Successful Production of an All-Female Common Carp(Cyprinus carpio L.)Population Using cyp17a1-Deficient Neomale Carp
Gng Zhi, Tingting Shu, Kungxin Chen, Qiyong Lou, Jingyi Ji, Jinfei Hung, Chung Shi,Xi Jin, Jingyn He, Donghuo Jing, Xueqio Qin, Wei Hu,e, Zhn Yin,e,*
a State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
b College of Advanced Agricultural Sciences, University of Chinese Academy of Sciences, Beijing 100101, China
c College of Fisheries, Huazhong Agricultural University, Wuhan 430070, China
d HAID Research Institute, Guangdong HAID Group Co., Ltd., Guangzhou 511400, China
e The Innovative Academy for Seed Design, Chinese Academy of Sciences, Beijing 100101, China
Keywords:Common carp Sexual dimorphism Growth cyp17a1 Sex steroids All-female population
ABSTRACT Due to sexual dimorphism in the growth of certain cultured fish species, the production of monosex fishes is desirable for the aquaculture industry.Nowadays,the most widely practiced technique available for the mass production of monosex fish populations is sex steroid-induced sex reversal. Here, a novel strategy for the successful production of all-female(AF)common carp(Cyprinus carpio L.),to take advantage of the sexual dimorphism in growth documented in this species, has been developed using genetic engineering via single gene-targeting manipulation without any exogenous hormone treatments. Male and female heterozygous cyp17a1-deficient common carp were first obtained using the clustered regularly interspaced short palindromic repeats/CRISPR-associated endonuclease 9 (CRISPR/Cas9) technique.An all-male phenotype for homozygous cyp17a1-deficient carp, regardless of the individuals’ sexdetermination genotypes (XY or XX), has been observed. A male-specific DNA marker newly identified in our laboratory was used to screen the neomale carp population with the XX genotype from the cyp17a1-deficient carp. These neomale carp develop a normal testis structure with normal spermatogenesis and sperm capacity. The neomale common carp were then mated with wild-type (WT) females(cyp17a1+/+ XX genotype) using artificial fertilization. All the AF offspring sample fish from the neomale-WT female mating were confirmed as having the cyp17a1+/-XX genotype,and normal development of gonads to ovaries was observed in 100.00%of this group at eight months post-fertilization(mpf).A total of 1000 carp fingerlings, 500 from the WT male and female and 500 from the neomale and WT female mating, were mixed and reared in the same pond. The average body weight of cyp17a1+/- XX females was higher by 6.60% (8 mpf) and 32.66% (12 mpf) than that of the control common carp. Our study demonstrates the first successful production of a monosex teleost population with the advantages of sexual dimorphism in growth using genetic manipulation targeting a single locus.
1. Introduction
The aquaculture industry has shown the most rapid growth in food production in the past several years[1].Monosex fish production is desirable in aquaculture,as some fish species exhibit sexual dimorphism in growth,with one sex growing faster than the other.Fish also exhibit a large variety of mechanisms of sex determination,including genetic sex determination(GSD)and environmental sex determination. However, sex determination in fishes is generally more plastic than that of most mammals. This phenomenon has been well demonstrated in a variety of fishes,in which phenotypic sex differentiation can be reversed by the administration of exogenous sex steroid hormones during sex differentiation,changing the phenotypic sex of individuals from their genotypic sex(such as neomale or neofemale fish). For example, it has been extensively reported that sex in fish can be reversed by the administration of exogenous estrogen (estradiol), androgen(testosterone),or inhibitors of aromatase or the androgen receptor[2–6]. Thus, the hormonal induction of sex reversal is one of the most widely practiced techniques for the mass production of monosex or sterile fish populations in the fishery industry. However, the production and maintenance of hormone-induced sexual reversal is unstable and laborious,with a relatively long husbandry period[6].In addition,there have been increasing concerns related to human health and environmental consequences due to the endocrine-disruption effects of hormone-related chemicals used in the fish farming production system [7]. Several studies have reported that the use of temperature and photoperiod with sex labile induction may serve as an alternative to regulate teleost sex.However,only a sex-biased population could be achieved with these manipulations [8,9]. Therefore, it is imperative to develop methods of sex reversal that utilize genetic engineering to obtain monosex or sterile fish populations.
The common carp (Cyprinus carpio L.) is the third most cultivated freshwater species and is widely cultivated worldwide for its growth traits and high economic value.It has been reported that the common carp exhibits XX/XY-type GSD. The growth of the common carp is sexually dimorphic, with the growth rate of females being at least 10% greater than that of males, especially after the juvenile stage [10,11]. Previous attempts to generate all-female (AF) populations were made in this institute by Wu et al. [12], who found that the offspring produced by gynogenesis with the XX genotype could be transformed into neomales after treatment with 17α-methyltestosterone. More importantly, the neomales,which are male in phenotype but XX in genotype,could produce AF offspring after artificial fecundation with wild-type(WT) females. In addition to the use of 17α-methyltestosterone,it has been extensively reported that sex control of the common carp can be reversed by the administration of the aromatase inhibitor letrozole and the estrogen receptor modulator tamoxifen[6].
Several sex-determination genes have been identified in fish species, including dmY [13], gsdfY [14], sox3Y [15], amhy [16,17],amhr2 [18], gdf6Y [19], dmrt1 [20,21], and sdY [22]. Consideration of these potential sex-linked markers and sex-determination genes is essential in the investigation of sex-determination mechanisms.However, the genes involved in sex determination in the common carp,which is an excellent model for sex-differentiation investigation for teleosts, have not been reported. Medaka is another wellknown freshwater fish model with an XX/XY GSD system [23].Cyp17a1, a member of the cytochrome P450 enzyme family, has been previously reported as a key enzyme in the synthesis of 17-hydroxyprogesterone, a precursor of testosterone and estradiol.Utilizing a natural scl mutant medaka strain containing a loss-offunction mutation in the P450c17 gene, it has been demonstrated that the loss of this gene results in the loss of secondary sexual characteristics (SSCs) in both scl mutant males (XY) and females(XX), but unimpaired spermatozoa development in scl mutant males (XY). Intriguingly, in the gonads of XX scl mutant medaka,spermatozoa and oocytes were seen at the diplotene stage (six months post-fertilization (mpf)) [24]. Recently, we generated cyp17a1-deficient zebrafish.An all-male phenotype capable of normal spermatogenesis and sperm was observed in the cyp17a1-deficient zebrafish.However,impaired male-typical SSCs and mating behavior have also been recorded[2,25,26].Combined with the observation in the scl medaka, these results confirm that sex steroids are indispensable for male-typical SSCs and mating behaviors[2,24,26]. Loss of the GSD system in a laboratory zebrafish model has been previously reported [27]. Therefore, it is interesting to know whether a similar phenotype as that seen in cyp17a1-deficient zebrafish, such as all-male homozygotes, which includes the potential neomale population, could be achieved in cyp17a1-deficient common carp with the reported GSD system.
