using reasonable experimental designs to obtain certain data through experiments, then fitting functional relationships between factors and response values using multivariate quadratic regression equations and finding optimal process parameters through the analysis of regression equations. Traditional single factor and orthogonal investigation can no longer meet experimental requirements, and the actual error is large, while response surface methodology which combines mathematics and statistics, facilitates rapid modeling, shortens optimization time, and increases confidence in engineering applications.
In this study, with ginger as the target substance and the curcumin yield as the investigation index, the main process parameters affecting the extraction of curcumin by supercritical CO2, i.e. , temperature, pressure and time, were optimized using the SC-CO2 technology and the Design Expert design software by the Box-Behnken response surface methodology (RSM), and a mathematical model of supercritical CO2 extraction of curcumin was established, providing a theoretical basis for the development and utilization of curcumin.
Materials and Methods
Experimental materials and reagents
The experimental material was ginger, which was purchased from the Tangshan farmers market in 2021. The ginger was soaked in cold water below 20 ℃ for 1-2 h, then rinsed with deionized water to remove sediment and inedible impurities, and crushed for use.
CO2 gas (purity>99.5%); reagents such as petroleum ether, methanol, diethyl ether, and NaOH, which were all analytically pure.
Instruments
HL-(5+1)L/50MPa-IIAQ supercritical CO2 fluid extraction equipment: Hangzhou Huali Pump Industry Co., Ltd.; JA1103 electronic analytical balance: Shanghai Haikang Electronic Instrument Factory; MNMF-1818 grain grinder: Hubei Bishan Machinery Co., Ltd.; RE52-2 rotary evaporator: Shanghai Huxi Analysis Instrument Factory Co., Ltd.; UV-3600 ultraviolet- visible spectrophotometer: Shimadzu, Japan.
Supercritical CO2 extraction
The supercritical extraction used an HL-(5+1)L/50MPa-IIAQ supercritical CO2 extraction equipment. A 20 g of sample was weighed into the extraction kettle for each test. The fixed conditions were as follows: ethanol concentration and flow rate of entrainer: 68 % and 0.5 ml/min, respectively, CO2 flow rate: 2 L/min , and outlet valve temperature: 70 ℃.
RSM experimental design
According to the existing experimental conditions and previous single-factor test results, the RSM experimental design was carried out with the curcumin yield as the index, and the three factors that had the greatest impacts on curcumin as independent variables, which were represented by X1, X2 , and X3 , respectively, and +1, 0, and -1 represented the high, medium, and low levels of each independent variable, respectively. The independent variables were coded according to the equation xi=(Xi-X0)/△X , where xi is the coded value of the independent variable, Xi is the true value of the independent variable, X0 is the true value of the independent variable at the central point of the experiment, and △ X is the change step size of the independent variable. The yield Y of curcumin by supercritical CO2 extraction was the response value, and the coding levels of the independent variables of the experiment are shown in Table 1.
X1=(xi-60)/5; X2=(xi-40)/10; X3=(xi-80)/10.
The quadratic polynomial equation fitted by the model by the least squares method was assumed as:
Y = A0+A1 X 1+A2 X 2+A3 X 3+A 12 X 1 X 2+A 13 X 1 X 3+ A 23 X 2 X 3 +A 11 X 21 +A 22 X 22 +A 33 X 23 (1)
In the formula: Y is the predicted response value; A0 is the constant term; A1, A2 and A3 are linear coefficients; A 12 , A 13 and A 23 are interaction coefficients; and A 11 , A 22 and A 33 are quadratic term coefficients.
Experiment of curcumin for scavenging free radical
Determination of reducing capacity
Determination method: Into a 10 ml stoppered test tube, 1 ml of sample solution, 0.2 ml of Na3PO4 buffer (0.25 mol/L, pH 6.6) and 0.5 ml of 1% K3[Fe(CN)6] were added. The reaction solution was heated in a constant temperature water bath at 50 ℃ for 20 min of reaction, and then rapidly cooled and added with 1 ml of 10% trichloroacetic acid solution. Then, 1.5 ml of the reaction solution was taken and added with 3 ml of distilled water and 0.2 ml of 1% FeCl3 solution. The obtained solution was mixed uniformly and determined 10 min later at 700 nm for absorbance, with distilled water as a blank control. The larger the absorbance value, the stronger the reducing capacity.
