Synthesis, Crystal Structure and Fluorescent Properties of a 1D Europium Coordination Polymer with 2,5-Furandicarboxylic Acid①
ZHANG Hn LI Xing MUHAMMAD Yseen XIE Hui-Feng ZANG Yi-Hn FANG Ming-Lu LIU Sho-Jie WANG Ho②
a (Beijing Key Lab of Special Elastomer Composite Materials, College of New Materials and Chemical Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617, China)
b (Institute of Chemical Sciences, University of Peshawar, Peshawar 25120, KP, Pakistan)
ABSTRACT By using solvothermal method, a one-dimensional chain compound[KEu2(FDCA)3(H2O)9·0.5(FDCA)] (1) was synthesized. Single-crystal X-ray diffraction data reveal that 1 crystallizes in monoclinic system, space group P21/n with a = 12.1996(1), b = 18.6454(2), c = 17.7123(2) Å, β = 98.8460(10)°, Dc= 1.753 g/cm3, Z = 2, V = 3981.03(7) Å3, R = 0.0544 and wR = 0.1511 for 7886 observed reflections with I > 2σ(I). In 1, three FDCA2- ligands construct a “23-crown-9-like” structure as the second building units (SBUs) to further form an infinite 1D chain. Meanwhile, the fluorescent result reveals that compound 1 can selectively and sensitively sense Fe3+by the fluorescence quenching.
Keywords: 2,5-furandicarboxylic acid, solvothermal synthesis, Fe3+, fluorescence quenching;
1 INTRODUCTION
Iron(III) is an indispensable trace element in human body as it plays key roles in the formation of hemoglobin and other normal metabolic processes[1,2]. However, Fe3+cannot be metabolized normally in human body and its excessive or deficient quantities cause serious diseases[3-5]. Compared with other detection methods, fluorescence sensing method has been widely concerned by researchers attributed to its high selectivity, operational convenience and sensitivity[6,7]. Therefore, it is important to develop a fluorescence sensor which can be used to detect low concentration of iron(III) ions.
Coordination polymers (CPs), as an inorganic-organic hybrid materials[8-11], have attracted extensive attention in gas adsorption[12], catalysis[13,14], drug delivery[15]and photochromic processes[16]. In addition to these, CPs as a new kind of fluorescence sensing materials have also attracted the interest of researchers[17]attributed to their high sensitivity and selectivity for specific type of metals[18]. Compared with transition metal coordination polymers (such as nickel and zinc CPs)[19,20], lanthanide coordination polymers (Ln-CPs) have more abundant structures, large stokes shift and a long luminescent lifetime due to the unique 4felectron layer of lanthanide metals[21-27], which significantly boost their performance.Based on the antenna effect, organic ligands can efficiently sensitize lanthanide ions through energy-transfer, and finally the lanthanide ions produce the luminescence[28]. These unique properties make Ln-CPs have different properties and applications compared with transition metal coordination polymers[29].
Herein, Ln-CP based on europium [KEu2(FDCA)3-(H2O)9·0.5(FDCA)] (1) was prepared by 2,5-furandicarboxylic acid, KNO3and Eu(NO3)3·6H2O. It was confirmed experimentally that 1 can emit intense fluorescence at 613 nm and possess high selectivity for Fe3+through fluorescence quenching.
2 EXPERIMENTAL
2. 1 Materials and methods
2,5-Furandicarboxylic acid, KNO3, Eu(NO3)3·6H2O and other chemicals were supplied by Hwrkchemical Co,. Ltd.Powder X-ray diffraction patterns were performed on a Rigaku D/Max-2500 diffractometer with a Cu-target tube.Infrared (IR) spectra were recorded on a Nicolet IS10 infrared spectrometer using KBr pellets. The fluorescence spectra,fluorescence lifetimes and sensing properties were measured with the FS5 fluorescence spectrometer (Edinburgh Instruments).
