Theoretical Measurements of Quantitative Effects Caused by Spectator Ligands on Palladium-catalyzed C-H Activation①
MA Xio-Si ZHOU Yong-Zhu,b② ZHANG Lei②
a (Department of Chemistry, School of Science, Tianjin Chengjian University, Tianjin 300384, China)
b (School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China)
ABSTRACT Ligands can definitely influence C-H activation at the metal center. A ligand not directly participating in the reaction is called a spectator ligand. We attempt to quantitatively characterize the effects of diverse spectator ligands on C-H activation at palladium. We designed a model palladium catalyst and selected an array of spectator ligands, such as methoxyl, amide, methyl, phenyl, cyanide, fluorine, chlorine, and several neutral ligands,and performed density functional theory calculations on the mechanism and energetics of C-H activation reactions of benzene with different catalysts. Univalent ligands have substantially larger effects than neutral ligands, and strongly σ-donating ligands (e.g., methyl and phenyl) severely hinder the C-H activation in progress. A ligand trans to the reaction site influences C-H activation more than that cis to the reaction site, indicating electronic effects to be at work.For example, the existence of a methyl ligand raises the barrier height of C-H activation by 6.4 or 14.4 kcal/mol when it is placed at the position cis or trans to the C-H activation site. The effects of poorly σ-donating ligands are not significant and similar to those of the κ1-acetate ligand. Some σ-donating and π-accepting ligands, such as cyanide and isonitrile, hinder the C-H activation trans to them but appear to facilitate the C-H activation cis to them. On the basis of molecular orbital analyses, a chemical model is proposed to understand the observed ligand effects. Lastly, the conclusions are applied to explain the plausible mechanism of the dehydrogenative Heck coupling.
Keywords: C-H activation, ligand effect, palladium catalyst, reaction mechanism, DFT calculation;
1 INTRODUCTION
Transition-metal-catalyzed C-H activation is one of the most widely investigated topics in modern organometallic chemistry, because it allows the direct conversion of C-H bonds into C-C or C-X bonds without the necessity of using pre-functionalized substrates[1]. Among many transition metals, palladium has been believed to be versatile in C-H activation[2,3], and numerous C-H functionalizations mediated by palladium catalysts have been reported in the literature,such as C-H/C-X coupling[4,5], C-H/C-H coupling[6,7], dehydrogenative cycloaddition[8,9], and so on. Current interests in the field of C-H activation include the control of regioand stereo-selectivity, exploration of bifunctional ligands and metal-ligand cooperative systems, and application of C-H activation in total synthesis[10,11].
Ligands have proved to influence C-H functionalizations,and in some cases they were capable of improving the reaction efficiency and/or rendering the selectivity. A ligand can act as either of two roles, namely the reacting ligand and the spectator ligand (Fig. 1). A ligand which is responsible for deprotonating the target C-H bond is a reacting ligand,while that merely coordinating with metal serves as a spectator ligand. The most widely used palladium catalyst is palladium acetate (Pd(OAc)2)[12]. Yu’s group established a kind of mono-N-protected amino acid (MAPP) ligands and demonstrated that they not only stabilized monomeric palladium complexes but also acted as proton abstractors for C-H cleavage[13,14]. In some cases, employment of external pyridine- or phosphorus-based ligands is crucial for Pd(OAc)2-catalyzed reactions[15,16]. Recently, chiral Nheterocyclic carbene (NHC) ligands have drawn attention of organic chemists in enantioselective C-H functionalizations[17]. On the above examples, MAPP ligands serve as reacting ligands responsible for scissoring the target C-H bond (Fig. 1a), while the other ligands of interest are simply spectator ligands (Fig. 1b).

Fig. 1. Two types of ligands affecting C-H bond cleavage. (a) MAPP serving as a reacting ligand; (b) group G serving as a spectator ligand
Despite plenty of mechanistic studies on transition-metalcatalyzed C-H activation in the literature[18-21], systematical investigation on the ligand effects, in a broader sense, has been rather rare. For example, the following questions have been unsolved: (1) What are the quantitative effects of spectator ligands on thermodynamic and kinetic parameters of C-H activation? (2) What are the unique geometrical and electronic characters of C-H activation in the presence of different spectator ligands?
In this work, we attempt to characterize the effects of an array of simple spectator ligands on palladium-catalyzed CH activation on the basis of density functional theory (DFT)calculations. Our strategy is that a palladium complex similar to Pd(OAc)2is chosen as the parent catalyst, and then,each of the spectator ligands is introduced to palladium by replacing one of the original ligands. Through comparison of the located mechanisms and energetics, the ligand effects can be measured.
2 COMPUTATIONAL DETAILS
All calculations were finished in the Gaussian 09 computational program[22]. Geometries were optimized with the B3LYP method[23], employing the double-ζvalence polarized DZVP basis set for all atoms[24,25]. The default selfconsistent reaction field polarizable continuum model was used with dichloroethane as solvent[26]. All the optimized geometries were subsequently subjected to frequency analyses, in order to calculate Gibbs free energies (298.15 K and 1 atm) and examine the number and motion of imaginary frequencies. To further refine the electronic energies obtained,single-point calculations were performed by using the B3LYP method with Grimm’s D3 correction[27]and the
Def2TZVPP basis set[28]for all atoms, on the basis of the same solvation model. The combination of the B3LYP method and a dispersion-corrected method has been widely used in mechanistic calculations[29,30]. The 3D geometries were made by the ChemCraft software.