In our present study,we aimed to generate AF common carp by using cyp17a1 knockout to generate neomale fish and subsequently performing artificial fecundation between control female fish and neomale fish. First, similar phenotypes to those observed among the cyp17a1-deficient zebrafish in terms of sex differentiation and sex characteristics—that is,all-male homozygotes without the male-typical SSCs—were also observed in the cyp17a1-deficient common carp. Second, the cyp17a1-/-XX genotype common carp(termed neomales), which developed as physiological males with normally developed testis structure, spermatogenesis, and sperm capacity, were mated with WT females (cyp17a1+/+XX). Third,the offspring fish produced from the artificial matings were examined for male-specific markers and subjected to histological analysis. As expected, the AF group offspring fish were all confirmed to be female with cyp17a1+/-XX genotype and developed as females with normally developed oocytes (100.00%, n = 81). Finally, a growth comparison was performed between 1000 fish from the AF and WT groups mixed at a 1:1 ratio and cultured in the same pond. Sex dimorphism in somatic growth was observed as early as 8 mpf, and the average body weight of the AF group fish was 6.60% higher than that of the WT control group. In summary, our study demonstrates the first successful production of an AF common carp population in the aquaculture industry without steroid hormone contaminants in the aquatic environment.
2. Materials and methods
2.1. Animals
The Yellow River carp used for all experiments in this study were purchased from a fish farm in Zhengzhou, Henan Province,China. All fish were reared and all experiments were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals and were approved by the Institute of Hydrobiology, Chinese Academy of Sciences (Section S1 in Appendix A).
2.2. Cyp17a1 knockout
The clustered regularly interspaced short palindromic repeats/CRISPR-associated endonuclease 9(CRISPR/Cas9)strategy was utilized for the cyp17a1 knockout. The guide RNAs (gRNAs) targeting the two sequences of the first exon of cyp17a1 in common carp are as follows: GGCATGAACAGAAAAGCCA and GGGAGTGATGGGAGGCTTGG. gRNAs for the two target sites were transcribed and injected into embryos with Cas9 recombination protein and buffer (Section S2 in Appendix A).
2.3. Male-specific marker identification
The male-specific marker was identified based on DNA sequencing, de novo genome assembly, reads mapping, and malespecific sequencing filtering, as previously described [28] (Section S3 in Appendix A).
2.4. Genotype examination
For genotype examination, genomic DNA was used as the template in this study.The primers used for the genotype identification of cyp17a1 are listed in Table 1,and procedures for DNA extraction are detailed in Section S4 in Appendix A.
2.5. Testis testosterone measurement
The concentration of testosterone in testis was measured, as previously described [2] (Section S5 in Appendix A). In brief, after sample preparation, the enzyme-linked immunosorbent assay(ELISA) buffer, wash buffer, ELISA standard, testosterone AChEtracer, and testosterone ELISA antiserum were prepared. The plate was set up according to the instructions. The plate was read at a wavelength between 405 and 420 nm, and a Cayman computer spreadsheet was used for data analysis.
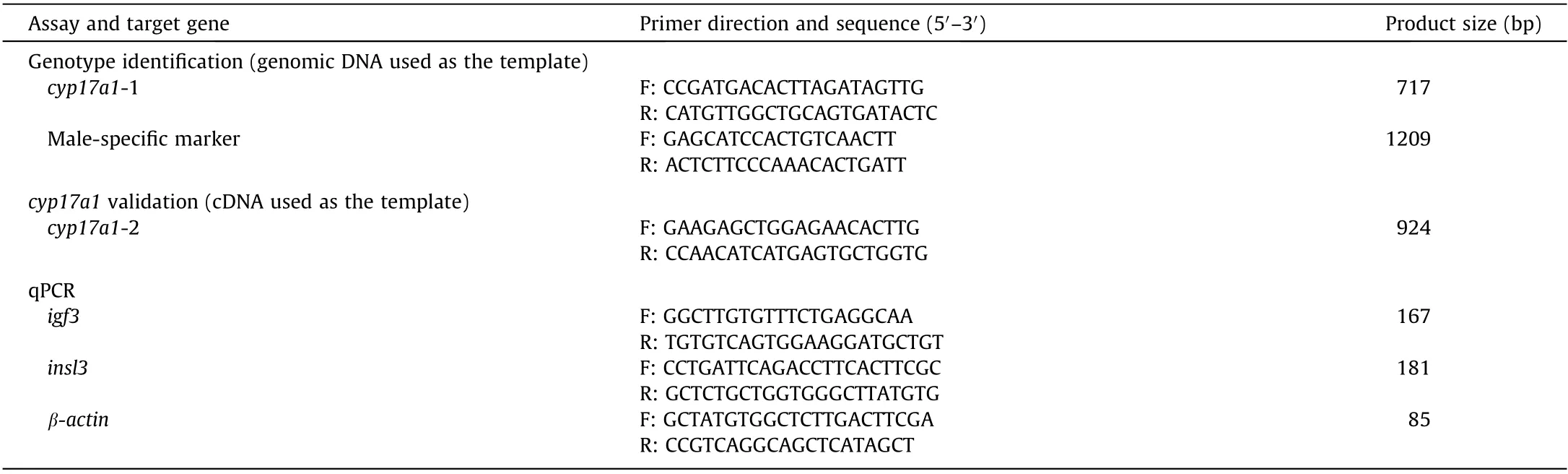
Table 1 Primers used in this study.
