Anti-obesity effect and UHPLC-QTOF-MS/MS based metabolite profiling of Solanum nigrum leaf extract
Zain Ul Aabideen, Muhammad Waseem Mumtaz✉, Muhammad Tayyab Akhtar, Muhammad Asam Raza,Hamid Mukhtar, Ahmad Irfan, Syed Ali Raza, Muhammad Nadeem, Yee Soon Ling
1Department of Chemistry, University of Gujrat, Gujrat, Pakistan
2Institute of Industrial Biotechnology, GC University Lahore, Lahore, Pakistan
3Department of Chemistry, College of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia
4Research Center for Advanced Materials Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia
5Department of Chemistry, GC University Lahore, Lahore, Pakistan
6Sabah Occupational Safety, Health and Environment Association, Lot33, 1st Floor, Block E, Iramanis Center, JalanLinatas, 88450, Kota Kinabalu,Sabah, Malaysia
ABSTRACT
Objective:To evaluate the antioxidant potential and pancreatic lipase inhibitory action of optimized hydroethanolic extracts of Solanum nigrum.
Methods:Optimized extraction for maximum recovery of metabolites was performed using a combination of freeze-drying and ultrasonication followed by determination of antioxidant and antiobesity properties. The ultra-high performance liquid chromatography equipped with mass spectrometry was used to analyze metabolite profiling of Solanum nigrum. Computational studies were performed using molecular docking and electrostatic potential analysis for individual compounds. The hypolipidemic potential of the most potent extract was assessed in the obese mice fed on fat rich diet.
Results:The 80% hydroethanolic extract exhibited the highest extract yield, total phenolic contents, total flavonoid contents along with the strongest 2,2-diphenyl-1-picrylhydrazyl scavenging activity,total antioxidant power, and pancreatic lipase inhibitory properties.The 80% hydroethanolic extract not only regulated the lipid profile of obese mice but also restricted the weight gain in the liver, kidney,and heart. The 80% hydroethanolic extract also reduced alanine transaminase and aspartate transaminase concentrations in serum.The effects of plant extract at 300 mg/kg body weight were quite comparable with the standard drug orlistat.
Conclusions:Solanum nigrum is proved as an excellent and potent source of secondary metabolites that might be responsible for obesity mitigation.
KEYWORDS: Solanum nigrum; Ultrasonication; Metabolite profiling; Total phenolic contents; Total flavonoid content;Antioxidant; DPPH; Total antioxidant power; Pancreatic lipase;UHPLC-QTOF-MS/MS; Antiobesity; Mice; Hypolipidemic;Molecular docking
Significance
The 80% hydroethanolic leaf extract of Solanum nigrum is proved as an effective source to treat obesity due to presence of various biologically active metabolites. The in vivo trials confirmed the anti-obesity properties of extract by modulation in lipid profile of obese mice. The findings are of immense significance which may be extended to improve naturopathic approach for obesity management and formulation of functional diet with medicinal attributes.
1. Introduction
Obesity (termed as “New World Syndrome”) is considered a serious global health problem worldwide. It is characterized by unnecessary deposition of fat in adipose tissues and other internal organs like the heart, liver, pancreatic islets, and skeletal muscles. It is responsible for several chronic disorders and metabolic disabilities such as hypertension, dyslipidemia, osteoarthritis, fatty liver disease, gallstones, obstructive sleep apnea, type 2 diabetes, heart failure, coronary artery disease, as well as breast, reproductive, and gastrointestinal cancer[1]. Obesity has been recognized as a lifestyle or nutritional disorder and is prevailing at an alarming rate both in developing as well as developed countries due to high caloric dietary intake and reduction in physical activities[2]. Pancreatic lipase secreted by the pancreas is involved in the digestion of fats into monoglyceride and free fatty acids for their uptake by enterocytes.Delayed indigestion and consequent low uptake of fat generally decrease fat accumulation in adipose tissues. Therefore, inhibition of pancreatic lipase is considered a vital target for mitigating obesity[3].Irregular pancreatic lipase activity may disturb the regular digestion of dietary fats into triglycerides that are distributed between adipose tissues and muscles, thus stimulating the fat deposition and leading to obesity development[4]. Animal, clinical, and epidemiological studies have revealed that chronic oxidative stress is responsible for the pathogenesis of many serious health-related complications including obesity. Oxidative stress triggers obesity by promoting the deposition of white fat and altering dietary intake[5]. Although several synthetic antioxidants that can reduce oxidative damage are available in the market, their safety always remains questionable due to adverse health impacts, which emphasize the need to search for safe and novel plant-based antioxidants[6].