3 RESULTS AND DISCUSSION
3. 1 Model catalyst and C-H activation process
Fig. 2 shows the designed parent Pd(II) catalyst, dimethylether-coordinated palladium acetate labeled as CAT in this paper, which has three ligands, namely aκ2-OAc ligand, aκ1-OAc ligand, and a dimethyl ether (OMe2) ligand.Replacement of theκ1-OAc ligand in CAT with another univalent ligand (X) or replacement of the OMe2ligand in CAT with another neutral ligand (Y) can generate an array of palladium catalysts with different spectator ligands. Theκ2-OAc ligand in CAT (and the other catalysts) is treated as the reacting ligand responsible for C-H bond cleavage. The C-H activation mechanisms and energetics mediated by different palladium catalysts will be compared in the following sections.
We first designed two C-H activation reactions of benzene catalyzed by CAT as given in Fig. 2, which differ in the position of the C-H activation site. If the C-H bond cleavage of benzene is induced by the O1 arm ofκ2-OAc,the reaction channel is named as the O1 pathway; similarly,the other reaction channel is named as the O2 pathway. The detailed mechanism and the corresponding free-energy variations are shown in Fig. 3. Only the structures on the O2 pathway are given for simplicity, because those on the O1 pathway are very similar.
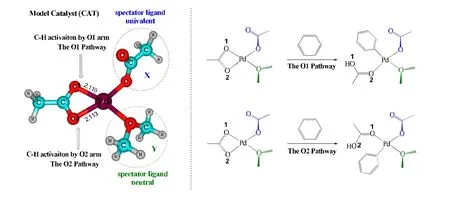
Fig. 2. Parent catalyst and C-H activation reactions

Fig. 3. Mechanism and free energies (in kcal/mol) for C-H activation of CAT and benzene. Only the structures on the O2 pathway are given
The located mechanism consists of two elementary steps,involving two transition states. In the first step, one of the two O arms ofκ2-OAc is removed by benzene through a ligand substitution via transition state TS-1. The interaction between palladium and benzene in INT-1 is a η2-type coordination. In the second step, the target C-H bond is deprotonated by the adjacent OAc group via transition state TS-2,which affords intermediate INT-2 with a normal Pd-C6H5coordinate bond.
The first step involves a free-energy barrier of 12.9 (O1 pathway) or 14.4 kcal/mol (O2 pathway), and INT-1 lies at 5.5 or 8.0 kcal/mol above the initial point. The free-energy barrier of the C-H activation step is calculated to be 13.2 or 13.4 kcal/mol. According to the steady-state approximation,however, the free-energy difference between TS-2 and the initial point should be assigned as the rate-determining activation barrier, which is computed to be 18.7 or 21.4 kcal/mol. The overall reaction needs to absorb free energies by 4.9 or 10.6 kcal/mol, indicating that it is unspontaneous under the standard condition. However, INT-2 only serves as an intermediate in the overall reaction rather than the final product, and subsequent transformations after INT-2 could release lots of free energies to achieve the overall spontaneity. It should be mentioned that benzene could also replace the OMe2ligand in CAT and the C-H activation step would be induced by the originalκ1-OAc group. Such a C-H activation pathway has also been characterized in our calculations, and the main results are provided in the Supporting Information. In fact, in this work we attempt to explore the effects of diverse spectator ligands in comparison to theκ1-OAc or OMe2ligand, and hence, the mechanistic pattern given in Fig. 3 will be more beneficial for fulfilling our research goal.
The three-dimensional structures of the proton-transfer transition states (TS-2) are given in Fig. 4. The located structures are consistent with the so-called concerted metalationdeprotonation (CMD) mechanism[18-20]and have a typical six-center cyclic structure, with H···O and H···C distances in the ranges of 1.288~1.313 and 1.327~1.343 Å, respectively. For the O2 pathway, the C···H distance is obviously longer than the O···H distance, while the two distances are relatively close for the O1 pathway, indicating that the proton-transfer transition state occurs later on the O2 pathway. Judging from the computed free-energy values and transition-state geometries, C-H activation should have more difficulty taking place at the positiontransto the originalκ1-OAc ligand (O2 pathway) in comparison to thatcisto the originalκ1-OAc ligand (O1 pathway).

Fig. 4. Three-dimensional geometries of TS-2 (bond length in Å)
3. 2 Spectator ligand effects: univalent ligands
In order to unveil the electronic effects of the spectator ligand X (univalent type) on C-H activation, we designed seven palladium catalysts by replacing theκ1-OAc ligand in CAT with a different univalent ligand, including methoxyl(OCH3), amide (NH2), methyl (CH3), phenyl (Ph), cyanide(CN), fluorine (F), and chlorine (Cl). The structures and compound names of these catalysts are defined in Fig. 5.

Fig. 5. Designed palladium catalysts with different univalent ligands (bond length in Å)
It is shown that the two Pd-O bonds coming from theκ2-OAc ligand are not of equal length, which is related to the nature of the introduced ligand X. Except for CAT-F, the Pd-O2 distance (transto X) is longer than the Pd-O1 distance (cisto X); especially, the two Pd-O distances differ by about 0.3 Å for both CAT-CH3and CAT-Ph. This phenomenon is attributed to the concept oftranseffect ortransinfluence in coordination chemistry[31], which states that aσ-donating or/andπ-accepting ligand tends to weaken itstranscoordinate bond. Thetranseffect is normally more significant than the correspondingciseffect. As a stronglyσ-donating ligand, both methyl and phenyl could exert a very significanttranseffect.