Determination of ·OH scavenging rate
Referring to the Fenton reaction system model, a substance with the ability to scavenge ·OH added into the reaction system (salicylic acid-ethanol solution 9 mmol/L, Fe 2+ 9 mmol/L, H2O2 8.8 mmol/L) will compete with salicylic acid for ·OH, which reduces the generation of colored substances. According to the fixed reaction time method, different concentrations of curcumin solutions were added to the same volume of reaction system, respectively, and the absorbance after adding different concentrations of curcumin solutions was measured at a wavelength of 510 nm, with distilled water as a blank control. The absorbance was substituted into the calculation formula of scavenging rate to calculate the capacities of various curcumin solutions with different concentrations to scavenge ·OH free radicals.
·OH scavenging rate (%)= (1- A1-A2 A3 ) ×100
In the formula, A 1 is salicylic acid-ethanol 0.5 ml+GPS 1.0 ml+Fe 2+ 0.5 ml+H2O2 5.0 ml; A 2 is salicylic acid-ethanol 0.5 ml+GPS 1.0 ml+distilled water 0.5 ml+H2O2 5.0 ml; and A 3 is salicylic acid-ethanol 0.5 ml+distilled water 1.0 ml+Fe 2+ 0.5 ml+H2O2 5.0 ml.
Determination of O -2·scavenging rate
O -2· was generated by pyrogallol auto-oxidation. Specifically, 4.0 ml of 0.05 mol/L Tris-HCl buffer (pH 8.2) was preheated in a 25 ℃ water bath for 20 min, and then added with 1 ml of the test solution and 1 ml of 25 mmol/L pyrogallol solution, respectively. After being mixed well, the reaction solution reacted in a 25 ℃ water bath for 5 min and then added with 100 μl of 8% HCl solution to terminate the reaction. Absorbance (A) was measured at a wavelength of 320 nm. A blank test was performed with 1 ml of distilled water instead of the test solution.
O -2·scavenging rate (%)= (1- A1-A2 A3 ) ×100
In the formula, A 1 is Tris-HCl 4 ml+GPS 1.0 ml+pyrogallol 2 ml; A 2 is Tris-HCl 4 ml+GPS 1.0 ml+distilled water 2 ml; and A 3 is Tris-HCl 4 ml+distilled water 1.0 ml+ pyrogallol 2 ml.
Determination of DPPH scavenging rate
DPPH free radicals are a kind of stable free radicals in 95 % ethanol solution, and have an absorption peak at 517 nm, which is purple. When free radical scavengers exist, the lone pair of electrons of DPPH is paired, the color becomes lighter, and the absorbance becomes smaller at the maximum absorption wavelength, and the color change has a stoichiometric relationship with the number of paired electrons. Therefore, it can be used to evaluate the scavenging of free radicals. During determination, 4 ml of the solution to be tested was added to 2 ml of 0.2 mmol/L DPPH free radical solution, and the absorbance (Ai) was measured at 517 nm after reacting in a water bath at 25 ℃ for 20 min. A blank test was performed with distilled water instead of the test solution.
DPPH scavenging rate (%)= (1- A1-A2 A3 ) ×100
In the formula, A 1 is GPS 4 ml+DPPH free radicals 2.0 ml; A 2 is GPS 4 ml+95% ethanol 2.0 ml; and A 3 is distilled water 4 ml +DPPH free radicals 2.0 ml.
Data processing
Design-Expert 7.0 was used for experimental design and data processing.
Results and Analysis
Box-Behnken experimental design and experimental results
Box-Behnken design was adopted for the multi-factor experimental design, and the experimental scheme and results are shown in Table 2. The 15 experimental points of the experimental scheme included 12 factorial points (1-12) and 3 center points (13-15). The purpose of repeating the center points was to estimate the pure experimental error of the whole experiment.
