Polyacrylamide gel migration after injection for breast augmentation:A case report
Rongshuai Yan,Yujie Lan,Zeyuan Lei,Yao Chen,Dongli Fan,Yiming Zhang ,Shaoliang Wang
Department of Plastic and Cosmetic Surgery,Xinqiao Hospital,Army Medical University,Chongqing 400037,China
Keywords:Polyacrylamide hydrogel Surgical complication Material migration Breast augmentation
ABSTRACT Polyacrylamide hydrogel (PAAG) was once considered a safe,reliable,and compatible injected filler and was widely used in breast augmentation,rhinoplasty,and other cosmetic surgeries.However,numerous complications,such as implant migration,have been observed after PAAG injections.Herein,we report a rare case of distant implant migration after PAAG injection for breast augmentation in which the material became displaced along the abdominal wall to the perineum and pelvic extraperitoneal space.After a well-prepared preoperative evaluation involving magnetic resonance imaging(MRI)and computed tomography(CT)examinations and threedimensional hologram,debridement surgery was performed to remove the injected material.After the operation,the patient was followed up for two years and was not scheduled for a second operation.Postoperative complications of breast augmentation after PAAG injection,especially gel migration,still affect thousands of patients.Once material migration occurs,surgical removal becomes difficult.Early diagnosis and treatment are recommended.
1.Introduction
“Amazingel”is the trade name of a polyacrylamide hydrogel(PAAG)used in China.It was approved by the National Medical Products Administration in December 2000 and has been used as a soft tissue filler for injected breast augmentation,rhinoplasty,cheek augmentation,temporal augmentation,and other cosmetic surgeries.However,it was banned for production,sale,and use in April 2006 because of its numerous complications.1,2During this period,over 200 000 people received PAAG injections for breast augmentation in China according to incomplete statistics.3Accordingly,the treatment of PAAG-related complications has become an important medical issue in the field of plastic surgery in China.4Approximately 300 clinical cases have been treated in our department in the past ten years.The migration of injected material is a common complication after PAAG augmentation mammoplasty;however,it is mostly confined to the breast and chest wall.In this paper,we report a rare case in which the material was displaced along the abdominal wall to the perineum and pelvic extraperitoneal space.
2.Case presentation
A 37-year-old woman was referred to our plastic surgery department with a chief complaint of a half-year history of a mass in the perineum.Eleven years ago,the patient underwent breast augmentation with PAAG injection at a local hospital.Two years ago,the patient underwent removal of the injected breast augmentation material and replacement with silicone prostheses in a private hospital.Six months ago,masses in the right abdominal wall and perineum appeared without any other discomfort and thus was not treated.Two months ago,masses in the left breast and induration of the right breast were observed.The patient was referred to a local cosmetic hospital and underwent puncture of the left breast to extract the effusion;however,the problem was not resolved.Thereafter,the patient visited several medical institutions with no resolution;therefore,the patient was referred to our department for further treatment.
On physical examination,the bilateral breasts were asymmetrical,prosthetic materials were palpable,bilateral breasts showed swelling,right pubes showed obvious bulging,and cystic masses were palpable on the right abdominal wall spanning from the umbilical plane to the right perineum;the mass was soft and moved with positional changes(obvious swelling in the right abdomen and perineum appeared in the standing position compared to the prone position)(Fig.1).