2. 2 Synthesis of [KEu2(FDCA)3(H2O)9·0.5(FDCA)] (1)
H2FDCA (0.2 mmol, 31.2 mg), KNO3(0.1 mmol, 10.1 mg)and Eu(NO3)3·6H2O (0.1 mmol, 44.6 mg) were added to 5 mL mixed solvent (Vethyleneglycol:Vethanol:VH2O= 1:1:1) in a 15 mL Teflon-lined reactor, and heated at 120 °C for 48 h. The reactor was then gradually cooled to 30 °C in 12 h and the products were filtered and washed thrice with ethanol. Finally,plenty of white crystals were air dried and collected under ambient conditions (yield: 40% based on Eu). FT-IR (KBr,cm-1): 3124 (b), 2979 (w), 2322 (w), 1577 (s), 1371 (s), 1222(w), 1168 (w), 1071 (w), 1047 (w), 1028 (w), 1016 (m), 970(m), 882 (w), 777 (s), 647 (m), 614 (m).
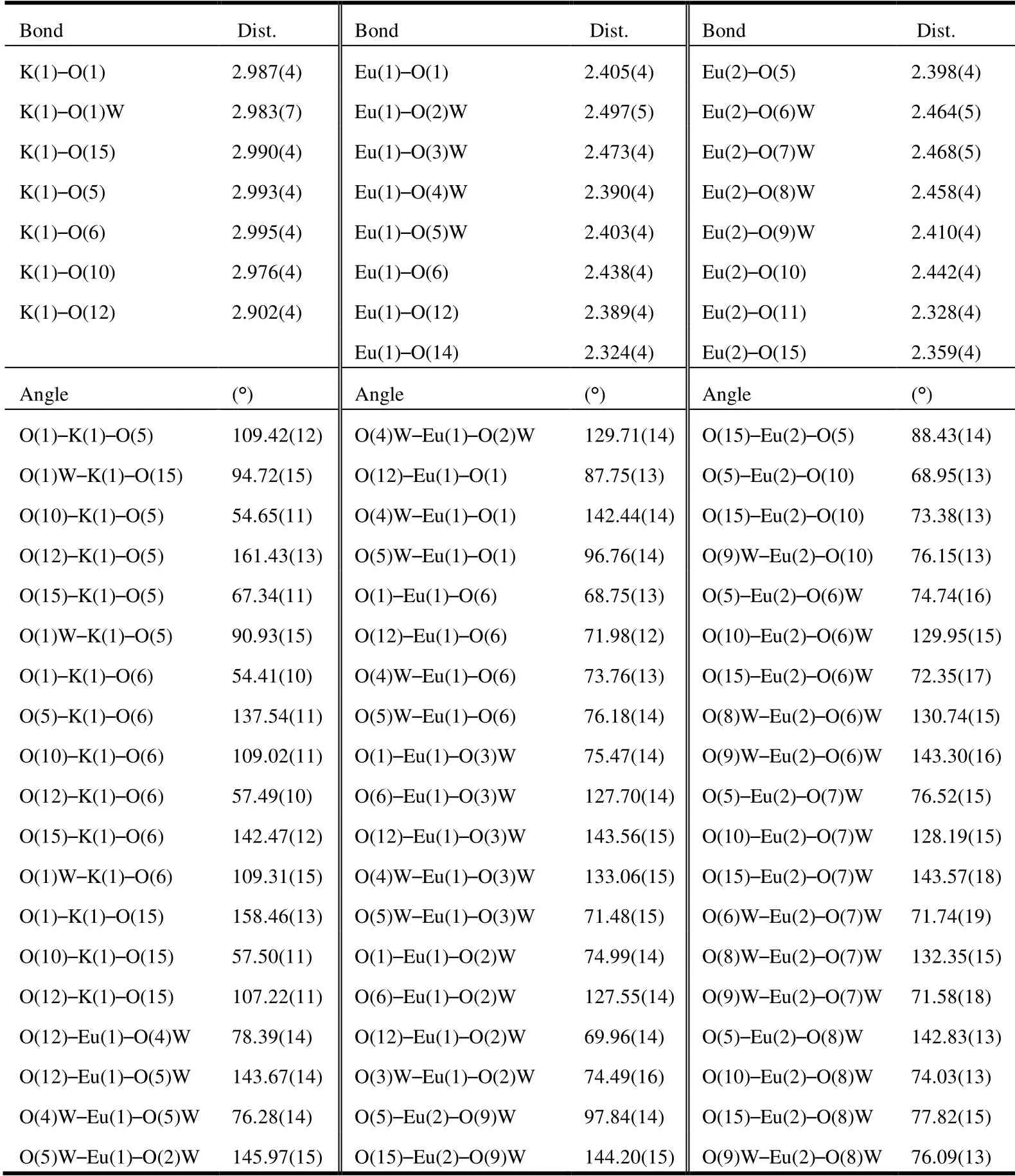
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
2. 3 X-ray structure determination
Single-crystal X-ray diffraction data for compound 1 were acquired on a Bruker-AXS SMART APEX2 CCD diffractometer at 293(2) K with MoKαradiation. The crystal data were analyzed using direct methods by the SHELXS program of SHELXTL-2014. In order to reduce the effect caused by disordered solvent molecules, SQUEEZE was applied. More details on the crystallographic studies of 1 are given in the CIF file while the main bond lengths and bond angles are offered in Table 1.