Model equation establishment and significance analysis
Software Design Expert was used to perform quadratic linear regression fitting on the experimental data in Table 2, obtaining the mathematical model:
Y= 7.25-0.29X1-0.27X2+0.32X3-1.01X 21-0.73X 22- 0.61X 23 -0.19X1X2+0.33X1X3-0.26X2X3 (3)
The significance test of the model coefficients is shown in Table 3 , and the variance analysis results of the model are shown in Table 4. It can be seen from Table 4 that the model was extremely significant ( P <0.01), the linear relationship between the dependent variable and the investigated independent variables was significant ( R 2=0.965 4), and the adjusted coefficient of determination of the model was R 2 Adj =0.903 2, indicating that the model could explain 90.32% of change in the response value. The fitting degree of the model was good, and the lack of fit term was not significant ( P >0.05), indicating that the quadratic regression equation obtained in this experiment could predict the response value well. From the significance test on the coefficients of the regression equation in Table 3, it could be seen that in the curcumin yield model, the primary terms of temperature X 1, pressure X 2, and time X 3 were significant; the quadratic terms X 21, X 22, and X 23 were extremely significant; and the interaction term X 1 X 3 was significant, but X 1 X 2 and X 2 X 3 were not significant.
Response surface analysis and optimization of curcumin yield in supercritical CO2 extraction
Under the condition of fixed extraction time of 80 min ( x 3=0), the response surface and corresponding contour of the effects of extraction temperature and pressure and their interaction on curcumin yield are shown in Fig. 1. It can be seen from Fig. 1 that the interaction between extraction temperature and extraction pressure was significant ( P <;0.05). In the low temperature region, the curcumin yield increased first and then decreased with the increase of the pressure, while in the high temperature region, with the increase of the pressure, the curcumin yield showed an increasing trend, and when the pressure reached a certain value, the curcumin yield changed gently, followed by a downward trend. It was inferred from this that when the temperature was constant, as the pressure increased, the density of the CO2 fluid increased, and the solubility of the CO2 fluid also increased. In the initial stage of extraction, the curcumin yield was extremely sensitive to the pressure change, and when the pressure exceeded 40 MPa, the curcumin yield began to decline. From the perspective of economy and safety, the extraction pressure of 35-40 MPa is appropriate. In the low pressure region, the curcumin yield showed a trend of first increasing and then decreasing with the increase of temperature, while in the high pressure region, the curcumin yield showed an increasing trend, and when the temperature reached a certain value, the curcumin yield changed gently and then decreased.