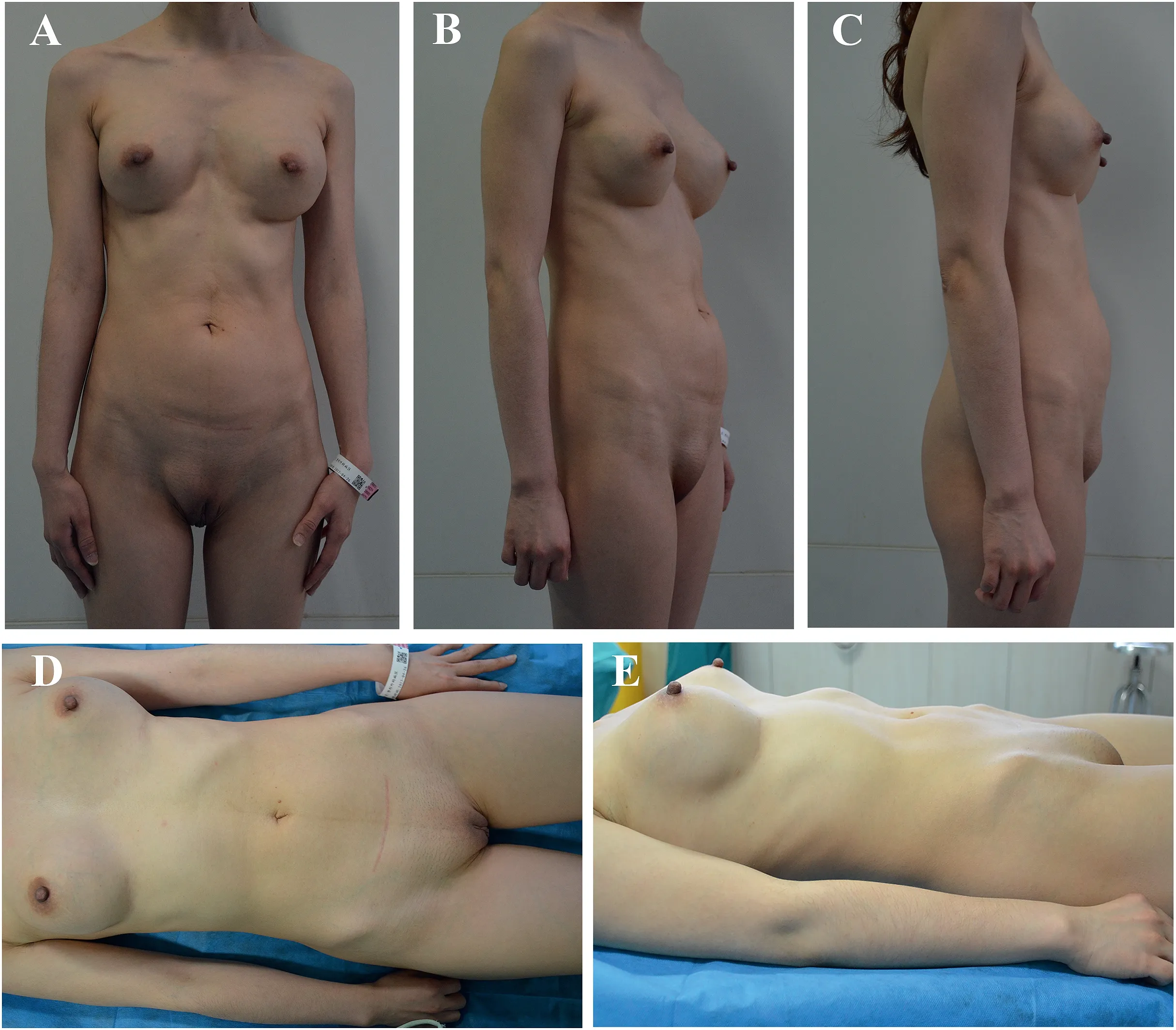
Fig.1.(A–C) Preoperative photos in the standing position.(D–E) Preoperative photos in the supine position.
Before the operation,magnetic resonance imaging (MRI) and computed tomography (CT) examinations were performed to assess the distribution and range of PAAG migration (Fig.2).The images demonstrated that the gel in the right breast had migrated along the right abdominal wall to the perineum and pelvis.A three-dimensional hologram showed communication between the perineum and pelvic mass,and the pelvic mass was adjacent to the right iliac vessels(Fig.3).