Various strategies are recommended worldwide to treat obesity including surgery, pharmacotherapy, exercise, and stringent diets.Contemporarily utilized synthetic anti-obesity medicine (orlistat)is a lipase inhibitor that blocks the breakdown of triglycerides to decrease the available amount of free fatty acids and monoglycerides for intestinal absorption[7]. However, this drug may exhibit side complications like nephrotoxicity, respiratory infection, dyspepsia,abdominal pain, oily stools, flatulence, as well as psychiatric and menstrual disorders[8,9]. Traditionally, medicinal plants or herbs have been utilized to control body weight and obesity in numerous countries. Oral consumption of medicinal plants, together with appropriate dietary changes, is one of the most popular strategies for the management of weight gain and obesity. The cholesterollowering and hypolipidemic impacts of plant extracts are well reported[10]. In recent years, the capability of several natural products has been explored for the management of obesity and it might be an excellent alternative option for the formulation of anti-obesity agents with limited or no side effects[11]. However, a significant number of medicinal plants claimed to exhibit anti-obesity effects in traditional medicinal systems, yet need to be explored on scientific grounds.
Solanum nigrum (S. nigrum) commonly known as black nightshade or makoi, a member of the family Solanaceae is extensively used in ethnomedicine for its anti-proliferative, hepatoprotective,anti-oxidative, anti-inflammatory, anti-obesity, and antidiabetic properties[12]. Leaves of S. nigrum are traditionally utilized for therapeutic purposes in many areas of the world including China,Pakistan, and Southern India to cure mouth ulcers, inflammation edema, cancer, insomnia, earache, fever, and pain[12,13]. The leaves of S. nigrum are reported to contain steroidal alkaloids, steroidal glycosides, and steroidal oligoglycosides[14]. Efforts are being made to identify novel bioactive constituents of S. nigrum and to explore their pharmacological role. Different analytical techniques (GC-MS,HPLC, and HPLC-MS, etc.) have been utilized for the identification and characterization of phytoconstituents from S. nigrum leaf extracts[14,15]. Among them, ultra-high performance liquid chromatography supported with efficient mass spectrometer (UHPLCQTOF-MS/MS) is recognized as a highly reliable analytical technique for the quick identification of bioactive metabolites in different parts of plants[10,16].
Current work was carried out to explore the antioxidant and antiobesity activity of the hydroethanolic (HE) leaf extracts of S. nigrum.Predominant bioactive constituents of S. nigrum leaf extract were identified using UHPLC-QTOFMS/MS. The molecular docking analysis of identified bioactive constituents against pancreatic lipase was performed to find the most probable inhibition site based on their binding energies.
2. Materials and methods
2.1. Chemicals and reagents
Ethanol, butylated hydroxyanisole (BHA), rutin, gallic acid, Folin Ciocalteu (FC) reagent, AlCl3, Tris-HCl buffer, Arabic gum, NaOH,NaNO2, pancreatic lipase, olive oil, acetone, CH3OH, C2H5OH,CaCl2, NaCl, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were mainly used. All utilized solvents, chemicals, and reagents were of high-quality research grade (Sigma-Aldrich, BDH & Merck).
2.2. Processing of plant material and extract preparation
Initially, healthy leaves of plants were collected from mature trees located in the territory of Azad Jammu & Kashmir, Pakistan. The leaves were subjected to species confirmation at the Department of Botany University of Gujrat, District Gujrat Pakistan. The plant material was also submitted vide specimen voucher UOGCHEM45/2018. The collected leaves were made dust-free and quenched immediately with liquid nitrogen to stop metabolism. The leaves were further lyophilized for 48 h at -68 ℃ before extraction.The freeze-dried leaves were converted into a powdered form of fine grain size (60-mesh-size) and stored at -80 ℃ in Ziplock packing until further experiments. The powder leafy material was then submerged in ethanol: water solvent system (100 mL each) of different concentrations ranging from 20% ethanol to pure ethanol with an interval of 20% each for 48 h. After that, mixtures were also vortexed for 2 h for better extraction of metabolites followed by ultrasonication for 30 min at 20 kHz (Soniprep 150 ultrasonicator MSE, UK). The obtained extracts were then centrifuged at 13 000 rpm for 10 min before filtration. The debris and plant material was removed by filtration apparatus connected with a vacuum pump.Excessive solvent was removed on rotary evaporator assembly under vacuum to maintain the structural integrity of phytoconstituents. The obtained extracts were lyophilized again and preserved at very low temperature for further studies.