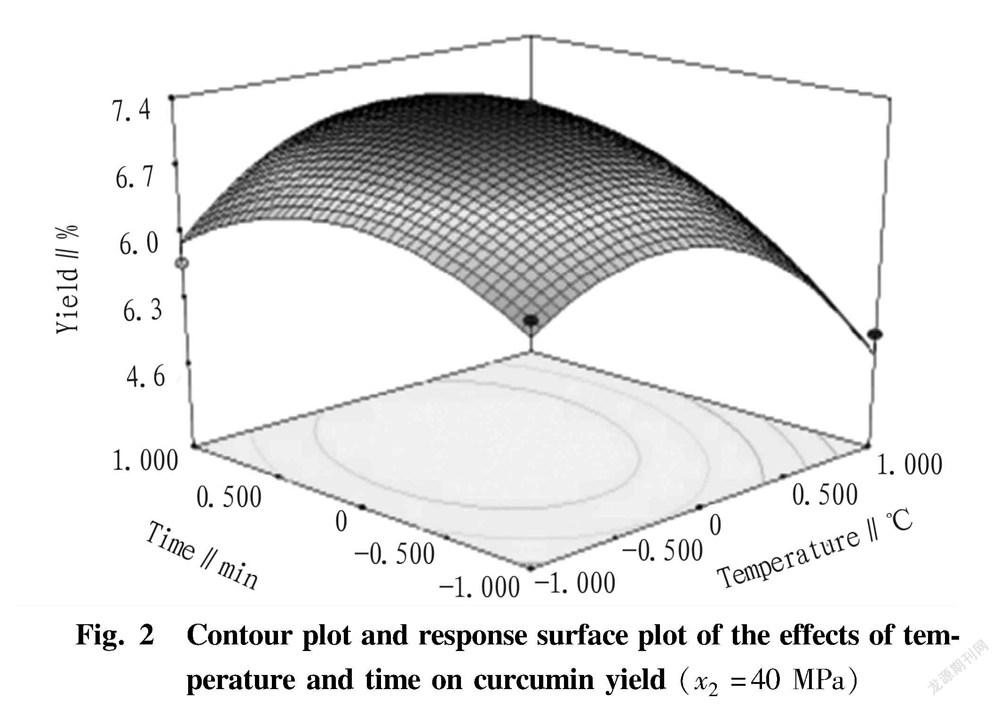
Under the condition of fixed extraction pressure of 40 MPa ( x 2=0), the response surface and corresponding contour of the effects of extraction temperature and time and their interaction on curcumin yield are shown in Fig. 2. As can be seen from Fig. 2, the interaction between extraction temperature and time was significant ( P <0.01). In the low temperature region, the curcumin yield increased first and then decreased with the increase of extraction time, while in the high temperature region, the curcumin yield increased gradually with the increase of time. In the short time region, the curcumin yield increased first and then decreased with the increase of the extraction temperature, while in the long time region, the curcumin yield showed an upward trend with the increase of the temperature, and when the temperature reached a certain value, the curcumin yield changed gently and showed a subsequent downward trend. It showed that a too-low or too-high temperature was not conductive to the curcumin yield. Therefore, the extraction temperature is generally selected in the range of 60-65 ℃ to achieve an ideal result.
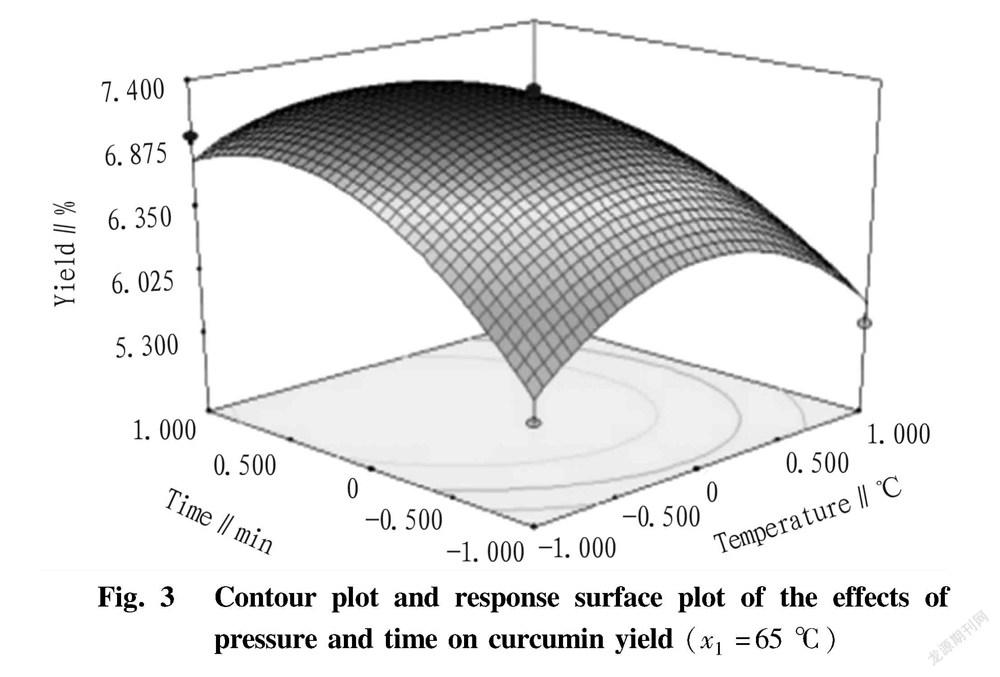
Under the condition of fixed extraction temperature of 65 ℃ ( x 1=0), the response surface and corresponding contour of the effects of extraction pressure and time and their interaction on curcumin yield are shown in Fig. 3. It can be seen from Fig. 3 that the interaction between extraction pressure and time was significant ( P <0.01). In the low pressure region, the curcumin yield increased with the increase of time, and when the extraction time reached a certain value, the curcumin yield changed slowly, followed by a downward trend, while in the high pressure region, the curcumin yield showed a slow upward trend with the increase of time. It showed that the extraction time was proportional to the curcumin yield in the initial period, and after a certain period of extraction, the extraction time had no significant effect in improving the curcumin yield. When the extraction time reached 85 min, the basic extraction was completed. In the short time region, the curcumin yield increased first and then decreased with the increase of pressure, while in the long time region, the curcumin yield changed gently with the increase of the pressure.