Although the C-H activation mechanisms mediated by these catalysts are similar to that using CAT as the catalyst,the energetic parameters vary widely with the nature of the spectator ligand. The computed rate-determining activation barriers (the difference between TS-2 and the initial point,like Fig. 3) and overall free-energy changes are collected in Table 1. We first discuss the kinetic effects exerted by the seven ligands. It is shown that CAT-F and CAT-Cl have free-energy barriers (19.1~19.5 and 21.1~21.2 kcal/mol)very close to CAT, and hence, the poorlyσ-donating ligands F and Cl appear not to hinder the C-H activation in progress.The catalysts CAT-OCH3, CAT-NH2, CAT-Ph, and CAT-CH3all make the C-H activation process harder. For the O1 pathway, the free-energy barrier increases from 20.7 to 25.1 kcal/mol in the order of CAT-NH2, CAT-OCH3,CAT-Ph and CAT-CH3. For the O2 pathway, the free-energy barrier increases from 25.3 to 35.8 kcal/mol in the order of CAT-OCH3, CAT-NH2, CAT-Ph and CAT-CH3. The O1 pathway is obviously more favorable both kinetically and thermodynamically than the O2 one for all of the catalysts. The severely retarding effect caused by both CH3and Ph should result from their stronglyσdonating ability and significanttranseffect. Generally, the free-energy barrier of C-H activation increases in the order ofκ1-OAc ≈ Cl ≈ F < OCH3< NH2< Ph < CH3. As anσdonating andπ-accepting ligand, the CN ligand displays a special behavior. The O1 pathway involves the lowest barrier of 18.3 kcal/mol, while the O2 pathway suffers from a high barrier of 25.2 kcal/mol, indicating that this ligand can promote the C-H activation at itscisposition but may hinder the reaction at itstransposition.
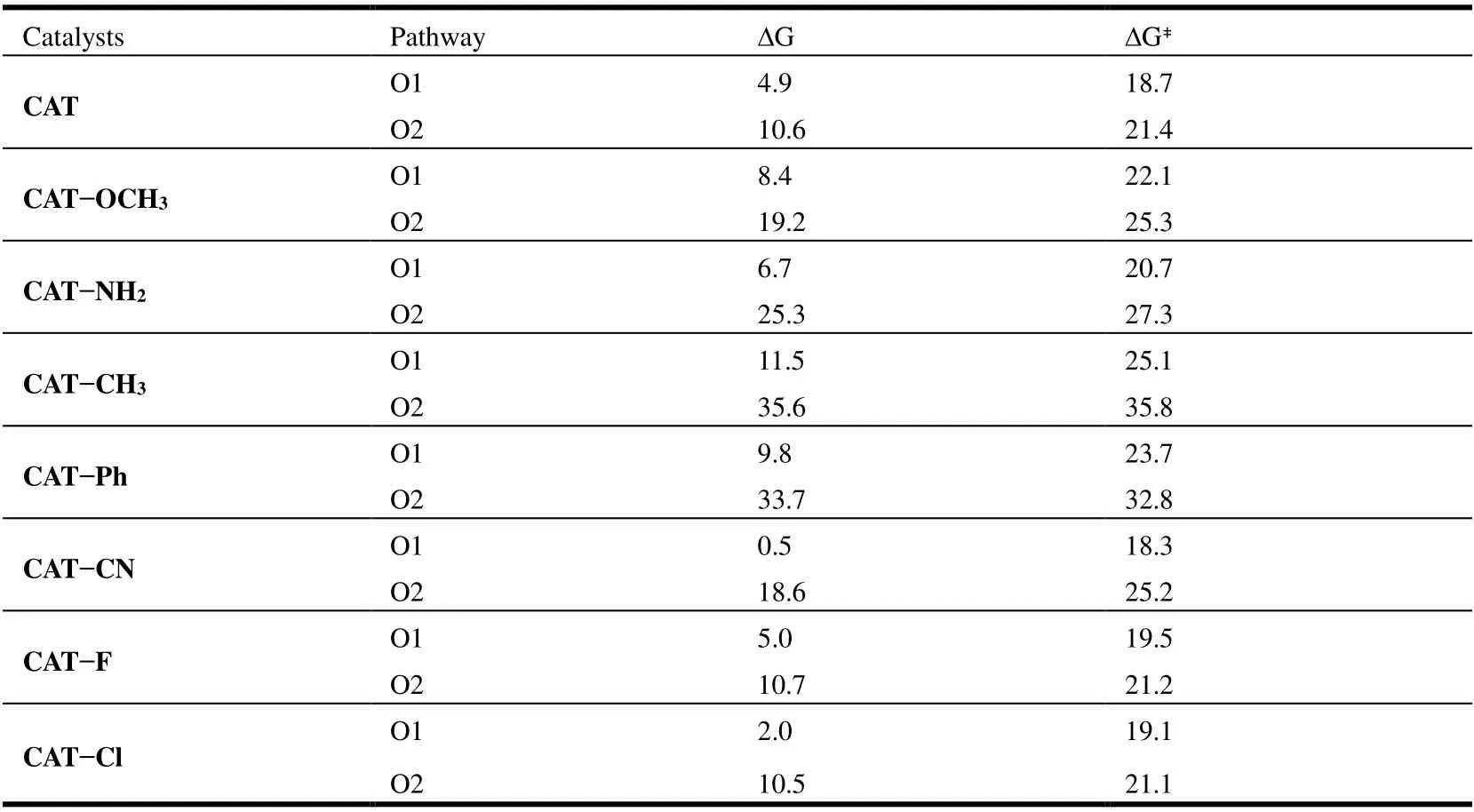
Table 1. Computed Free-energy Changes (ΔG) and Barriers (ΔG‡) for C-H Activation Reactions Mediated by Different Catalysts (in kcal/mol)
The thermodynamic parameters display a similar trend. In comparison to CAT, the introduction of OCH3, NH2, Ph or CH3makes the C-H activation process more endothermal in terms of free energies, especially for Ph or CH3. The effect on the O2 pathway is more evident than that on the O1 pathway. In contrast, CAT-F and CAT-Cl have very similar thermodynamic parameters to CAT. As an exception, the O1 pathway mediated by CAT-CN is near free-energy neutral(ΔG= 0.5 kcal/mol), while the O2 pathway is strongly endothermal by 18.6 kcal/mol.