2. 4 Fluorescence experiment
The solid sample was ground into powder for luminescence experiment. 3 mg well-grounded powder was immersed in 3 mL ethanol solution of MNOx(Mm+= K+, Cd2+,Mg2+, Mn2+, Ni2+, Ca2+, Zn2+, Cu2+, Fe3+, 1 mmol/L) and treated ultrasonicated for 5 min. Then, the fluorescence response in the range of 550~650 nm was monitored under 310 nm excitation.
3 RESULTS AND DISCUSSION
3. 1 Characterization of [KEu3(FDCA)3 (H2O)9·0.5(FDCA)]
(1)molecules in a bicapped triangular prism, similar to Eu(1).The bond lengths of Eu-O are in the 2.328(4)~2.497(5) Å range. Interestingly, three FDCA2-ligands form a“23-crown-9-like” and the K+ion locates in the hole of the“crown ether” (Fig. 1c). The “crown-like” unit connects four Eu ions, and neighboring Eu ions constitute a binuclear unit through -O-C-O- from two FDCA2-ligands (Fig. 1d). Such binuclear units are further bridged by “crown-like” unit into an infinite 1Dchain (Fig. 1e).
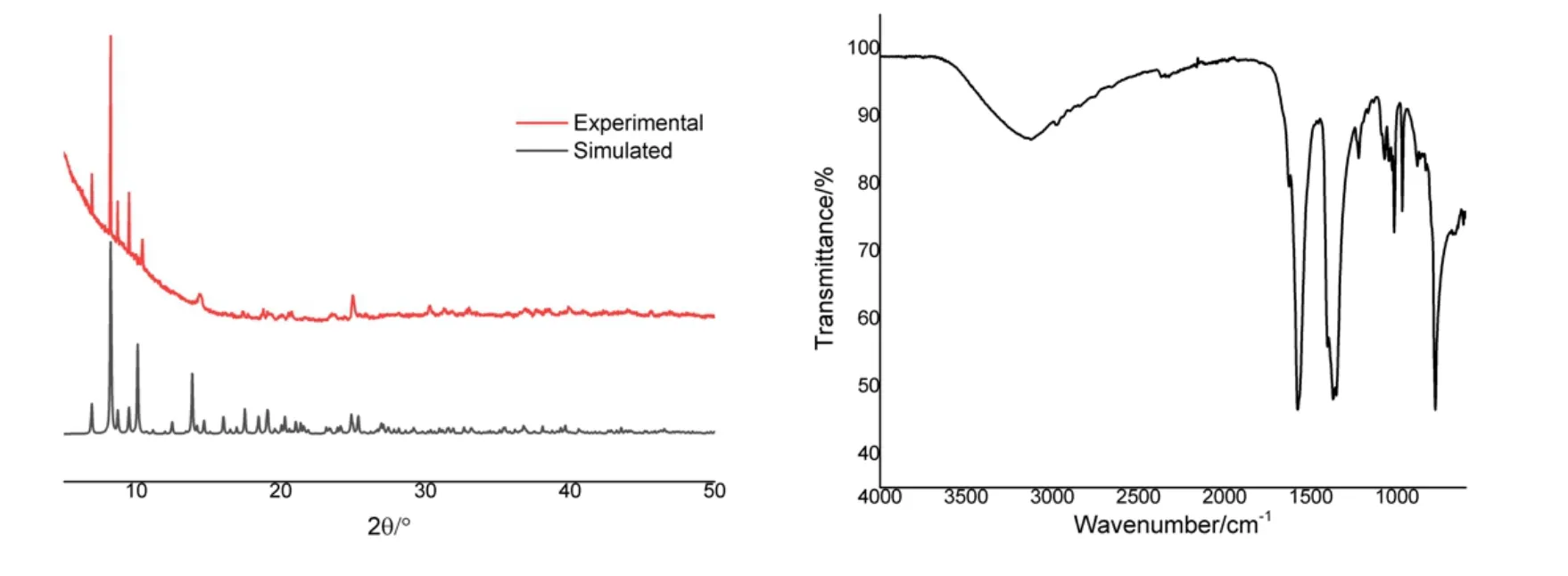
Fig. 2. PXRD patterns (left) and IR spectra (right) for 1
Crystallographic analysis exhibits that 1 crystallizes in theP21/nspace group of monoclinic system. The asymmetric unit contains two Eu3+ions, one K+ion, three FDCA2-, a-half free FDCA2-and nine coordinated H2O molecules (Fig. 1a). The Eu(1) ion locates in a distorted bicapped trigonal prismatic coordination environment completed by four carboxylato oxygens from four FDCA2-ligands and four oxygens from four H2O molecules (Fig. 1b). The Eu(2) ion is coordinated by four carboxylato oxygens (O(5), O(10), O(11), O(15))from four FDCA2-ligands and four oxygens from four H2O