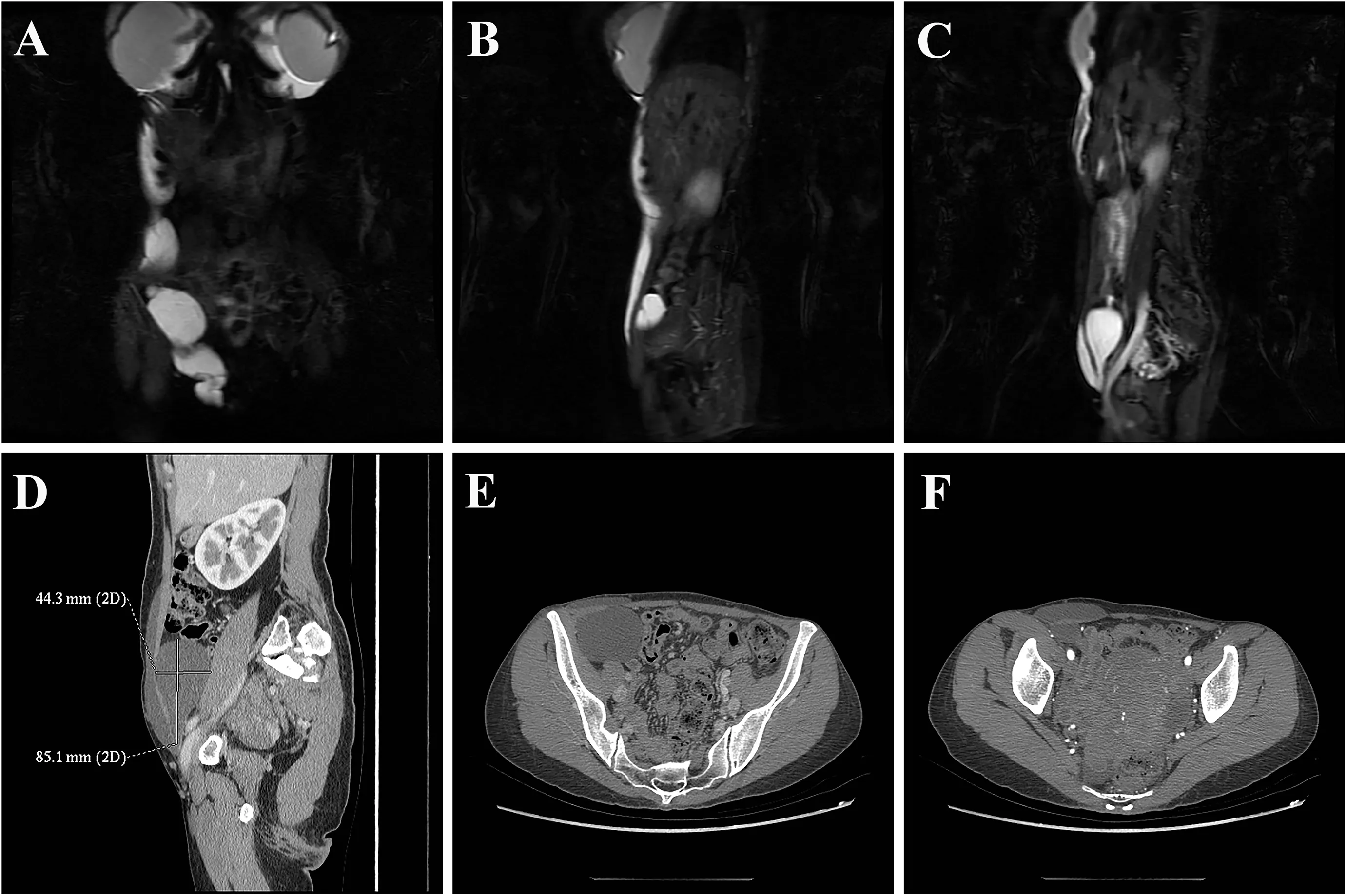
Fig.2.(A–C) MRI showing long T1 and long T2 abnormal signal intensities around the prosthesis and within the soft tissues of the right abdominal wall,extending down to the perineum and the right anterior part of the pelvis;the signal strengths were similar.(D–F)Enhanced CT revealing cystic low-density shadows under the skin of the right abdominal wall,the right perineum,and the right anterior part of the pelvis.There was communication between the perineum and the pelvic mass.