Performing the first-order partial derivative of the three independent variables of the three-dimensional nonlinear regression equation and setting them to zero, the conditions for higher curcumin yield were obtained: X 1=-0.076, X 2=-0.226, X 3=0.290 , with which the response value was Y =7.34. The conditions were converted into actual parameters, that is, at a temperature of 64.6 ℃, a pressure of 37.7 MPa, and a time of 82.9 min, with which the curcumin yield was as high as 7.34%. Under the optimal conditions, the experiment was verified and repeated three times. The average curcumin yield was 7.36%, and the relative deviation between the two was far less than 5%. Therefore, the extraction process obtained by response surface methodology is stable and reliable, and has certain reference value.
Model validation
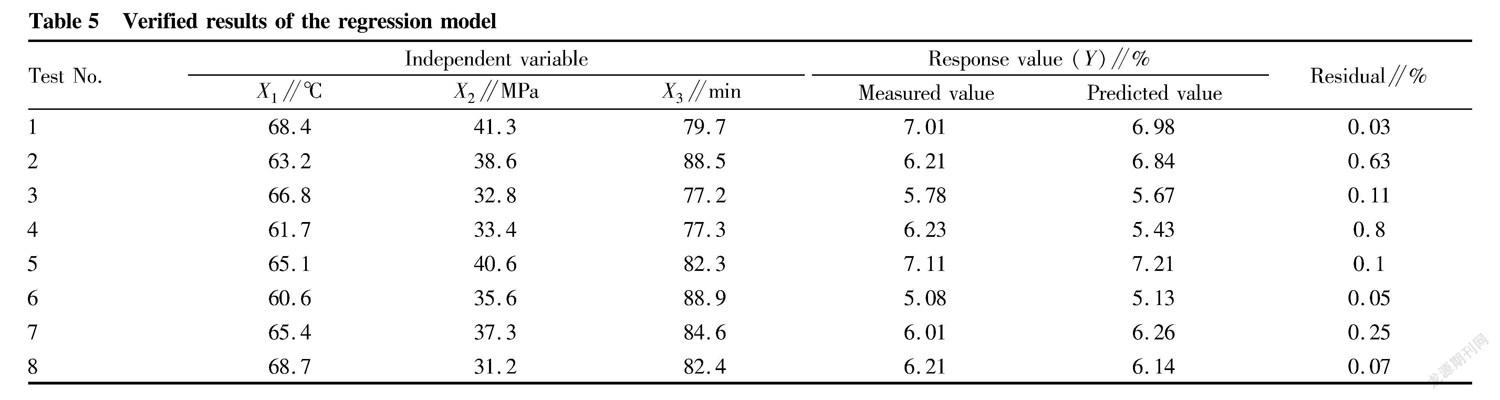
In order to better apply the supercritical CO2 extraction process of curcumin to practice, the parameters in the above model were tested and verified to obtain a better regression model. Through above-mentioned analysis, verification tests were carried out according to 8 groups of parameters of x1, x2, x3 in table 5, and prediction was performed with the 8 groups of extraction process parameters by the regression model. The test results were analyzed by Design-Expert software. The correlation coefficient between the measured values and the predicted values was 0.948 7 ( P <0.01), and the average relative error was 0.26 %, which proved that the model could well evaluate the effect of supercritical CO2 extraction of curcumin.