2.6. Histological analyses
Anatomical examination and histological analysis(hematoxylin and eosin staining)were performed on the gonads of cyp17a1+/+XY fish,cyp17a1-/-XY fish,cyp17a1+/+XX fish,and cyp17a1-/-XX fish.The sectioning and staining procedures were performed as previously described[29](Section S6 in Appendix A).Scale bars are provided in each image.
2.7. RNA extraction and complementary DNA (cDNA) synthesis
RNA extraction and cDNA synthesis were performed as previously described[2](Section S7 in Appendix A). The cDNA was utilized for the genotype identification of cyp17a1 and quantitative real-time polymerase chain reaction (qPCR) of igf3 (insulin-like growth factor 3) and insl3 (insulin-like 3). The primers used for cyp17a1 genotype confirmation with cDNA as the template are listed in Table 1.
2.8. Quantitative real-time polymerase chain reaction (PCR)
A Bio-Rad real-time system (Bio-Rad Systems, USA) was used according to the manufacturers’instructions(Section S8 in Appendix A). The primers used for the qPCR of igf3, insl3, and β-actin are listed in Table 1.
2.9. Scanning electron microscope
Sperm morphology was performed with a scanning electron microscope (SEM) according to the detailed description provided in Section S9 in Appendix A.
2.10. Statistical analysis
Data were analyzed using GraphPad Prism 8 software (Graph-Pad software, USA). All results are presented as means ± standard deviation (SD). Differences were assessed using Student’s t-test.For all statistical comparisons,results were considered statistically significant at P < 0.05.
3. Results
3.1. Identification of the male-specific DNA marker of common carp
One of the candidate male-specific sequences containing 1209 basepairs (bp) was obtained based on comparative analyses between the female and male assembled genomes of the common carp. This DNA fragment was subjected to male-specificity validation with PCR assays with DNA samples of the common carp from homogeneous and non-homogeneous populations from various sources(Section S10 in Appendix A).The assessment with 1%agarose gel electrophoresis of the male specificity of the amplified PCR products perfectly matched DNA samples with the gender patterns of the common carp (Fig. S1 in Appendix A). The primers used for the genotype identification of this male-specific marker in our present study are listed in Table 1. The male specificity of the PCR products was validated with our anatomical analyses with more than 100 common carp samples from homogeneous and nonhomogeneous populations from common carp samples collected from various cultured regions. The specific PCR product amplified from male common carp samples was identical and consistent based on our Sanger sequencing results. However, this confirmed male-specific DNA sequence has not yet been matched with any common carp genomic DNA sequence submitted in GenBank previously. No significant potential coding region has been identified within this male-specific DNA fragment either.
3.2. Generation of cyp17a1-deficient common carp
The putative cyp17a1 mRNA sequence is 1533 bp long,encoding 510 amino acids. Utilizing the CRISPR/Cas9 strategy, F0 cyp17a1 knockout fish with the mutation in two target sites were generated by the microinjection of Cas9 protein and cyp17a1 gRNAs(Fig. 1(a)). Eleven F0 male fish with effective cyp17a1 mutation at one year post-fertilization(ypf)were hybridized with WT females.In the F1 population, offspring derived from WT females and F0 males (marked with electric marker number 700497) were obtained and examined;3 bp insertions were noted in the first target site of cyp17a1 and 14 bp deletions were noted at the second target site (Fig. 1(b)). The F1 cyp17a1 heterozygotes at 1 ypf were incrossed, and the cyp17a1 homozygote from the F2 population was obtained. Polyacrylamide gel electrophoresis (PAGE) of the cyp17a1 target PCR and imaging after ethidium bromide staining were used for the examination of the cyp17a1 mutation status,as the heterologous chain in the cyp17a1 PCR product of the heterozygote could be identified after denaturation and renaturation. In contrast, a single band was observed in the cyp17a1 PCR product of the cyp17a1+/+fish and cyp17a1-/-fish,and the heterologous chain was observed after mixing with WT cyp17a1 PCR products in the second round of PCR (Fig. 1(c)). The PCR products produced using the genome and cDNA as templates were both used for mutation validation. The results demonstrated that the mutant line only retained eight correct amino acids and, after 45 incorrect amino acids, premature stopping occurred (Fig. 1(d)).
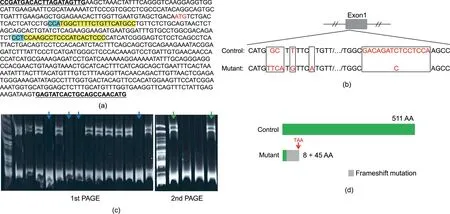
Fig. 1. Knockout of cyp17a1 in common carp. (a) The cyp17a1 sequence from the genomic DNA of common carp. The primers for screening were designed according to the sequence that has been underlined and shown in bold. The protospacer adjacent motif region (NGG) sequences are highlighted in blue, and the designed target sites are highlighted in yellow.The start codon ATG of cyp17a1 is shown in red font.(b)Schematic representation of control cyp17a1 and cyp17a1 mutant alleles.The mutations in the cyp17a1 at the first and the second target sequences are shown in red font.(c)Genomic DNA was used for PCR assay,and the PCR products from DNA of fish were genotyped using PAGE.The PCR products from DNA of cyp17a1+/-fish could be identified in the 1st PAGE,and the PCR products from DNA of cyp17a1+/+fish and cyp17a1-/-fish(marked with blue arrows in the 1st PAGE)could be identified after mixing with the cyp17a1 PCR products of the DNA of WT fish during the 2nd PAGE(marked with green arrows in the 2nd PAGE).(d)Schematic representation of the putative peptides of control and mutant Cyp17a1.The truncated protein contains 8 amino acids(shown in green)that are identical to those of the control Cyp17a1 protein and 45 miscoding amino acids (shown in gray) sequenced and predicted from the cyp17a1 transcripts derived from the cyp17a1 mutant fish. The premature stop codon TAA in the mutant line is shown in red font. AA: amino acids.