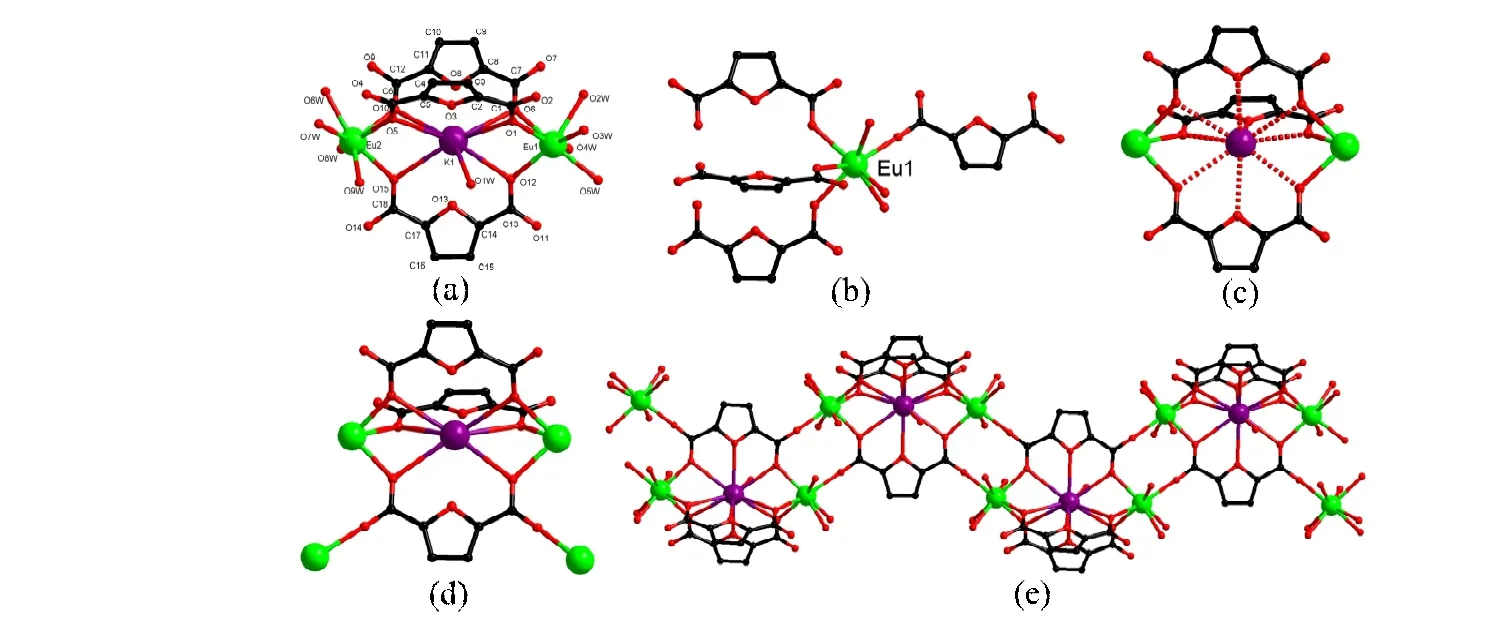
Fig. 1. (a) Asymmetric unit of 1 (The free FDCA2- ligand is omitted for clarity). (b) Coordination environment of Eu1.(c) “23-crown-9-like” structure. (d) “23-crown-9-like” structure bridges four Eu ions. (e) 1D chain of 1
3. 2 PXRD and IR spectra
The PXRD was performed at room temperature to demonstrate the phase purity of the obtained samples. Fig. 2 shows that the peak positions of compound 1 in PXRD pattern have good coincidence with the simulated pattern, indicating that the purity of samples was up to requirement. In left of Fig. 2,the peak at 882 cm-1is attributed toγ(C-H) of the furan rings.Theν(C-O-C) on the furan rings results in a moderate peak at 1071 and 1222 cm-1. Furthermore, the strong and sharp bands at 1375, 1571 cm-1correspond to theνs(COO-) andνas(COO-), respectively. The broad absorption band around 3124 cm-1is ascribed to characteristicν(O-H) of coordinated water molecules.
3. 3 Thermal stability

Fig. 3. TG curve of 1
Thermogravimetric analysis (TGA) study was performed under N2atmosphere at 25~700 °C to evaluate the thermal stability of 1. As depicted in Fig. 3, the initial weight loss of about 15.69% from 27 to 175 °C (calcd.: 15.51%) attributes to the detachment of coordinated water molecules.
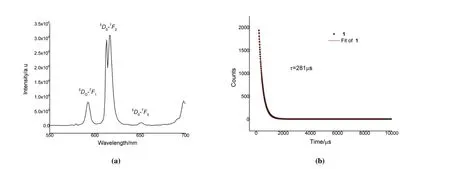
Fig. 4. (a) Emission spectra of 1 in solid state; (b) Fluorescence lifetime of 1
3. 4 Luminescent properties