The transition-state geometries are provided in Fig. 6. As the free-energy barrier increases, in general, the difference of the C···H and O···H distances becomes larger and the transition state occurs later. For example, the transition state on the O2 pathway occurs later than that on the O1 pathway for all of the catalytic systems, because the C···H distance is obviously longer than the O···H distance for the O2 pathway.Note that the imaginary frequencies of TS-2-CH3 and TS-2-Ph on the O2 pathway are only -187.0and -493.2icm-1, respectively, since they occur quite late on the reaction coordinates.
In summary,σ-donating ligands, such as OCH3, NH2, Ph,and CH3, could hinder the C-H activation in progress, and the effects of CH3and Ph are evident. As thetranseffect is more significant than thecisone and the small CH3group causes a large difference, they should mainly be electronic rather than steric effects.
Fig. 7 provides a chemical model to explain the observed ligand effects. The effects of theσ-donating ligand can be rationalized by parts (a) and (b). The lone-pair orbital of this ligand could overlap with the fragment orbital having the largest component of the developing Pd···Ph bond. A small amount of electron density flows to the bonding region of Pd···Ph, and the consequent electron accumulation would increase electrostatic repulsion to hinder the C-H activation and Pd-C bond formation. It is understandable that the back overlap of the ligand is more effective than the side overlap,and hence, thetranseffect must be more evident than theciseffect. For the CN ligand residing at thecisposition, the developing Pd···Phσ-lobe could be in conjugation with theπsystem of CN, through space overlap, to stabilize the Pd···Ph bonding.