3.3.All-male homozygotes with impaired gonadal steroidogenesis and male-typical SSCs at 1 ypf resulted from cyp17a1 depletion
Figs.2(a–d)displayed the aexamination of fish gonads.All-male homozygotes of the cyp17a1-/-common carp at 6 mpf were also observed at 1 ypf (Figs. 2(b) and (d)). The common carp exhibits sexual dimorphism in somatic growth; females grow significantly faster than males [10,11]. The body weights of the fish from the F2 population corroborated the fact that female carp grow faster than male carp in both cyp17a1+/+fish and cyp17a1+/-fish(Fig. 2(e)). However, the sexual dimorphism in somatic growth was eliminated after cyp17a1 depletion, as the body weights of the cyp17a1-/-XX fish were comparable to those of the cyp17a1-/-XY fish (Fig. 2(e)). It was notable that the fish with the cyp17a1+/-XX genotype showed a 32.66%greater body weight than that of the control group (cyp17a1+/-XX fish: (750.6 ± 277.2) g vs cyp17a1+/+fish:(565.8±166.6)g).Meanwhile,statistical analyses of the number and ratio of the fish in each genotype were performed in the F2 population, including carp with the genotypes cyp17a1+/+,cyp17a1+/-, and cyp17a1-/-. We found that the derivation ratio in total matched the law of inheritance (Mendel’s law) (cyp17a1+/+fish: 20.77%; cyp17a1+/-fish: 51.38%; and cyp17a1-/-fish:27.85%) (Table 2).
Although the levels of testis testosterone significantly decreased in cyp17a1-/-fish (Fig. 2(f)), the sperm morphology of the cyp17a1-/-XX fish and cyp17a1-/-XY fish did not differ from that of the cyp17a1+/+XY fish (Figs. 2(g–i)). Tubercles on the operculum and pectoral fin have been reported as male-typical SSCs[30]. When fish from the F2 population at 1 ypf were examined(Fig.3),tubercles on the operculum and pectoral fin were observed in the control male fish(Fig.3(a))but not in the control female fish(Fig. 3(c)) or cyp17a1-/-fish, regardless of the sex chromosome pattern (Figs. 3(b) and (d)).
3.4. Generation of the AF common carp population
The sexual dimorphism in the somatic growth of the common carp,which causes female carp to be more valuable than male,suggests that the establishment of AF populations from the hybridization between cyp17a1+/+XX fish and cyp17a1-/-XX fish(neomale)would make a significant contribution to yield improvement in aquaculture. Identification of genotype and histological analysis of gonadal differentiation of fish from AF population were shown in Fig. 4. As expected, all offspring from the aforementioned parents were identified as AF, as male-specific markers were not observed in the AF populations at five days post-fertilization(dpf) (100.00%, n = 84) (Fig. 4(a), rows 13–96). In addition, the gonads of the AF populations derived from the hybridization between cyp17a1+/+XX fish and cyp17a1-/-XX fish(neomale)were dissected and subjected to histological analysis at 8 mpf; all were identified as having developed into ovaries (100.00%, n = 81)(Figs. 4(c) and (d)).
3.5. Growth comparison between fish from the control and AF groups
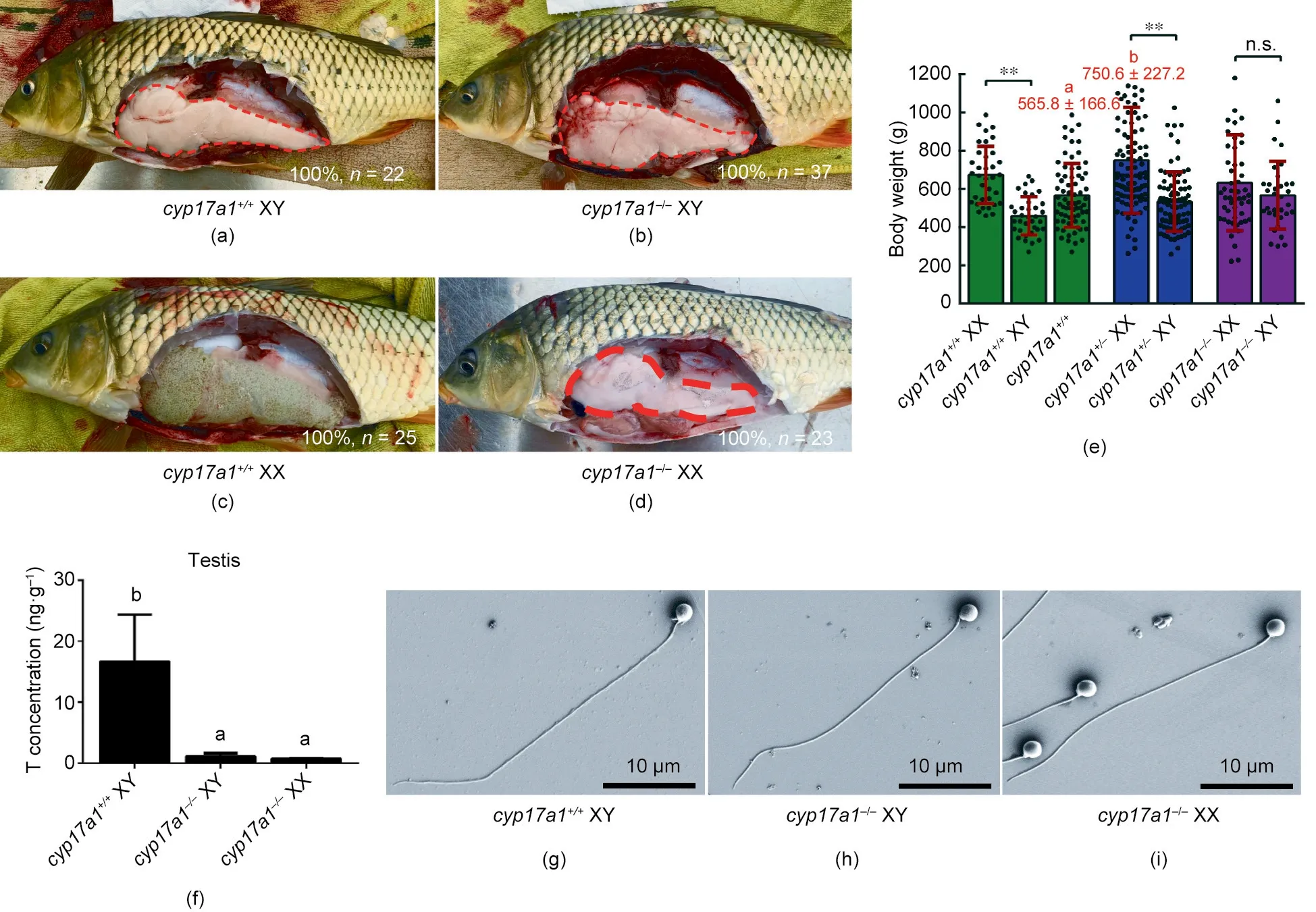
Fig. 2. Depletion of cyp17a1 resulted in all-male homozygous fish at 1 ypf. (a–d) Anatomical examination of fish gonads. (e) Body weight comparison of the fish with the aforementioned genotypes.(f)Measurement of testis testosterone levels in the cyp17a1+/+XY fish,cyp17a1-/-XY fish,and cyp17a1-/-XX fish.(g–i)Morphologic analysis of spermatozoa. T: testosterone. The letters, a and b, in the bar chart (e) and (f) represent significant difference. n.s.: no significance; **: P < 0.01.