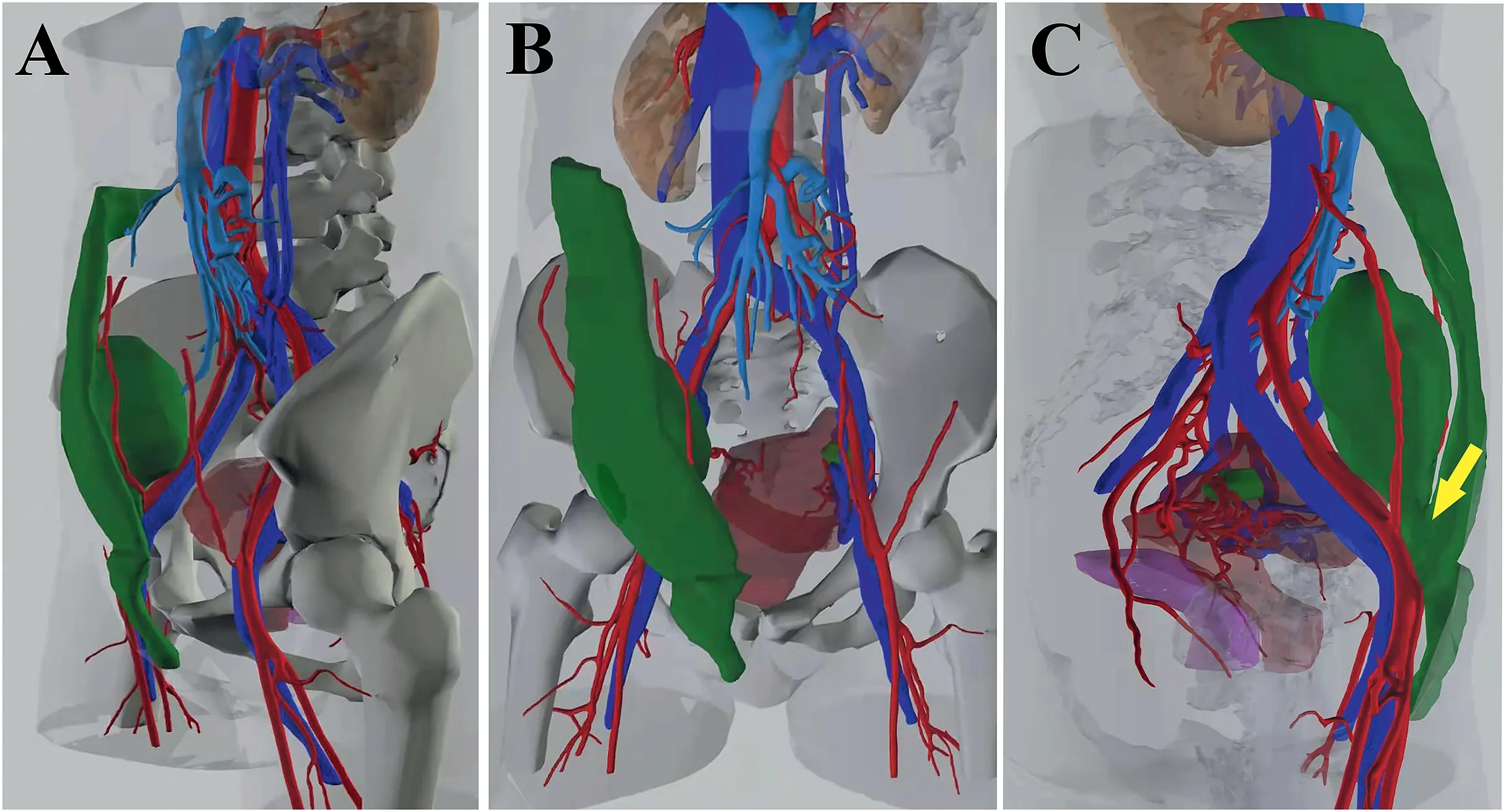
Fig.3.Three-dimensional holograms showing communication between the perineum and the pelvic mass (yellow arrow).The pelvic mass was adjacent to the right iliac vessels.