2.3. Total phenolic and flavonoid contents
An already reported protocol with slight modifications was employed for the evaluation of total phenolic contents (TPC)[17]. The extracts were dissolved in CH3OH and mixed with FC reagent. A fresh 20% Na2CO3solution was prepared. The mixture of extract and FC stayed for 5 min and 4 mL of freshly prepared Na2CO3solution was added to it. The mixtures were incubated for 90 min at ambient temperature. For quantitative estimation, 750 nm wavelenghth was selected for absorbance measurement. Same set of experimental run was made using gallic acid to compute the results. A gallic acid standard curve was prepared for phenolic content measurement. TPC were calculated regarding gallic acid and presented as gallic acid equivalent (mg GAE/g).
A well-established method based on complex formation by AlCl3was utilized for estimation of total flavonoid contents (TFC) of extracts with minor modifications[18]. Methanolic solutions of all extracts were prepared in pure methanol. After that, reaction mixtures were prepared by mixing 0.10 mL of NaNO2(0.5 M), 0.15 mL of AlCl3·6H2O (0.3 M) and 3.4 mL of MeOH (30%) followed by adding prepared methanolic extract (200 μL). The mixtures were allowed to stay for 5 min followed by the addition of 1 mL of NaOH(1 M) and absorbance was measured at 510 nm. Rutin was used as standard and results were expressed as milligrams of rutin equivalent in each gram of understudy dried plant extracts (mg RE/g DE).
2.4. DPPH radical scavenging assay
The different S. nigrum extracts were evaluated for free radical scavenging potential which was determined as per the previously described DPPH radical scavenging assay with slight modification[19]. For this, DPPH reagent was dissolved in methanol and understudy extracts were mixed with this methanolic DPPH solution. The samples were shaken for proper mixing and allowed to incubate for 20 min at 35 ℃ for reaction completion and absorbance was measured at 517 nm. The percent scavenging effect of each extract was computed according to the formula below.
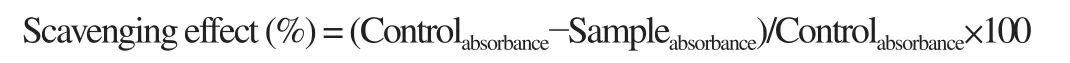
BHA was used as a standard synthetic antioxidant and the findings were presented as IC50(μg/mL). The analysis was carried out in a triplicate manner.
2.5. Total antioxidant power (TAP) assay
TAP of different S. nigrum extracts was computed according to a well-established phosphomolybdenum assay with slight changes[20].This method is based on the reduction principle, which involves a change in the oxidation state of Mo (Ⅵ) to Mo (Ⅴ) by the active ingredients of a particular extract. This reduction results in the appearance of green color due to the phosphate-Mo(Ⅴ) complex for which absorbance is checked. In brief, specific extract amount(0.15 mL) was mixed in solution [comprising 0.5 M H2SO4+ 30 mM Na3PO4+ 5 mM (NH4)2MoO4]and incubated at 90 ℃ for 90 min. After that, the mixtures were allowed to cool down to ambient temperature and absorbance was measured at 765 nm.
2.6. Inhibition of pancreatic lipase
The in vitro porcine pancreatic lipase inhibition (PPL) by extracts was assessed to determine the anti-obesity activities. Plant extracts were first mixed with PPL dissolved in Tris-HCl buffer (0.01 M),followed by adding a mixture of olive oil and Arabic gum using a homogenizer. A modified method was adopted for the evaluation of PPL inhibition of the plant extracts[21]. For this, all extracts were immersed in pure methanol and allowed to react with PPL solution.The resulting mixture was incubated at 4 ℃ for 30 min, followed by adding 2 mL of substrate solution and re-incubated for 30 min at 37 ℃. Ethanol and acetone mixture (1:1) was added and titrated with a solution of NaOH (0.02 M) to complete the neutralization reaction.The NaOH solution was used to neutralize the free fatty acids that were released in the reaction mixture by the action of the enzyme.The percent inhibition was computed using the mathematical relation given below.