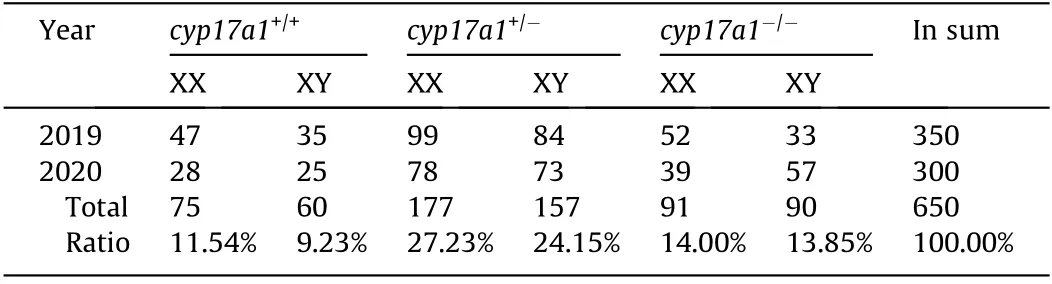
Table 2 Number and ratio of fish in each genotype identified in 2019 and 2020.
A total of 500 fish from the AF group (cyp17a1+/-XX genotype)were reared in the same pond with 500 fish from the control group after hatching. At 8 mpf, the body weights of 56 male control fish(Fig.5(a)),70 female control fish(Fig.5(b)),and 92 fish from the AF group(Fig.5(c))were randomly selected,sampled,and statistically analyzed. The body weights of the control males (cyp17a1+/+XY)and control females (cyp17a1+/+XX) again perfectly reflected the more rapid growth of female common carp over male carp at 8 mpf (control male: (1.348 ± 0.2428) kg vs control female: (1.459± 0.2830) kg). Although the control group had an average body weight of (1.410 ± 0.2705) kg per fish, the growth performance of the fish from the AF group was significantly improved (1.503 ±0.2127) kg (Fig. 5(d)).
4. Discussion
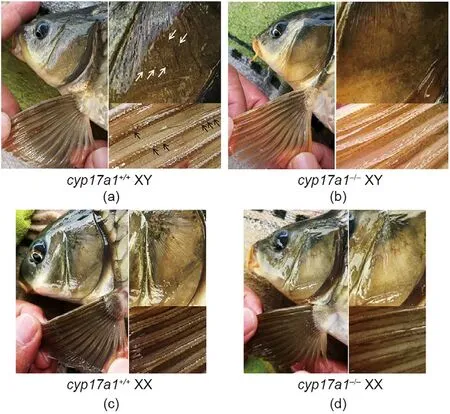
Fig. 3. Gross appearance of tubercles on the operculum and pectoral fin.(a) cyp17a1+/+ XY fish; (b) cyp17a1-/- XY fish; (c) cyp17a1+/+ XX fish;(d) cyp17a1-/- XX fish. The white arrows indicate the operculum tubercles. The black arrows indicate the pectoral fin tubercles.