After a well-prepared preoperative evaluation with multidisciplinary consultation,debridement surgery was performed to remove the injected material with the assistance of general surgery.The operation was performed with small incisions extending from the submammary plica to the mid-axillary line of the abdominal wall and groin.Ultrasound-guided positioning was used to fully understand the scope of material displacement and shaping.During the operation,the silicone prostheses were removed from both breasts,and the PAAG in the breast prosthesis cavity was removed under direct vision.After repeated irrigation,a drainage tube was placed for continuous vacuum suction,and the incisions were sutured layer by layer.At the right groin area and the right abdominal wall near the mid-axillary line,small incisions were made,and the injected material and degenerated tissues were cleared by repeated irrigation with negative-pressure suction,followed by adequate drainage,suturing to block the displacement channel,postoperative compression bandaging,and continuous negative-pressure suction.The material migrated into a deep position within the pelvic extraperitoneal space which was observed intraoperatively,and the channel was turned along the muscle space with irregular tissue intervals.Complete eradication of the material in the first stage was difficult,and the incidence of postoperative inguinal hernia was high.Moreover,the patient had a strong desire for an aesthetically pleasing incision.After talking with relatives of the patient,the sinus tract in the right groin area was blocked and obstructed in the first stage,and the mass in the pelvic extraperitoneal space was removed during elective surgery.One week after the operation,the wound healed,and the swelling in both breasts,right abdominal wall,and perineum significantly improved (Fig.4).The patient was followed up at the outpatient clinic after discharge.Regular ultrasound examinations revealed no significant increase in pelvic mass size.The patient had no symptoms and was not scheduled for a second operation.