At room temperature the solid state luminescent properties of 1 are investigated. As shown in Fig. 4a, Eu(III) can be significantly sensitized and exhibit four sharp fluorescence peaks withλEx= 310, attributed to the transitions of5D0→7F1(592 nm),5D0→7F2(613 and 616 nm), and5D0→7F3(652 nm). The intense bands are observed at 613 and 616 nm,which produced intense red luminescence. The fluorescence lifetime of solid state 1 is investigated, which fit the single exponential decay functionI=I0exp(-t/τ) (τ= fluorescence lifetime) (Fig. 4b)[30], whereI0andIare the fluorescence intensity at time 0 andt. The fitting result of 1 could conclude that the fluorescence lifetime isτ= 281 μs.
3. 5 Sensing of metal ions
With the fore-idea that Eu-complex has luminescent properties, its luminescent abilities towards metal ions are investigated. Fig. 5a shows that 1 displays differentiated luminescence response for various metal ions. When adding K+and Cd2+, the luminescence intensity of 1 is slightly enhanced,while the addition of other metal ions produced varying degrees of quenching effects, among which Fe3+ion has the most drastic effect. These results suggest that compound 1 shows remarkable selectivity to Fe3+. The quenching effect of Fe3+on compound 1 can be seen more significantly (Fig. 5b).
The anti-interference ability is also necessary for sensor, so a series of anti-interference experiments are demonstrated. As shown in Fig. 6, the powder of 1 is well dispersed to the other metal solution, and the luminescence intensity of 1 has rapidly decreased after introducing Fe3+ions. Therefore, 1 has a remarkable anti-interference ability to Fe3+ions even if there are other coexisting metal ions.