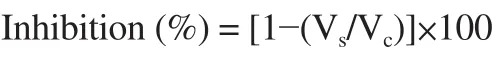
Where, Vcand Vsare the volumes of NaOH used in titration for control and sample, respectively.
2.7. Metabolite fingerprinting
Based on in-vitro antioxidant and enzyme inhibitory assays, the most potent extract was selected and used for metabolite profiling.The extract was added in aqueous CH3OH to form a suspension,followed by its filtration via a (0.45 μm) poly-tetrafluoroethylene filter. The UHPLC system was equipped with a degasser, binary pump system, and an auto-sampler. ACQUITY UPLC HSS T3 (C-18) column of 100 mm×2.1 mm×1.8 μm dimensions was used and the temperature was kept constant at 40 ℃. For elution, binary gradient mobile phase composed of water (0.1% formic acid) as solvent A and acetonitrile as solvent B was used with the change in composition. The composition of the mobile phase varied during an experiment in the pattern of 0 min, 1% B; 0.5 min, 1% B; 16.00 min,35% B; 18.00 min, 100% B; 20.00 min, 1% B. The mobile phase flow was kept at 0.6 mL/min with a sample volume of 1 μL. The chromatograph was equipped with a hybrid mass spectrometer by Waters (Vion IMS QTOF). For ionization purposes, an ion source was utilized both in positive and negative electrospray ionization mode, whereas the operational and reference capillary voltages were set to 1.50 kV and 3.00 kV, respectively. Likewise, the source and desolvation gas temperature was adjusted to 120 ℃ and 550 ℃,respectively with a desolvation gas flow of 800 L/h. The cone gas(nitrogen >99.5%) flow of 50 L/h was also maintained. Data was received in high-definition MSE (HDMSE) mode between m/z 50-1 500 at 0.1 s/scan. The CE of 4 eV was used for low energy scans and the CE ramped from 10 to 40 eV was used for a high energy scan.Moreover, Argon (99.999%) was employed as collision-induceddissociation gas.
2.8. Molecular docking studies and computational analysis
Identified bioactives were subjected to docking studies using Molecular Operating Environment (MOE 2016.08). The PPL structure was downloaded, and compounds were subjected to docking into the active site regions. Briefly, for docking analysis,the conformations (ligand-enzyme) were generated to produce the binding energy data based upon the most feasible interactions. The hydrophilic and hydrophobic compatibilities at different residues of various amino acids were examined. The results were appraised for docking, and analysis of their surfaces was carried out with graphical representation utilizing MOE and discovery studio visualizer.Computational studies on charge transfer at the molecular level were also done. Similarly, frontier molecular orbitals (FMOs) and highest occupied molecular orbitals energies were also determined.The energy gaps (HOMOs-LUMOs-Egaps) along with molecular electrostatic potential (MEP) and ionization potential (IP) for every identified metabolite were also examined.
2.9. In vivo hypolipidemic study
The mice model was used to assess the effect of plant extract on serum lipid parameters and related biochemical indicators. A total of 30 albino mice of 6-week age were procured from the animal house of GC University Lahore. The conditions of animal house were maintained at (28.0±2.0) ℃ with average humidity of (62.75±3.25)% throughout the experiment. The ad libitum supply of water and food remained continue as per treatments (ARRIVE guidelines 2.0). The husk in container was replaced on daily basis. The lignocaine (10 mg/mL) was applied as local anesthesia for blood collection from lateral vein of tail. No pain feel by animals was observed during blood collection. For dissection, after applying local anesthesia, the intraperitonial injection of meloxicam was done. The mice were allowed to stay in new conditions for 10 d for proper adaptation.Mice were provided with a high-fat diet (HFD) and water supply.
The HFD was consisted of corn starch (40%), protein (25%), starch(10%), vegetable oil (2.5%), sucrose (5%), vanaspati ghee (1%), cow milk butter (10%), coconut oil (2%) and cholesterol (4%).