Scavenging effects of curcumin on free radicals
Study on the reducing capacity of curcumin
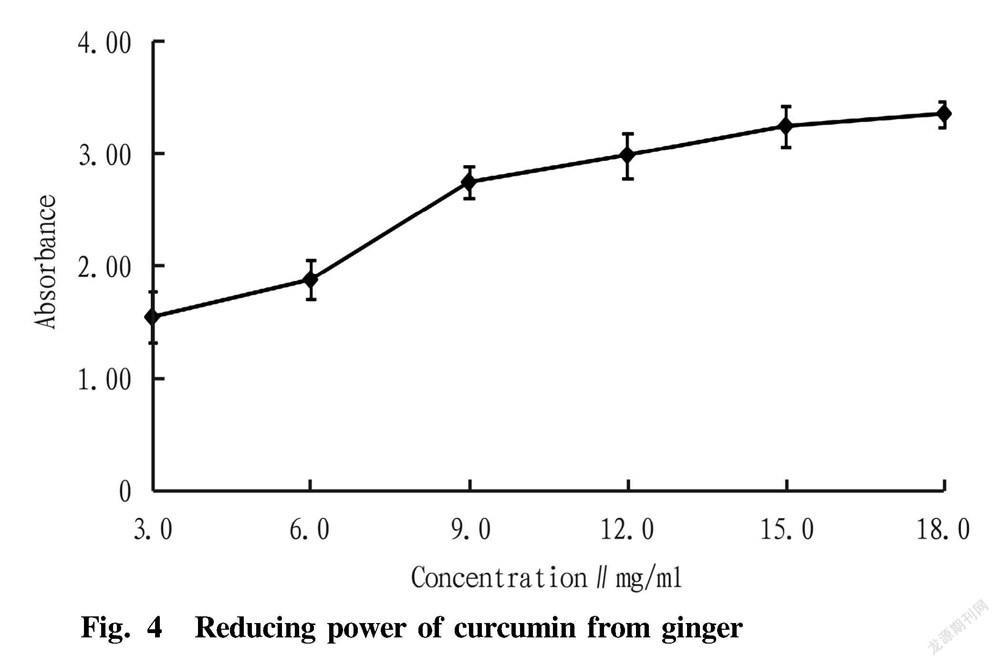
The determination of reducing capacity is to test whether the sample is a good electron supplier, and the sample with a strong reducing capacity should be a good electron supplier. The stronger the reducing capacity, the stronger the oxidation resistance. The reducing capacity results of curcumin are shown in Fig. 4.
As shown in Fig. 4, with the increase of the concentration, the reducing capacity of curcumin was continuously enhanced. When the concentration of curcumin reached 18.0 mg/ml, the absorbance value reached about 3.4, which proved that curcumin had a certain reducing capacity.
Scavenging effect of curcumin on ·OH
As shown in Fig. 5, in the concentration of 3.0-18.0 mg/ml, the scavenging rate was linear with the GPS concentration. The linear equation was y= 4.390 5 x +8.733 3 (R 2=0.999 4). According to the linear equation, the IC 50 (mass concentration of curcumin when scavenging 50% of free radicals) was 9.40 mg/ml.
Scavenging effect of curcumin on O -2·
As shown in Fig. 6, in the concentration of 3.0-18.0 mg/ml, the scavenging rate had a linear relationship with the concentration of curcumin. The linear equation was y=3.409 5x+19.214 (R 2=0.999 2). According to the linear equation, the IC 50 was 9.03 mg/ml.