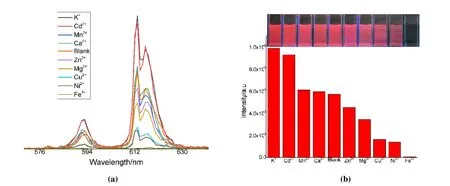
Fig. 5. (a) Luminescence spectra of 1 dispersed in ethanol solutions of different metal ions λEx = 310;(b) the 5D0 → 7F2 transition (613 nm) intensities of 1 when dispersed in various metal ions aqueous(insert, photographs of 1 under UV lamp after immersed in different metal ions solution)
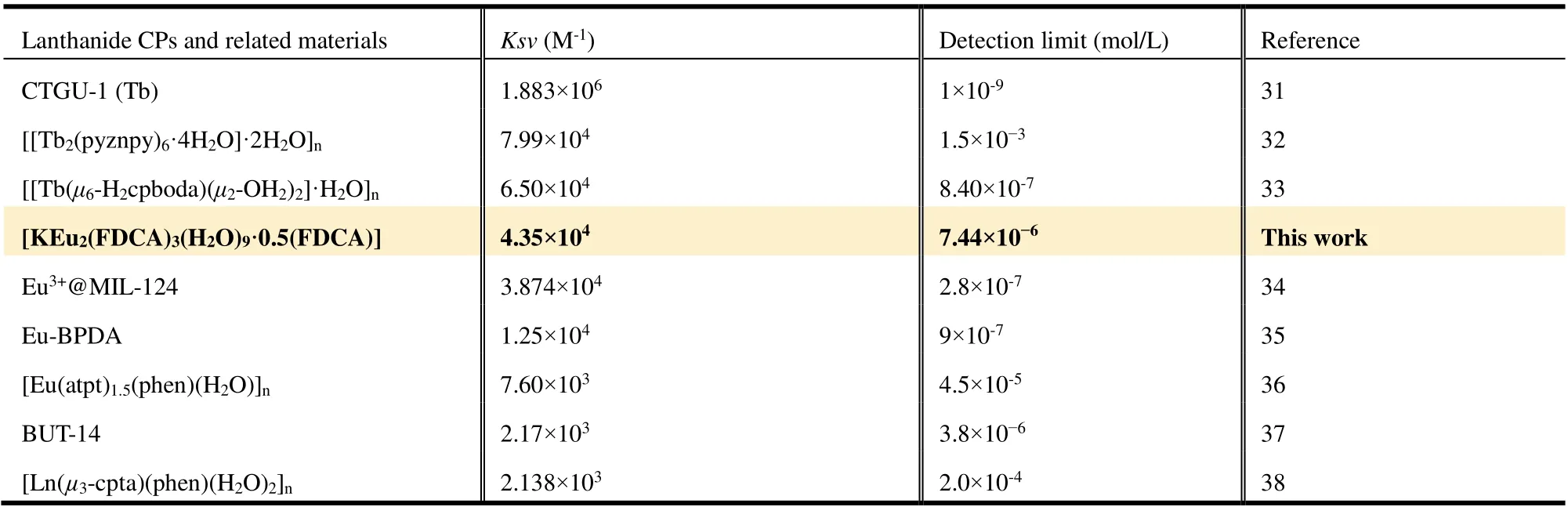
Table 2. Comparison of the Sensing Performance of Compound 1 with the Reported Ln-CPs for the Sensing of Fe3+
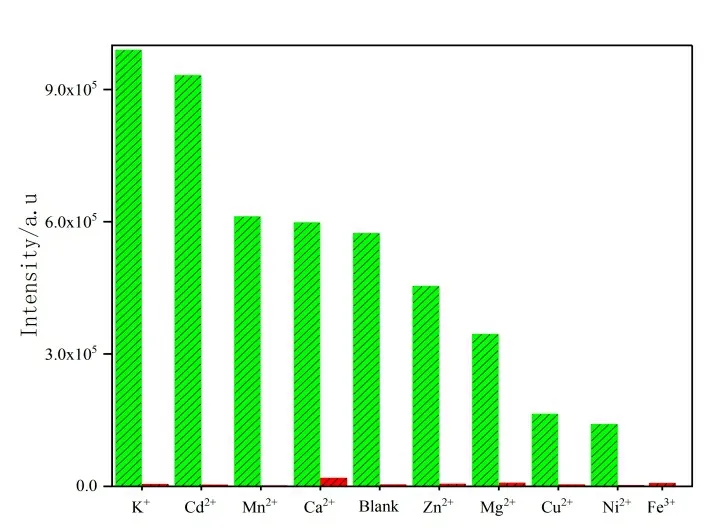
Fig. 6. Fluorescence variation of 1 to different metal ions (green bars luminescence intensities with different metal ions; red bars,luminescent intensities after the addition of Fe3+ ion)

Fig. 7. Luminescence spectra of 1 suspension with various concentrations of Fe3+
Further, luminescent quantitative titration experiments were performed to better understand the sensitivity of 1 for Fe3+.The fluorescence intensities of 1 are weakened step by step with a corresponding increase in the fluorescence quenching efficiency when increasing the contents of Fe3+in the ethanol solution (Fig. 7 and Fig. 8a). The quenching phenomenon can be explained by the Stern-Volmer (S-V) formula:I0/I=Ksv[M]+ 1 (Ksv= quenching effect constant), whereI0andIare the luminescent intensities of 1 and 1-Fe3+, respectively, and [M]is the concentration of Fe3+in solution. The quenching curves can be fitted by the S-V equation, and show good linearity at lower concentrations from 0 to 0.08 mM (Fig. 8b).Ksvvalues of Fe3+ions are calculated to be 4.35 × 104M-1, which are close to that of reported Ln-CPs for detecting Fe3+ions (Table 2)[31-38]. The equationI0/I=Ksv[Fe3+] + 0.73 (R2= 0.97),which is similar to the idealS-Vequation, shows