The HFD treatment was carried out for 8 weeks to induce obesity especially in terms of body weight. Out of 30 mice, 6 mice were selected as normal diet groups (NDG) and were provided with a normal chow diet. The mice fed on HFD were weighed and grouped(each group comprising of 6 mice each) into a high-fat group (HFG),half extract dose group (HEG, plant extract at 150 mg/kg body weight), full extract dose group (FEG, plant extract at 300 mg/kg body weight) and orlistat treated group (ORL, treated with 50 mg of orlistat as standard drug)[22]. The orlistat and plant extracts were administrated orally through gavage once in 24 h. These groups were further treated for 8 weeks. The diet and water consumption were noted every 24 h and their average values were reported. The dietary intake (g/mouse/day), water consumption (mL/mouse/day), and faecal fat contents (%) were reported for each group and compared.After an 8-week treatment, mice blood was collected and analyzed for lipid profile including total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides(TG). Hemoglobin (Hb), alanine transaminase (ALT) and aspartate transaminase (AST) were also determined. The 8 h fasted mice were sacrificed by applying chloroform as anesthesia to collect vital organs (liver, kidney, and heart) to determine their fresh comparative weights.
2.10. Statistical analysis
Standard deviation was (±) applied to the data obtained during the study. One-way ANOVA (analysis of variance) was performed to analyze the difference of means. The Minitab 17.0 software was used. Differences were considered to be statistically significant with P<0.05.
2.11. Ethical statement
A prior ethical approval from Institutional Ethical Committee of GC University Lahore with No. GCU / II B / 06 dated 1st Jannuary 2019 was obtained clearly stating that no radiations and genetically modified organism were used in study. Further, experiments were conducted in presence of trained and skilled person under due protocols. Mice were handled with great care and kept in polypropylene containers/cages lined with fine husk.
3. Results
3.1. Extract yield
In the current work, the extraction method was optimized by utilizing HE solvent systems of different compositions, and the effect of every composition on percent yield was assessed (Table 1). The extract yield percent of different S. nigrum extracts came in the range from (16.88±0.02)% to (23.08±0.03)% with a maximum extract yield of (23.08±0.03)% for 80% HE leaf extract and a minimum extract yield of (16.88±0.02)% for 20% HE.

Table 1. Extract yields, TPC, and TFC of different Solanum nigrum (S.nigrum) extracts.
3.2. Total phenolic and flavonoid contents of extracts
TPC in leaves of S. nigrum (20%-80% HE and pure ethanolic extracts) are presented in Table 1. It showed that 80% HE extract contained significantly higher TPC [(161.73±3.53) mg GAE/g extract](P<0.05) and TFC [(77.54±1.18) mg RE/g extract]than that of 60%, 40%, 20% HE, and pure ethanolic extracts (P<0.05) (Table 1).
3.3. DPPH scavenging assay
The IC50values of DPPH radical scavenging activities of S. nigrum extracts and BHA (positive control) are shown in Figure 1A.Antioxidant potential of 80% HE was strongest [IC50=(44.05 ± 1.80)μg/mL]among all extracts.