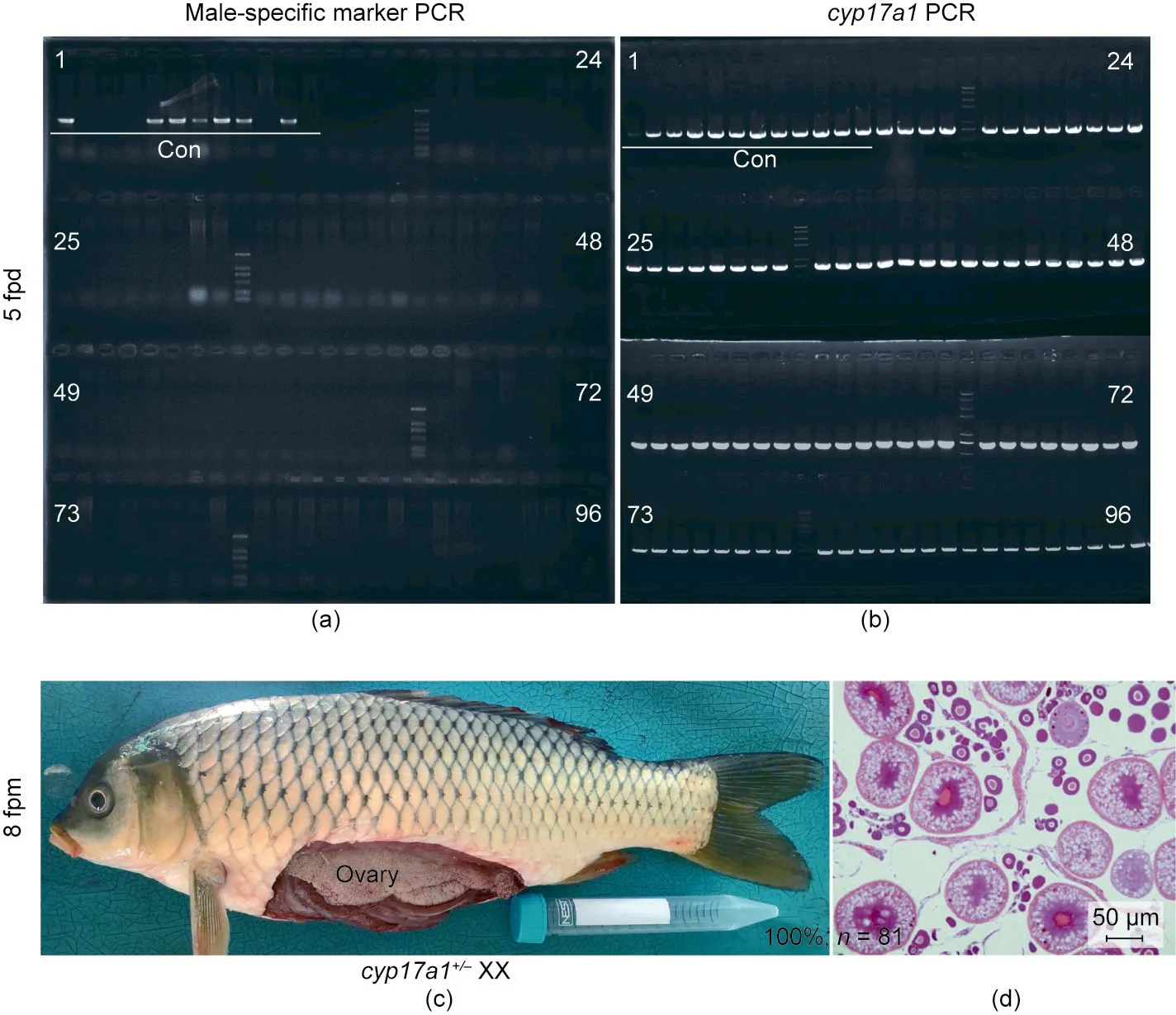
Fig. 4. Identification of genotype and histological analysis of gonadal differentiation of fish from AF population. (a) Sex identification with male-specific marker. (b) The identification of cyp17a1 served as control. The lane numbers 1, 24, 25, 48, 49, 72, 73, and 96 were marked on the agarose gel electrophoresis images. Lanes 1 to 12, PCR products of male-specific marker with DNA of control fish.Lanes 13 to 96,PCR products of cyp17a1 with genomic DNA of fish from AF population. (c, d) The representative image of the anatomical and histological examination of gonads of fish from AF population (cyp17a1+/- XX) at 8 mpf. Con: control.
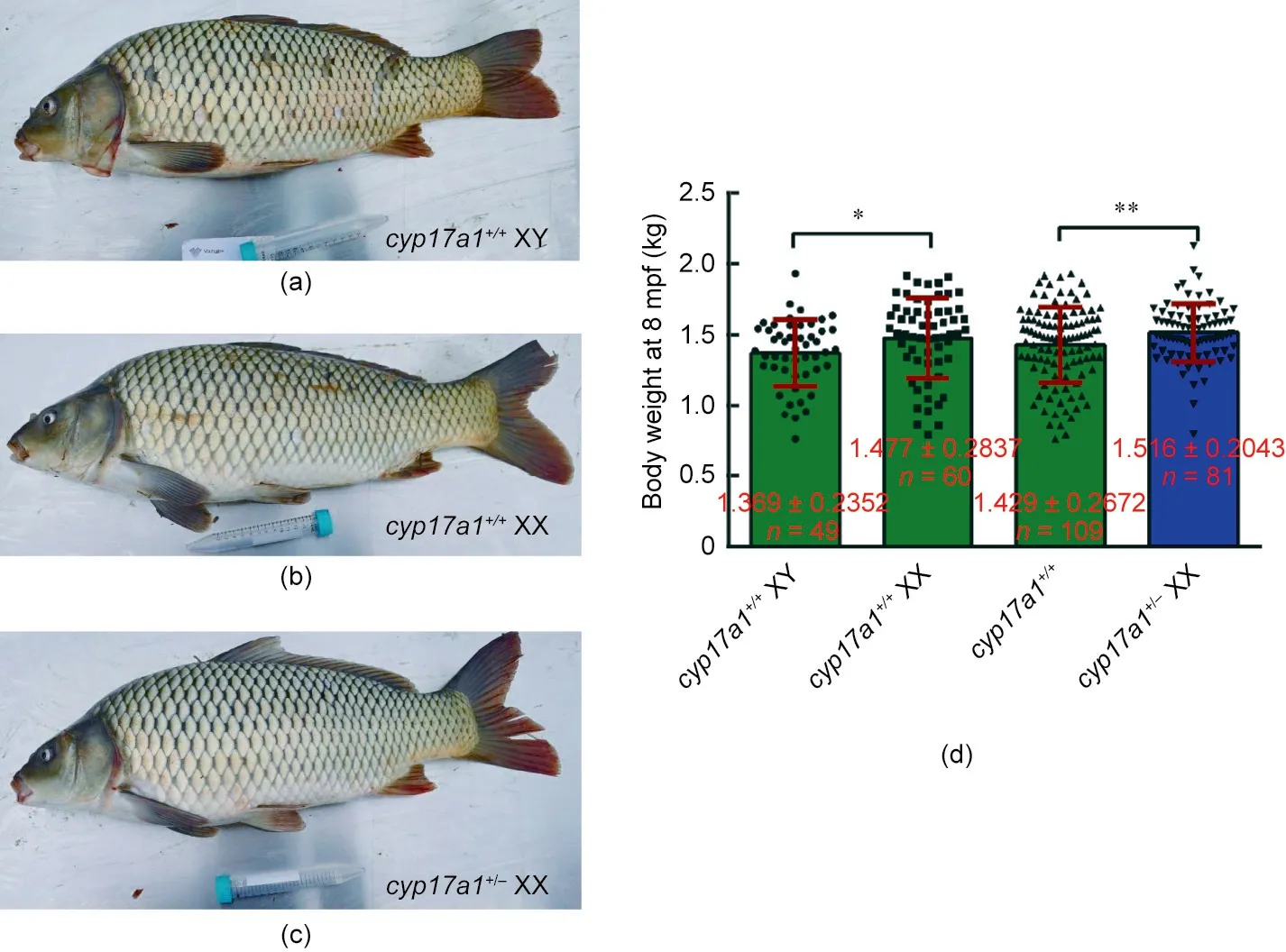
Fig. 5. Somatic growth comparison between fish of the AF and control groups at 8 mpf. (a–c) Representative images of the general appearance of the fish. (d) Body weight comparison of fish was determined with 109 fish of control group and 81 fish of AF group. *: P < 0.05; **: P < 0.01.