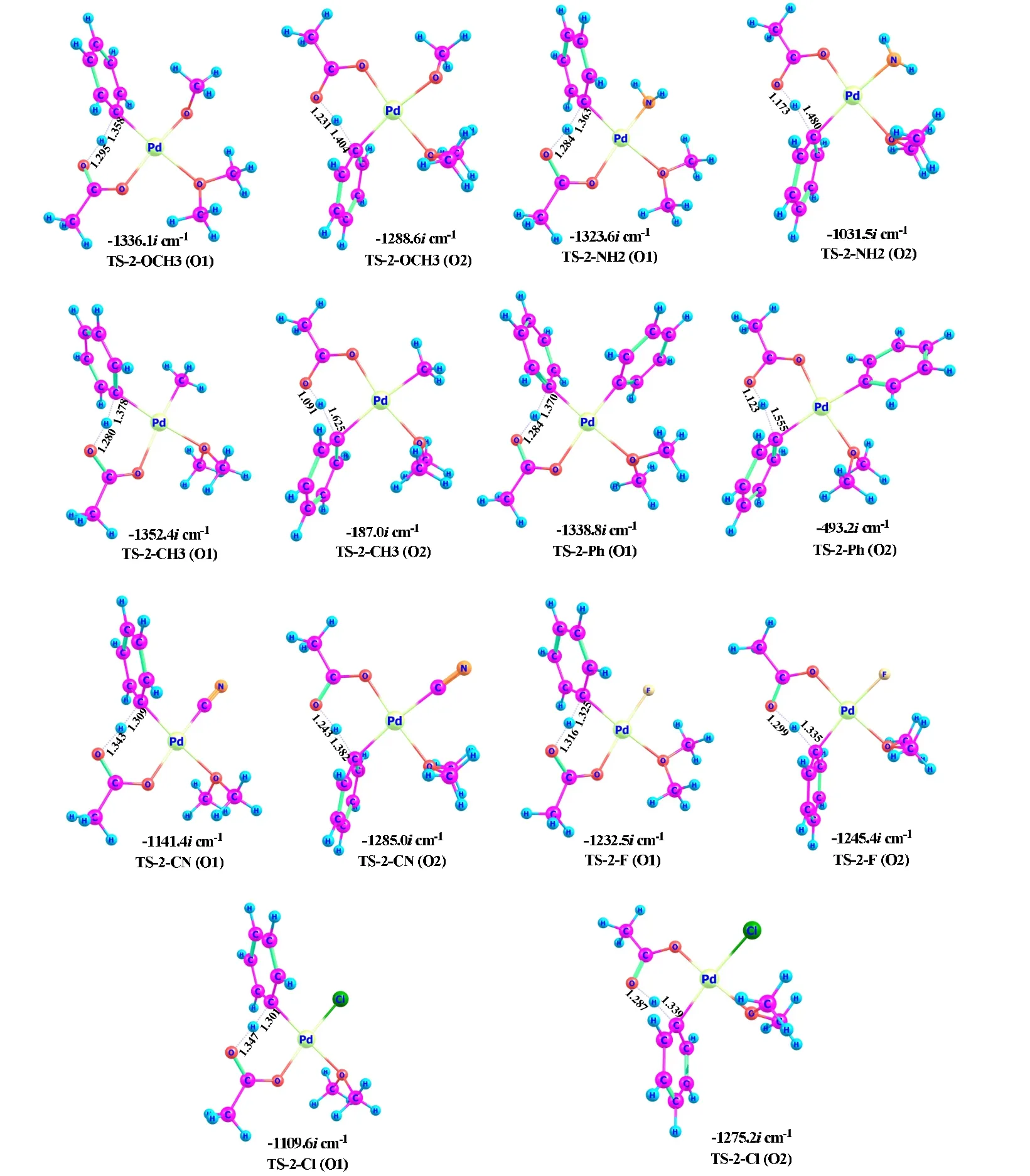
Fig. 6. Three-dimensional geometries of the proton-transfer transition states (bond length in Å)
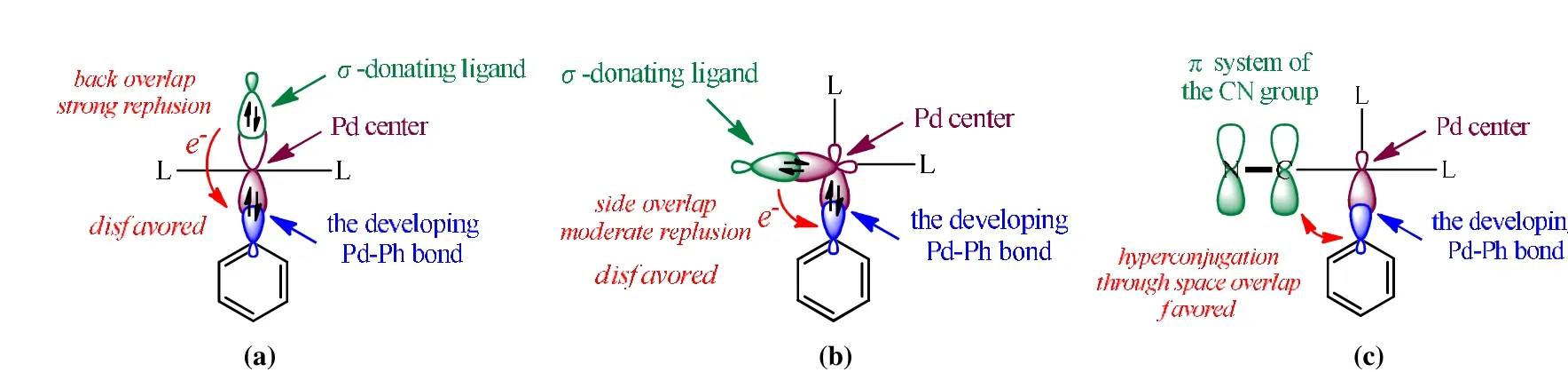
Fig. 7. A general model to explain the observed ligand effects on C-H activation
The proposed chemical model is supported by the calculated Kohn-Sham orbitals of some typical transition states(Fig. 8). The HOMOs of both TS-2-CH3 and TS-2-Ph indicate an overlap repulsion between the Pd···Ph and Pd-C bonds for both the O1 and O2 pathways. The HOMOs of TS-2-CN have similar feathers. For the O1 pathway,however, TS-2-CN has a low-lying orbital exhibiting a hyper-conjugation between the Pd···Cσ-lobe and the C≡Nπ-lobe. The characters of these selected orbitals are generally in line with the proposed chemical model.
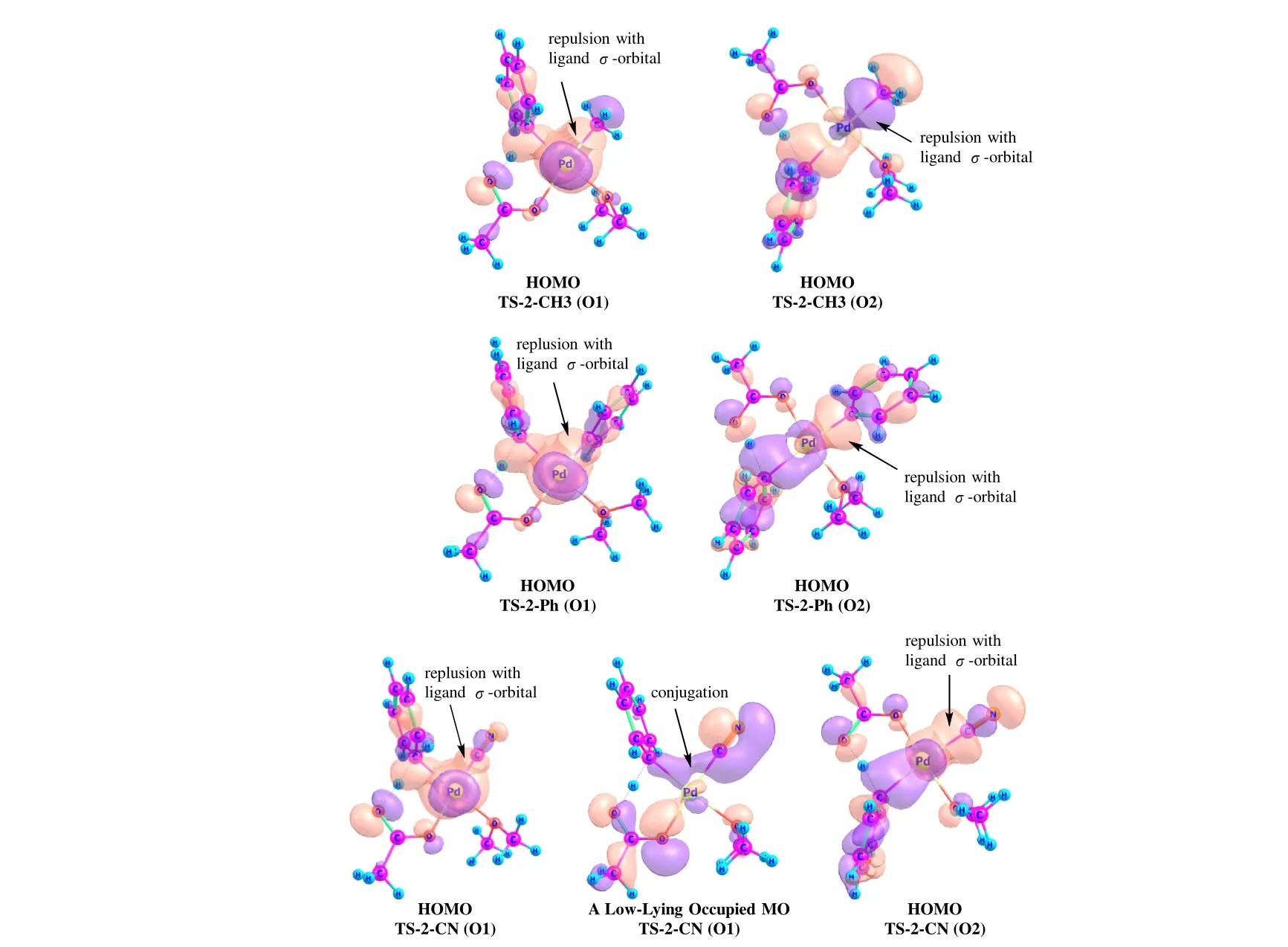
Fig. 8. Some Kohn-Sham orbitals of the selected transition states of C-H activation
3. 3 Spectator ligand effects: neutral ligands
In order to explore the effects of the spectator ligand Y(neutral type) on C-H activation, we designed another seven palladium catalysts by replacing the OMe2ligand in CAT with a different neutral ligand, including amine (NMe3), phosphine (PMe3), dimethyl sulfide (SMe2), ethene (CH2=CH2),ethyne (CH ≡CH), N-heterocyclic carbene (NHC), and isonitrile (CNR). The structures and compound names of these catalysts are defined in Fig. 9.