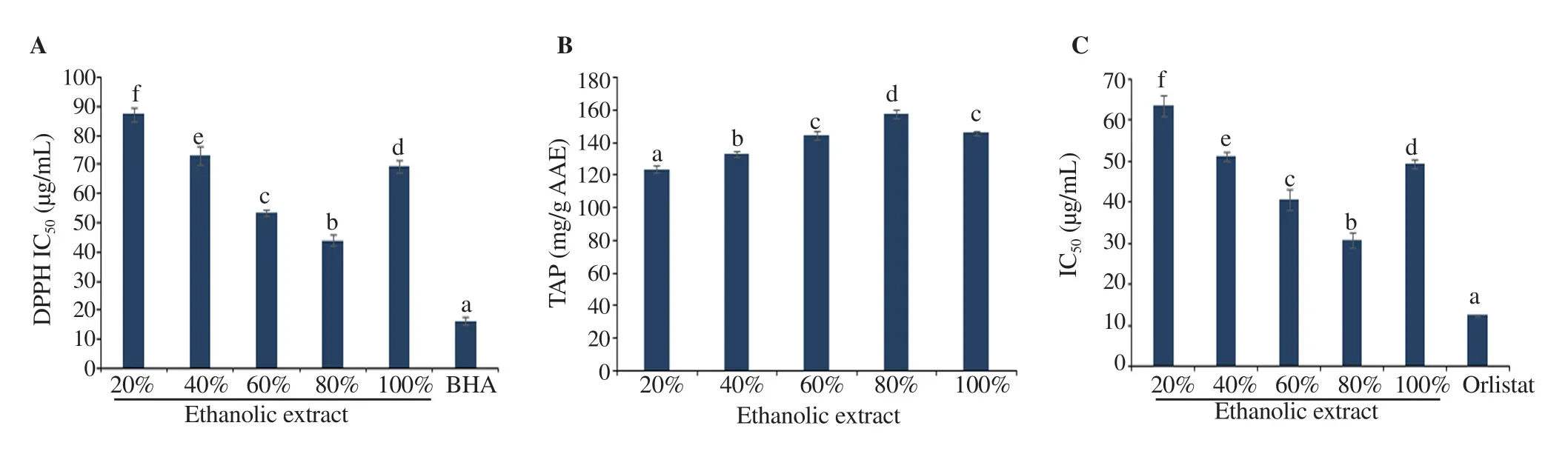
Figure 1. DPPH radical scavenging potential (A), total antioxidant power (TAP) analysis results (B), and pancreatic lipase inhibition (C) of different S. nigrum leaf extracts. Values having different letters are significantly different (P<0.05). BHA: butylated hydroxyanisole.
3.4. TAP assay
TAP of various S. nigrum extracts by phosphomolybdenum assay is shown in Figure 1B. As indicated from the results, 80% HE S.nigrum leaf extract showed significantly higher TAP [(157.36±2.90)mg AAE/g DE](P<0.05).
3.5. Pancreatic lipase inhibitory activity of S. nigrum extracts
The S. nigrum extracts were also subjected to check their inhibitory potential against pancreatic lipase and results are illustrated in Figure 1C. Results revealed that 80% HE S. nigrum extract exhibited significantly (P<0.05) stronger inhibitory effect towards pancreatic lipase [IC50=(30.67±1.72) μg/mL]than 60%, 40%, 20% HE, and pure ethanolic S. nigrum extracts.
3.6. Phytochemical characterization using UHPLC-QTOFMS/MS
The most potent S. nigrum leaf extract (80% HE) was analyzed for the identification or characterization of prominent bioactive molecules by UHPLC-QTOF-MS/MS. The chromatogram obtained is presented in Supplementary Figure 1, showing the base peaks of compounds. Mass spectral information of the identified phytoconstituents such as observed masses, predicted formulas,mass errors, and MS/MS fragments are given in Table 2. Whereas compound-specific fragmentation patterns are displayed in Supplementary Figure 2.

Table 2. Mass spectral information of all identified compounds in 80% HE leaf extract of S. nigrum.
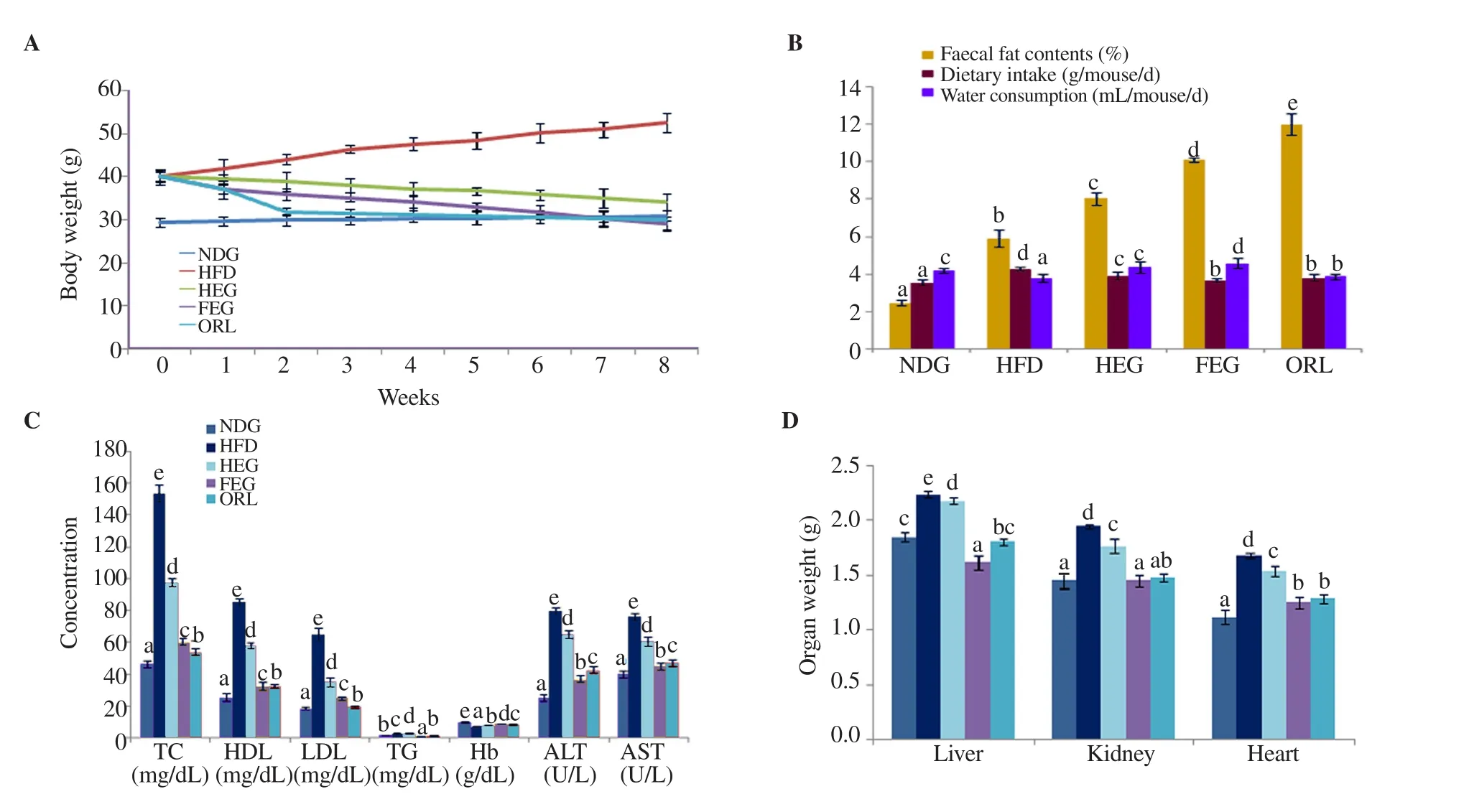