In the present study,cyp17a1 knockout was performed in common carp,and the developmental consequences with regard to sexual traits were assessed at 5 mpf, 6 mpf, and 1 ypf. We failed to confirm the male specificity of the two DNA fragments in common carp reported previously[31,32].Therefore,we had to carry out our own comparative analyses between the female and male assembled common carp genomes. Our newly identified male-specific DNA marker(Section S10 and Fig.S1 in Appendix A and Table 1)is a valuable tool to screen out neomale common carp with the XX genotype from the all-male cyp17a1-deficient carp.This ensures the selection process of neomale carp (cyp17a-/-XX) for artificial fecundation with WT female carp (cyp17a1+/+XX), and efficiently generates an AF carp population(Fig.4).Combined with our previous work with the zebrafish model,we demonstrated that the roles of sex steroids in regulating gonad development and differentiation are similar in the family Cyprinidae, regardless of the GSD background. We also demonstrated that the average body weight of fish from the AF group was significantly higher than that of fish from the control group at 8 mpf(Fig.5).The successful production of an AF common carp population using neomale cyp17a1-deficient fish suggests a potential alternative to steroid hormone induction for the utilization of sexual dimorphism in growth.
In the 1980s,Wu et al.[12]reported that gynogenesis and treatment with 17α-methyltestosterone could be effectively used to produce neomales. Subsequently, neomales, which are male in phenotype but XX in genotype,were found to produce AF offspring after the artificial fecundation of WT female fish.Recently,AF carp populations obtained by crossing normal females with neomales produced via gynogenesis and methyltestosterone treatment have been reported [33,34]. In addition, it has been reported that sex reversal of the common carp can be induced by the administration of letrozole and tamoxifen [6]. To establish AF populations,methyltestosterone and aromatase inhibitors (fadrozole or letrozole)have been widely used to generate neomales in several aquaculture species, including the gynogenetic yellow drum [35],Atlantic halibut (Hippoglossus hippoglossus L.) [36], mandarin fish(Siniperca chuatsi) [37], brook trout (Salvelinus fontinalis) [38], yellow catfish (Pelteobagrus fulvidraco) [39], and sablefish (Anoplopoma fimbria) [40].
In the present study,based on our previous studies on the zebrafish model, a genome-editing approach was utilized to investigate the mechanism by which sex steroids regulate gonad development and differentiation in common carp, as well as the potential application thereof in neomale generation. Through the generation of neomale common carp produced by cyp17a1 depletion,a novel sex-control technique for the generation of an AF population for aquaculture was established. In the past decades,Chinese scientists have produced many monosex varieties in several farmed fish. However, traditional methods for the production of AF populations in the common carp are complicated by the generation of a substantial number of physiologically stable neomales,which limits large-scale production. Moreover, steroid hormonerelated chemical residues and their endocrine-disruption effects are of concern [7]. Compared with the traditional hormone induction method, it is easier to mass-produce physiologically stable neomales from the offspring derived from the mating between heterozygous individuals, without the need for any special treatments. Furthermore, the significantly higher average body weight of fish with the cyp17a1+/-XX genotype observed in two consecutive field comparative trials demonstrates the effectiveness and practicability of our technological scheme for mass AF production and the exploitation of sexual dimorphism in the growth of common carp aquaculture (Figs. 2 and 5). Intriguingly, sexual dimorphism in growth was not observed among the cyp17a1-/-XY and cyp17a1-/-XX fish (Fig. 2(e)). These results demonstrate that the sexual dimorphism in common carp growth is based on sex steroid-mediated ovarian differentiation.
Defective spermatogenesis has been observed in androgen signaling-deficient zebrafish caused by the depletion of ar [41–43], cyp11a2 [44], or cyp11c1 [45]. However, an all-male phenotype with unaffected testicular development and spermatogenesis,but loss of male-typical SSCs,has been observed in adult cyp17a1-deficient zebrafish and common carp, even with significantly impaired steroidogenesis. We think that the presence of normally developed testis with mature spermatozoa despite androgen insufficiency may be due to other compensatory factors, as many studies have demonstrated that zebrafish progestin signaling,which is produced in the testis, as well as other androgenic steroids, might be alternative signals for spermatogenesis promotion and maintenance. Further study is still needed to support this hypothesis. Similar to the observation of spermatogenesis in the gonads of XX scl mutant medaka, which also carry oocytes at the diplotene stage (6 mpf), the cyp17a1-/-XX common carp in the present study exhibited apparent intersex gonads at 5 mpf (Section S11 and Fig. S2 in Appendix A). However, at 5 mpf, a higher level of apoptosis was observed in the gonad tissue of the cyp17a1-/-XX common carp. Later, uniform testicular development and spermatogenesis were observed in the gonad tissue of cyp17a1-/-XX carp at 6 mpf (Section S12 and Fig. S3 in Appendix A). These observations are in agreement with those for the cyp17a1-deficient zebrafish, which showed testis differentiation and increased apoptosis at 30 dpf,a critical period for gonadal differentiation. Based on our observations in the two cyp17a1-deficient cyprinid fish(zebrafish and common carp),oocyte development was completely halted, unlike the phenotype observed in the scl mutant medaka [24]. The difference in gonadal differentiation observed between the cyp17a1-deficient cyprinid fish (zebrafish and common carp) and scl mutant medaka may be due either to differences in the role of estrogen in the determination of ovarian differentiation or to the observation for the medaka not being late enough. Hypertrophic testes with a significantly increased number of spermatozoa were observed in both cyp17a1-deficient zebrafish and cyp17a1-deficient common carp. Previously, we have demonstrated that the upregulation of pituitary Fshβ, which may have resulted from the absence of effective negative feedback regulation of the sex steroids, stimulated testis development and spermatogenesis in cyp17a1-deficient zebrafish,as the additional knockout of fshβ in the cyp17a1-deficient zebrafish resulted in normalized testis development and spermatozoa number [2]. The hypertrophic testis in the cyp17a1-/-common carp at 6 mpf may also be attributed to the potential upregulation of gonadotropins in the pituitary, since the target genes of fshβ,igf3, and insl3 were significantly upregulated in the testis of cyp17a1-/-fish, compared with the expression in the cyp17a1+/+fish (Fig. S3).