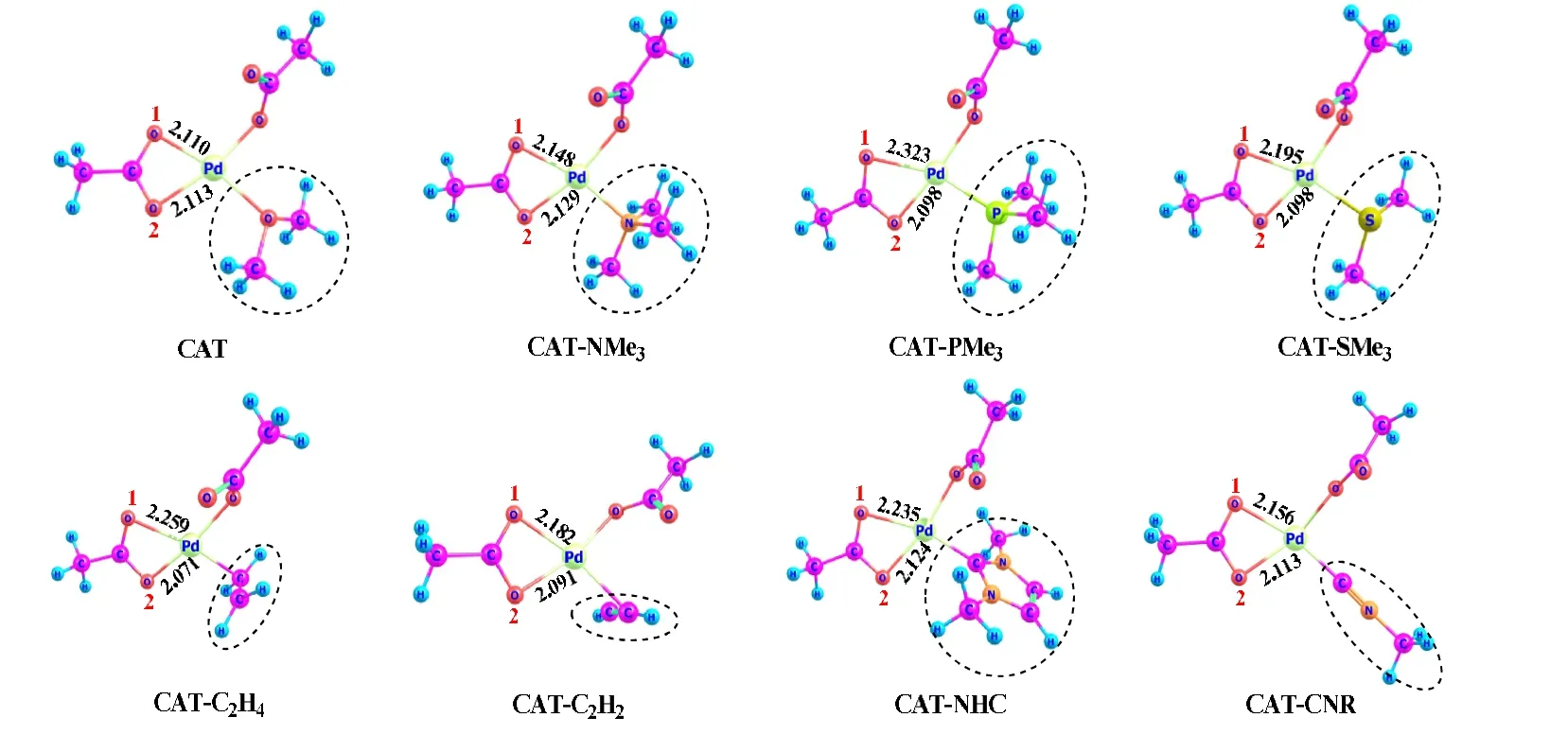
Fig. 9. Designed palladium catalysts with different spectator ligands (bond length in Å)
The neutral ligand Y exerts atranseffect on the O1 pathway, since it resides at the positiontransto the O1 arm ofκ2-OAc. The computed rate-determining activation barriers and overall free-energy changes are collected in Table 2.These ligands each hinder the C-H activation process as compared to the weaklyσ-donating ligand OMe2, but their effects are less evident than those caused by the univalent ligands. In comparison to CAT for the O2 pathway, the NMe3ligand substantially increases the barrier height by 6.6 kcal/mol, while all of the other ligands, except CNR, result in a small increase of the barrier height (by 1.4~3.3 kcal/mol). The different effects of NMe3and PMe3on the O2 pathway should be caused by steric effect rather than electronic effect, since the Pd-N and N-C bonds are shorter than Pd-P and P-C, which makes the phenyl and NMe3groups more crowded. For the O1 pathway, however, CAT-PMe3involves a high barrier of 28.4 kcal/mol and CAT-NMe3shows a much lower barrier of 23.6 kcal/mol because PMe3has strongerσ-donating ability than NMe3, and the former ligand could exert a more significanttranseffect on the O1 pathway to hinder the C-H activation in progress. The different behaviors of PMe3and NMe3on the O1 and O2 pathways indicate the combined electronic and steric effects to be at work. For catalysts CAT-SMe2, CAT-C2H4, and CAT-C2H2, the free-energy barriers of the O1 and O2 pathways are close to each other.