Figure 2. Bodyweight changes (A), faecal fat contents, dietary intake and water consumption (B), lipid profile, hemoglobin (Hb) levels, alanine transaminase(ALT) and aspartate transaminase (AST) concentrations (C), and organ weights (D). TC: total cholesterol, HDL: high-density lipoproteins, LDL: low-density lipoproteins, TG: triglycerides; NDG: normal diet group; HFD: high-fat diet group; HEG: half extract dose group (plant extract at 150 mg/kg body weight),FEG: full extract dose group (plant extract at 300 mg/kg body weight); ORL: orlistat treated group (50 mg of orlistat as standard drug). Values having different letters are found significantly different (P<0.05).
Chromatographic information indicated that a variety of compounds were present in the extract. A majority of compounds belong to diversified classes of phenolic acids, flavonoids and related subclasses.
3.7. Molecular docking studies
Efficacy of the phytochemicals against lipase inhibition is presented in Table 3. Many of the identified phytochemicals showed lower binding energy than that of standard compound orlistat and showed better binding capacity with pancreatic lipase. The binding energies were in the range of -9.673 to -16.549 kJ/mol. Cannabiscitrin was the top-scoring compound with the lowest binding energy (-16.549 kJ/mol).

Table 3. Binding energies with pancreatic lipase and nature of interactions.
The ribbon diagrams of orlistat and cannabiscitrin (top-scoring compound) onto active site of pancreatic lipase are presented in the Supplementary Figure 3. The ribbon diagram elucidated that both compounds were embedded deep into the active pocket of lipase.3D interaction plots of other extract components are presented in Supplementary Figure 4. The binding profiles of docked compounds revealed significant interactions with key residues at active pocket of pancreatic lipase. The pancreatic lipase (1LPB) activity primarily depends upon catalytic tirade which comprises SER152, HIS263,and ASP176 residues. Among these three, SER152 is the most vital residue for lipase activity. The compounds that can interact with these residues in particular with SER152 can inhibit the lipase activity.