that the interaction of Fe3+with compound 1 is controlled by the concentration diffusion[3,4]. The detection limit (LOD) of compound 1 for detecting Fe3+calculated by the ratio of 3δ/slope is 7.44 × 10-6M, and the sensing ability of compound 1 is comparable with that of some previously reported Fe3+fluorescent sensors (Table 2)[31-38]. Such highKsvvalue and low detection limits indicate that Fe3+has strong quenching effect for compound 1, thereby confirming its highly applicable nature for the detection of Fe3+in practical applications.

Fig. 8. (a) Quenching efficiency curve of 1 for Fe3+-EtOH, (b) S-V plot of 1
The recycling performance is very important for a good sensor. Thus, we investigate the recycling performance of compound 1, which is simply obtained by soaking in ethanol for 24 h. The quenching efficiency of 1 to Fe3+is not significantly reduced after five cycles (Fig. 9), which indicates that 1 can be used as a repeated and stable probe to detect Fe3+in ethanol solution. It also proves that the framework remains stable after recognition Fe3+ions of 1 and the quenching is not due to the collapse of the framework.
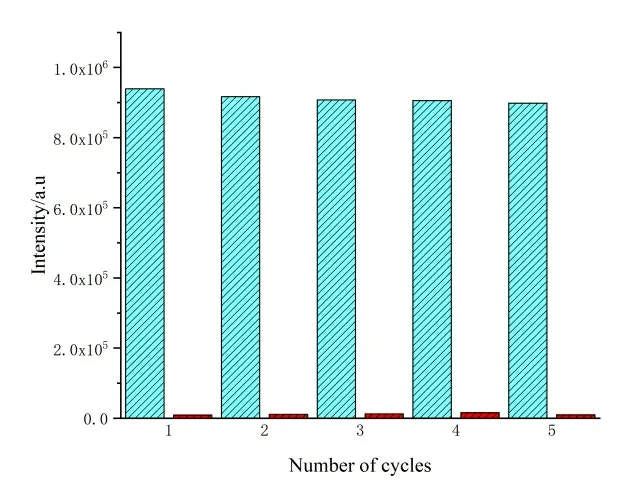
Fig. 9. Recycling experiments for recognition of Fe3+(blue bars, the luminescence intensity of 1 before added Fe3+; red bars, the luminescence intensity of 1-Fe3+)
4 CONCLUSION
In summary, polymer [KEu2(FDCA)3(H2O)9·0.5(FDCA)](1) is synthesized via solvothermal approach. Compound 1 exhibits a one-dimensional chain with “23-crown-9-like”SBUs and possesses high selectivity and sensitivity for the sensing of Fe3+by luminescence quenching mechanism withKSV= 4.35 × 104M-1and LOD = 7.44 × 10-6M.
- 结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①