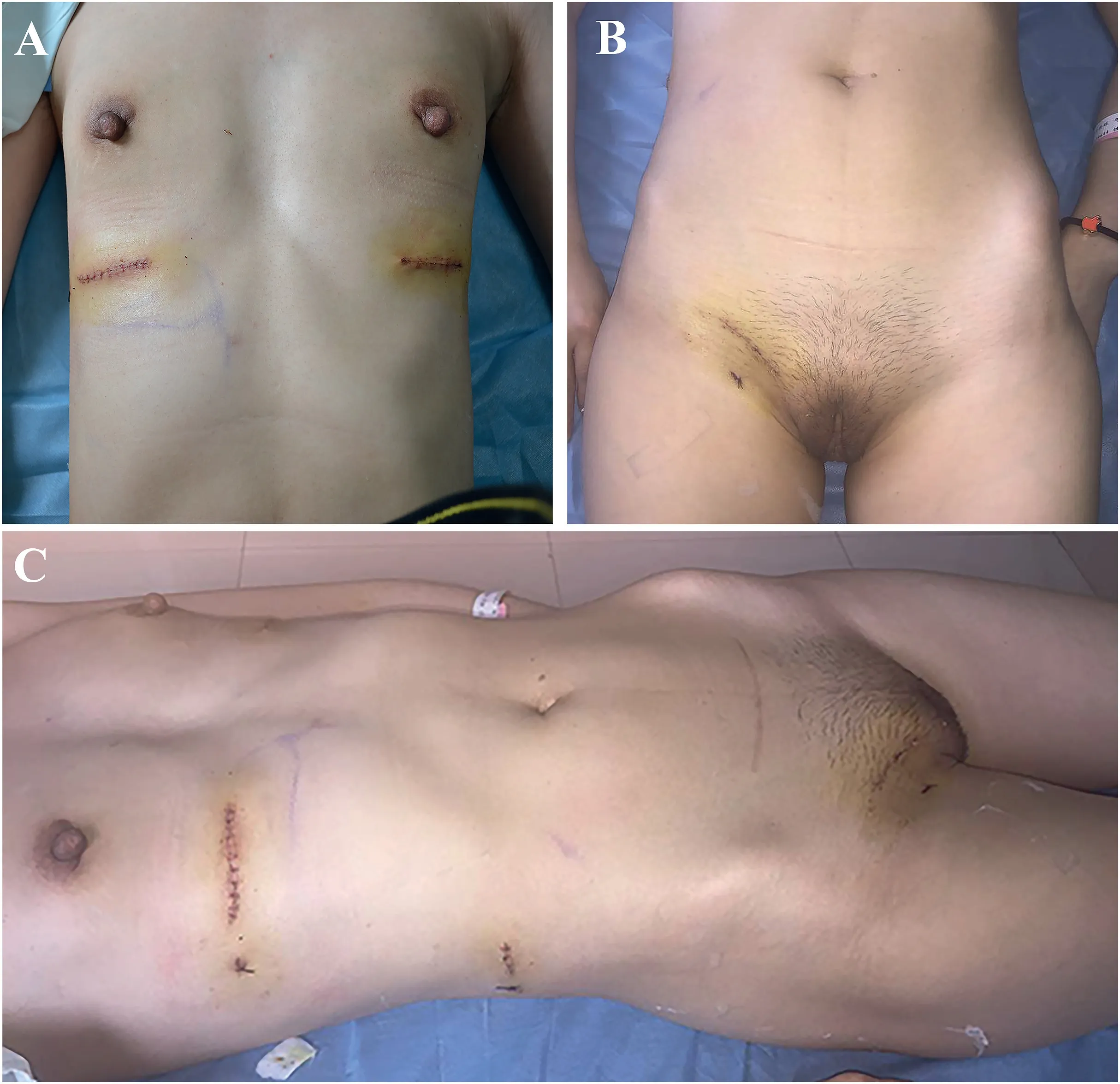
Fig.4.Postoperative day 7 after suture removal showing significant improvement.
3.Discussion
PAAG is a polymer composed of acrylamide and methacrylamide that has been found to be colorless,water soluble,and viscous.5PAAG as a soft tissue filler has been widely used in cosmetics in China,especially in augmentation mammoplasty.Later,it was banned by the China’s National Medical Products Administration in 2006 due to long-term complications.The safety of PAAG remains controversial.It has been found to be nontoxic and noncarcinogenic in several animal studies.6However,recent studies have reported cases of cancer development following PAAG injection7–9and long-term degradation of monomers in the body resulting from carcinogenicity and neurological and reproductive toxicity.10The reported complications accompanied by PAAG injection for breast augmentation include pain,induration,breast deformation,distant migration,and infection,2,10causing tremendous stress for patients both physically and psychologically.When the situations described above occur,surgical removal of the injected material should be the preferred approach.Distant migration of the injected material proves more complicated,as it results from disruption of the fibrous capsule on the surface of the injected material under the action of gravity or muscle contraction and other external forces.11Thus,the treatment of distant migration is often difficult and requires multiple incisions.This case report describes a patient with a peculiar distant migration.The injected material had migrated to the lateral abdominal wall and perineum along the intermuscular space and subcutaneous tunnels and extended into the pelvic extraperitoneal cavity along the weak area of the abdominal wall in the superficial and deep inguinal rings.Cystic masses were also present.To date,no similar cases have been reported.