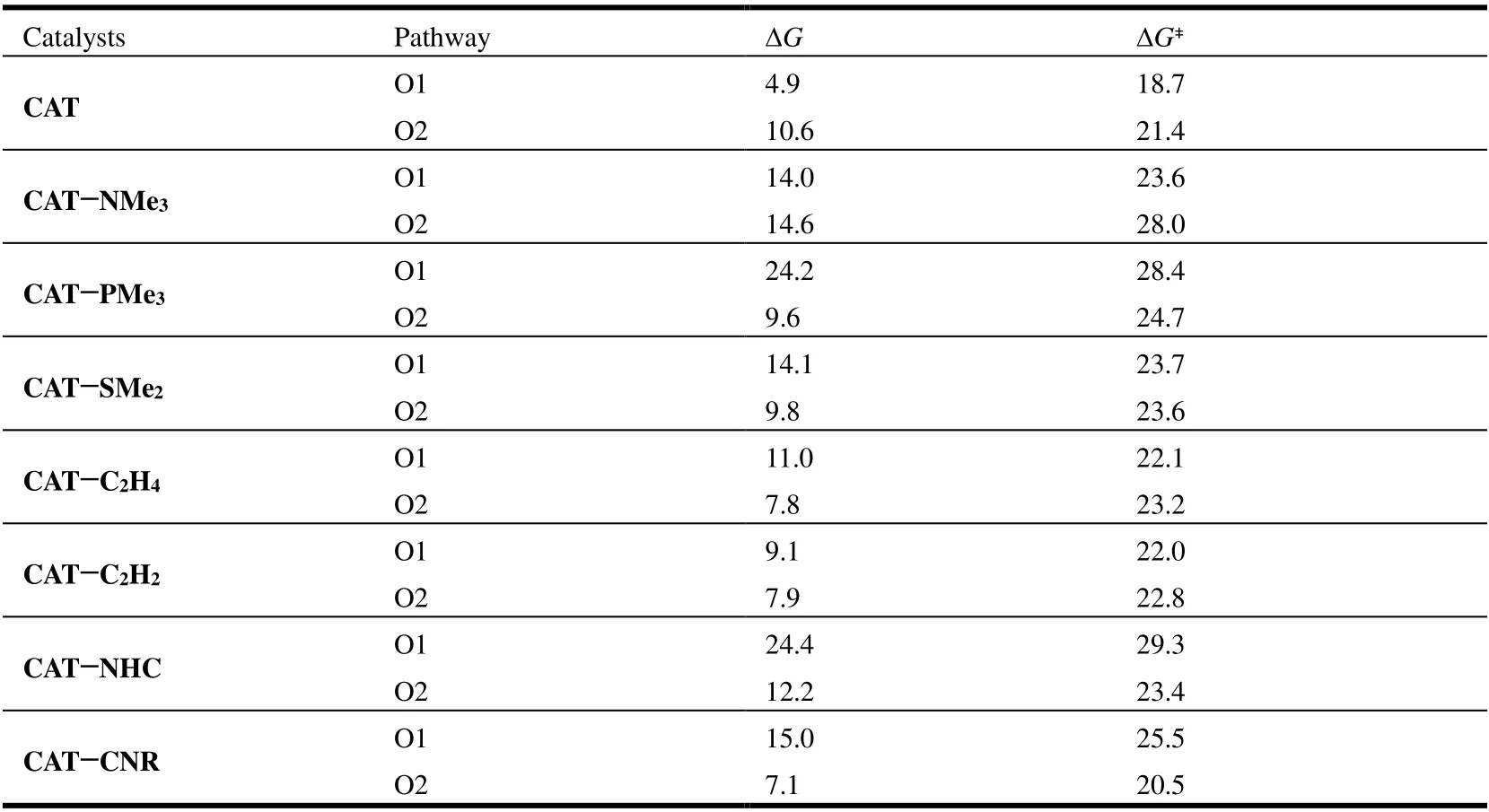
Table 2. Computed Free-energy Changes (ΔG) and Barriers (ΔG‡) for C-H Activation Reactions Mediated by Different Catalysts (in kcal/mol)
It seems that the catalyst CAT-NHC severely hinders the C-H activation on the O1 pathway, with the free-energy barrier of 29.3 kcal/mol that is even higher than that of CAT-PMe3, and hence, thetranseffect of the NHC ligand should be significant. The thermodynamic aspect displays a similar trend. These indicate that the employment of a chiral NHC ligand in palladium-catalyzed C-H functionalizations could deliver the stereo-selectivity but might lower the reactivity towards C-H activation. Notably, as aσ-donating andπ-accepting ligand, the CNR ligand appears to behave similarly to the CN ligand discussed before, since it could promote the O2 pathway (cisto CNR) but would hinder the O1 pathway (transto CNR).
In order to save space, some of the transition-state geometries and typical molecular orbitals are given in the supporting information. For NMe3and PMe3systems, the calculated Kohn-Sham orbitals of the transition states indicate an overlap repulsion between the Pd···Ph and Pd-C bonds for both O1 and O2 pathways. The transition state of the NHC system on the O1 pathway has a similar high-lying molecular orbital. The transition state of the CNR system on the O2 pathway is featured by a conjugation between theπ-bond of CNR and the developing Pd···Phσ-lobe.