In recent years, several knockout species have demonstrated female-to-male sex reversal,such as cyp19a1a and cyp17a1 knockout zebrafish and tilapia, suggesting that these sex steroids are indispensable to the regulation of gonadal differentiation [2,3].Even so,the phenotypes caused by cyp17a1 mutation in mammals are highly diverged with those caused by cyp17a1 mutation in fish;for example, in both humans and mice, the cyp17a1 mutation causes disorders in sex development and delayed sexual maturation [46–49]. In medaka, another fish model that uses the XX/XY GSD system, scl mutant fish carrying a cyp17a1 mutation also exhibited loss of male-typical SSCs and mating behaviors but unimpaired spermatozoa development, as evidenced by both XX and XY mutants exhibiting gonads with spermatozoa [24]. When comparing the observations on cyp17a1-deficient zebrafish, an experimental strain without an identified GSD system [27], with those of cyp17a1-deficient medaka and carp, it can be seen that the phenotypes of the three cyp17a1 mutant teleosts are quite similar with respect to SSCs, spermatogenesis, and testicular development.These results indicate that a difference may exist in the roles of sex steroid hormones in gonadal development between teleosts and mammals, regardless of the presence of a GSD system. Moreover, the present study demonstrates that the XX/XY sexdetermination system of the common carp is sex steroiddependent(Table 2 and Fig.4).These findings enabled us to establish a novel sex-control strategy for culture fish in aquaculture.The AF carp population produced by this genetic approach contains one copy of an edited cyp17a1 locus.An optimized strategy to establish a high-ratio neomale population has been established(Section S13 and Fig. S4 in Appendix A). In the future, we will try to produce sterilized, rapid growth, and AF triploid common carp by crossing our diploid cyp17a1-/-neomale carp with a tetraploid female carp,following the methods set out in previous studies [50]. The future AF triploid carp will reduce ecological risk due to their inability to reproduce, which would prevent the drifting of the artificial cyp17a1 locus within the carp population.
5. Conclusions
The manipulation of cyp17a1 depletion can be an effective approach to produce cyp17a1-/-XX neomales with proper testicular differentiation and spermatogenesis. Although the neomales exhibit external female SSCs phenotypes, large amounts of viable sperm can be easily collected from mature neomales that can then be successfully used to fertilize mature eggs collected from WT females. In addition, together with our newly identified malespecific DNA marker, mature neomale common carp can easily be screened out from cyp17a1-deficient all-male carp for use as the paternal parent fish for the generation of an AF population.This enables the production of a cyp17a1+/-XX AF population via routine in vitro artificial fertilization procedures used in aquaculture.The adoption of this genetic approach can be used to produce neomale common carp with a few nucleotides missing and one loss-offunction gene.This novel genetic approach provides an alternative method to mass-produce stable AF populations of common carp by exploiting the sexual dimorphism in carp growth in a manner beneficial for aquaculture. It also avoids the issue of endocrinedisruption effects caused by the endocrine residues released by traditional steroid hormone-induced sex-reversal methods. It is still necessary to sterilize these fish to prevent genetic drift of the mutant cyp17a1 locus within the carp population.
Acknowledgments
This work was supported by the National Key Research and Development Program, China (2018YFD0900205) to Zhan Yin;the National Natural Science Foundation, China (31972779 and 31530077)to Gang Zhai and Zhan Yin;the Pilot Program A Project from the Chinese Academy of Sciences (XDA24010206) to Zhan Yin; the Youth Innovation Promotion Association of CAS(20200336)to Gang Zhai;and the State Key Laboratory of Freshwater Ecology and Biotechnology(2016FBZ05)to Zhan Yin.We thank Yuan Xiao and Zhenfei Xing for providing the scanning electron microscope (SEM) service (Analysis and Testing Center, Institute of Hydrobiology, CAS).
Compliance with ethics guidelines
Gang Zhai, Tingting Shu, Kuangxin Chen, Qiyong Lou, Jingyi Jia,Jianfei Huang, Chuang Shi, Xia Jin, Jiangyan He, Donghuo Jiang,Xueqiao Qian,Wei Hu,and Zhan Yin declare that they have no conflicts of interest or financial conflicts to disclose.
Authors’ contributions
Gang Zhai and Tingting Shu conducted most of the experiments for this work.Gang Zhai prepared all the figures and wrote the draft.Qiyong Lou,Jingyi Jia,Jianfei Huang, Chuang Shi,Xia Jin, and Jiangyan He provided help in genotyping, fish breeding, and rearing.Kuangxin Chen and Wei Hu provided help in the identification of male-specific markers. Donghuo Jiang, Xueqiao Qian, and Zhan Yin provided insights for this work, and initiated and supervised the research team. Zhan Yin and Gang Zhai administrated the project and revised the paper. All authors approved the final manuscript and agreed to be accountable for all aspects of this work.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eng.2021.03.026.
- Engineering的其它文章
- Editorial for the Special Issue on 6G Requirements, Vision, and Enabling Technologies
- The Drive for Electric Motor Innovation
- Direct Air Carbon Capture Takes Baby Steps—Giant Strides Are Needed
- Start-Ups Seek to Accelerate Path to Nuclear Fusion
- 6G:Ubiquitously Extending to the Vast Underwater World of the Oceans
- Industrial Wireless Control Networks: From WIA to the Future