The reasons for distant implant migration after augmentation mammoplasty with PAAG injection are unknown but are correlated with factors such as injection volume,injection level,gravity,and mechanical forces and may also be related to the body’s response to foreign materials.Surgical removal of an implant is the most common procedure for complications.
In addition to the complexity and variability of surgical removal,postoperative breast deformity is a major concern for surgeons and patients.Based on the treatment of a large number of cases in the past ten years,our clinical experiences are as follows:preoperative imaging examinations,especially MRI examination,should be improved12;the distribution and range of migration should be clarified;and the medical conditions and requests of patients need to be fully considered when making surgical plans.Surgery should not blindly pursue complete removal;if so,unacceptable outcomes may occur,such as postoperative breast deformities and incision scars.The overall objective of treatment should be to completely clear the injected material and capsule in the primary breast lesion,exclude potential tumor risk,and consider the contour and shape of the breast.If the fibrous capsule on the surface of the injected material is thick with induration in the breast and if a solid mass is present,ideal surgical results can be obtained using marginal incision of the areola or inferior fold incision to dissect the capsule and completely remove the mass(Fig.5).If the foreign capsule on the surface of the injected material is thin or free or if even distant migration is present with the injection being jelly-like in a large area,complete surgical removal is almost impossible,and a small incision combined with suction and repeated irrigation (Fig.5) is often more acceptable to patients.Moreover,endoscopy combined with waterjet debridement can be used if the conditions permit.After the operation,continuous negative-pressure suction and compression bandaging can accelerate the intracavity tissue adhesion.Regular postoperative follow-ups are critical.Patients should be instructed to undergo ultrasound and other imaging examinations during regular clinic visits.Prompt treatment is required,provided that redness,swelling,masses,and other symptoms occur again.
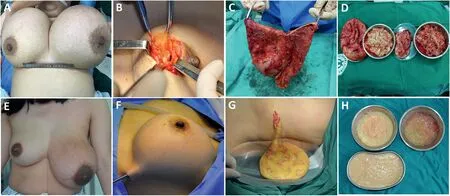
Fig.5.(A–D)Marginal incision of the areola completely resecting the capsule and necrotic tissue.(E–H)Small incisions combined with suction for repeated irrigation.
4.Conclusion
Postoperative complications of breast augmentation after PAAG injection,especially gel migration,still afflict thousands of Chinese females.Once material migration occurs,surgical removal is more difficult.Therefore,we recommend that all medical institutions use formal breast augmentation fillers instead of PAAG.Patients who have undergone breast augmentation with PAAG injection should regularly visit medical institutions for comprehensive examinations early and receive regular treatment to remove the injected material,instead of visiting after complications have occurred.
Ethics approval and consent to participate
The need for ethical approval and consent to participate was waived as this is a case report.
Consent for publication
The patient gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests.
Authors’contributions
Yan R:Writing-Original Draft,Funding acquisition.Lan Y:Data curation,Investigation.Lei Z:Methodology.Chen Y:Investigation.Fan D:Supervision.Zhang Y:Resources,Project administration.Wang S:Supervision,Conceptualization.
Acknowledgments
This work was supported by the Chongqing Higher Education Teaching Reform Project(grant no.193373).
 Chinese Journal of Plastic and Reconstructive Surgery2022年1期
Chinese Journal of Plastic and Reconstructive Surgery2022年1期
- Chinese Journal of Plastic and Reconstructive Surgery的其它文章
- Androgen-related disorders and hormone therapy for patients with keloids
- Prospective application of poloxamer 188 in plastic surgery:A comprehensive review
- A systematic review of the treatment of lower eyelid retraction and our attempt of a dermal-orbicularis oculi suspension flap
- Using the parietal branch of superficial temporal vessels:A good approach to total ear replantation
- Misdiagnosis of malignant meningioma in subcutaneous soft tissue of the forehead:A case report
- External application of a Nocardia rubra cell wall skeleton in the treatment of diabetic foot ulcers