3. 4 Application of the ligand effects to mechanistic analyses on Heck couplings
Dehydrogenative Heck reactions of arenes with alkenes furnish arylalkenes by direct C-H/C-H coupling. It was well established that the favorable pathway consists of three successive processes, including C-H activation of the arene,migratory insertion of the alkene, andβ-H elimination (see Fig. 10, left part). This generally accepted mechanism demonstrates that dehydrogenation of the alkene is normally achieved byβ-H elimination rather than C-H activation.Theoretically, the same arene-alkene cross-coupling could be realized through a different pathway, involving two C-H activation events and a reductive elimination step (Fig. 10,right part). On this pathway, after the C-H activation at the arene, the alkene would also undergo a C-H activation step to form an intermediate containing both the Pd-alkenyl and Pd-aryl bonds. Both two mechanisms shown in Fig. 10 are the typical PdII/Pd0/PdIIcatalytic cycles.
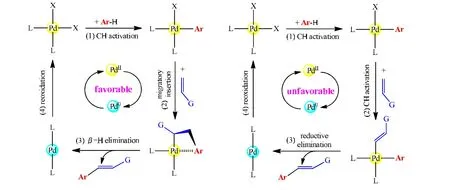
Fig. 10. Reaction pathways for dehydrogenative Heck couplings: the conventional mechanism (left) and a new mechanism (right)
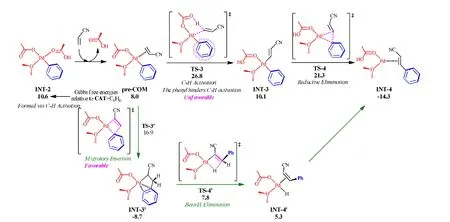
Fig. 11. Structures and free-energy variations (in kcal/mol) for the coupling between benzene and acrylonitrile through two different pathways
On the basis of the conclusions drawn in this work, we can explain why the conventional mechanism is plausible and the other mechanism is not. The C-H activation at the arene leading to an arylpalladium intermediate is always the first process on the whole catalytic pathway, but the following transformations are different. On the newly proposed pathway, the alkene is engaged in a C-H activation step instead of a migratory insertion step. However, the second C-H activation step at the same palladium center should be difficult due to the fact that the aryl ligand formed from the first C-H activation would hinder the C-H activation in progress. The strongly retarding effect of the phenyl ligand on C-H activation, as observed before, makes this pathway unlikely to be competitive.
In order to support the above assumption, we performed DFT calculations on the two pathways (Fig. 11). The following discussion starts from INT-2, which is the intermediate product of the C-H activation of benzene with CAT. Using acrylonitrile as the alkene partner, we first characterize the conventional mechanism. The ligand exchange between acrylonitrile and acetic acid generates the precursor complex pre-COM. The cross-coupling between acrylonitrile and benzene results from the migratory insertion step via TS-3′,which affords INT-3′. Then, one of the two hydrogen atoms on the methylene group is transferred to palladium by theβ-H elimination step via TS-4′, delivering INT-4′ with the product 2-phenyl acrylonitrile. On the other pathway, pre-COM undergoes the C-H activation of acrylonitrile through CMD mechanism via TS-3, generating INT-3 with twocisPd-C bonds. The cross-coupling between the aryl and alkenyl groups is finished by the reductive elimination step via three-center transition state TS-4. TS-3′ is much more accessible than TS-3, because the total free-energy barrier of the C-H activation of acrylonitrile reaches at 26.8 kcal/mol and that of the migratory insertion step is merely 16.9 kcal/mol. Hence, the pathway involving two C-H activations could be ruled out. In the viewpoint of elementary steps, the C-H activation of acrylonitrile has the free-energy barrier of 18.8 kcal/mol (compare pre-COM to TS-3), while that of benzene with CAT is merely 13.2~13.4 kcal/mol (compare INT-1 with TS-2 in Fig. 3). These support that the palladium-catalyzed C-H activation would be hindered by a stronglyσ-donating spectator ligand.
4 CONCLUSION
The electronic and steric environments of a transition metal could be tuned by their spectator ligands. This work aims at providing a quantitative measurement of the effects of diverse spectator ligands on palladium-catalyzed C-H activation. The main conclusions are summarized as follows:
(1) The univalent ligand generally influenced C-H activation more than the neutral ligand.
(2) Theσ-donating ligand hindered the C-H activation in progress, and the retarding effect became more evident as the electron-donating ability of the ligand increased.
(3) Theσ-donating ligandtransto C-H activation site exerted a larger effect than thatcisto the C-H activation site.
(4) Asσ-donating andπ-accepting ligands, both cyanide and isonitrile hindered the C-H activation at theirtransposition,while they facilitated the C-H activation at theircisposition.
(5) A chemical model was proposed to rationalize the observed ligand effects and was subsequently applied to mechanistic analyses on dehydrogenative Heck couplings.
- 结构化学的其它文章
- Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
- Discovery of Benzimidazole Derivatives as Novel Aldosterone Synthase Inhibitors: QSAR, Docking Studies, and Molecular Dynamics Simulation①
- QSAR Models for Predicting Additive and Synergistic Toxicities of Binary Pesticide Mixtures on Scenedesmus Obliquus①
- Preparation, Crystal Structure and Fungicidal Activity of N-(5-(benzofuranol-7-oxymethyl)-1,3,4-thiadiazol-2-yl)amide Compounds①
- Antibiotic Silver Particles Coated Graphene Oxide/polyurethane Nanocomposites Foams and Its Mechanical Properties①
- Planar Tetracoordinate Carbon in 6σ + 2π Double Aromatic CBe42- Derivatives①