Scavenging effect of curcumin on DPPH
As can be seen from Fig. 7, in the concentration of 3.0-18.0 mg/ml, the scavenging rate had a linear relationship with the concentration of curcumin. The linear equation was y =5.009 5 x +9.733 3 ( R 2=0.999 3). According to the linear equation, the IC 50 was 8.04 mg/ml.
Conclusions
① The process model of supercritical CO2 extraction of curcumin was established by response surface methodology (RSM). The adjusted coefficient of determination of the model was R 2 Adj = 0.903 2 , which indicated that the model could explain 90.32% of the change of the response value. The fitting degree of the model was good, and the lack of fit was not significant ( P >0.05), which indicated that the quadratic regression equation obtained in this study could well predict the law of curcumin yield changing with each parameter.
② Through software Design Expert, the process of supercritical CO2 extraction of curcumin was designed and optimized by the Box-Behnken experimental design method. The results showed that the effects of extraction temperature, pressure and time on the extraction yield of curcumin were all significant ( P <0.05); and the interaction between temperature and time was significant ( P < 0.05), while the interaction between temperature and pressure and between pressure and time was not significant ( P >0.05).
③ The optimum process conditions for supercritical CO2 extraction of curcumin were as follows: extraction temperature 62.6 ℃, pressure 37.7 MPa, and time 82.9 min, under which the polysaccharide yield was 7.34%. The test proves that the extraction process parameters of curcumin are reasonable and reliable.
④ Curcumin had a certain reducing capacity, and showed strong scavenging capacities to ·OH, O -2· and DPPH, and its IC 50 values were 9.40, 9.03 and 8.04 mg/ml, respectively.
References
[1] WU T. Research of extract of ginger ( Zingiber officinale Roscoe) in the preservation of silver carp ( Hypophthalmichthys molitrix )[J]. Journal of Yangtze University: Natural Science Edition, 2010, 7(1): 79-82. (in Chinese).
[2] GROSSO C, FERRARO V, FIGUEIREDO AC, et al. Supercritical carbon dioxide extraction of volatile oil from Italian coriander seeds[J]. Food Chemistry, 2008, 111(1): 197-203.
[3] ZAIDUL ISM, NORULAINI NAN, OMAR AKM, et al. Supercritical carbon dioxide (SC-CO2) extraction and fractionation of palm kernel oil from palm kernel as cocoa butter replacers blend[J]. Journal of Food Engineering, 2006, 73: 210-216.
[4] ARIASM, PENICHET I, YSAMBERTT F, et al. Fast supercritical fluid extraction of low and high-density polyethylene additives comparison with conventional reflux an automatic soxhlet extraction[J]. J Supercrit Fluids, 2009, 50(1): 22-28.
[5] ELIANA VC, GEORGE JMR, JOAO ACT, et al. Optimization of acid hydrolysis from the hemicellulosic fracton of of Eucalyptus grandis residue using response surface methodology[J]. Bioresource technology, 2007, 98: 422-428.
[6] ZHANG JW, FU DF, PENG QJ, et al. Optimized conditions for production of xylose by acid-hydrolysis of rice straw based on response surface methodology[J]. Transactions of the Chinese Society of Agricultural Engineering, 2009, 25(11): 253-257. (in Chinese).
[7] KAHRAMAN F. The use of response surface methodology for prediction and analysis of surface roughness of AISI4140 steel[J]. Material and Technology, 2009, 43(5): 267-270.
[8] BAS D, BOYACI IH. Modeling and optimization I: Usability of response surface methodology[J]. Journal of Food Engineering, 2007, 78 (3): 836-845.
Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Molecular Cloning and Bioinformatics Analysis of crp Gene in Vibrio alginolyticus
- Breeding Technology of Sunflower Inbred Lines with Four Generations in One Year
- Artificial Plant Seeds and Their Application
- Breeding of a Water-saving Drought-resistant Two-line Hybrid Rice Variety Wanliangyou 1008
- Development of Landscaping and Selection of Contemporary Landscaping Plants
- The Register and Characteristics of Chinese Key Protected Wild Plants in Dabie Mountains National Nature Preserve, Hubei Province