The comparison of binding modes of top scored phytochemicals with orlistat showed many similarities. The binding profile of orlistat revealed that it was hydrogen-bonded with TYR114 and PHE77 (at the distance of 2.68Å and 2.64Å, respectively) and it was also able to interact with SER152, TYR114, PRO180, PHE215, ILE78, ALA260,ARG256, ALA259, and LEU264 through hydrophobic interactions.Cannabiscitrin that exhibited the lowest binding energy had a hydrogen bond with the most important amino acid residue SER152 at a distance of 2.50Å, and it was also interacting with ALA260,ALA259, ILE78, and ARG256 through hydrophobic interactions similar to the orlistat. Hibiscetin-3-O-glucoside which showed second-lowest binding energy showed hydrogen bonding at PHE77,HIS151, ASP79, and THR255 while it had nonbonding interactions with SER152, ILE78, ARG265, ALA260, and ALA259 making a strong ligand-protein complex. Amongst other phytochemicals identified in S. nigrum extract, isoetin-7-O-β-D-glucopyranosyl-2'-O-α-D-glucopyranoside and ellagic acid were also able to make a hydrogen bond with SER152. Based on findings, it can be depicted that phytochemicals extracted from S. nigrum can considerably inhibit the activity of pancreatic lipase. The docking studies provide an open end for further studies to fruitfully explore the importance/use of S. nigrum extract as a natural anti-obesity medicine.
3.8. Electronic properties and single electron transfer mechanism
The charge density distribution of HOMOs and LUMOs of molecules is shown in Supplementary Figure 5.
The energies of FMOs and HOMO-LUMO energy gaps (Egaps) are very informative segments to understand the electronic nature of biologically active substances. The energies of the HOMOs (EHOMOs,EHOMOs-1), LUMOs (ELUMOs, ELUMOs+1), and Egapsof various compounds at B3LYP/6-31G** level along with electron affinity and IP values are presented in Table 4.

Table 4. Ground state HOMO energies (EHOMO), LUMO energies (ELUMO),HOMO-LUMO energy gap (Egap), electron affinity (EA), and ionization potential (IP) of studied compounds (eV).
In Supplementary Figure 6, the MEP mapped for isolated compounds was demonstrated in a color scheme. The red and blue colors were used as demarcation between regions of positive and negative potential. The red color represents the region of higher negative potential and the blue color specifies the region of higher positive potential. The negative and positive potential regions are suitable for electrophilic and nucleophilic attack, respectively. The MEP decreasing order was depicted to be blue > green> yellow> orange > red. Here the blue and red colors show considerable attraction and repulsion, respectively. The supplementary information depicted that oxygen atoms of hydroxy, keto, carboxylic, or methoxy groups along with -Cl, would exhibit negative electrostatic potential,whereas hydrogen atoms associated with hydroxyl/methyl groups would show positive electrostatic potential. So, results revealed that in the case of nucleophilic or electrophilic attack, considerable repulsion would be expected at oxygen/-H atoms while significant attraction would be expected at -H/oxygen atoms, respectively.
The phenol was the standard used in presented work. Moreover, it has been ascertained that IP values of understudy compounds were lower than phenol. Based on IP values, it can be predicted that the understudied compounds would exhibit good antioxidant property.
3.9. In vivo hypolipidemic study
3.9.1. Body weights, fecal fat contents, dietary intake, and water consumption
The results of body weight changes (g) upon treatment are given in Figure 2A. The findings of faecal fat contents (%), food intake, and water consumption of obese mice are given in Figure 2B. After eight weeks of dietary manipulation, the body weight of NDG animals was increased from (29.43±1.10) g to (31.05±2.03) g. Whereas, the body weights of HFD mice were increased from (40.25±1.04) g to(52.66±2.29) g. After treatment, plant extract at 300 mg/kg body weight (FEG) efficiently reduced the body weight by 44.51% which was very close to the results of orlistat (42.85%) when compared to the final weight of HFD mice. The half dose of the extract (HEG)reduced the body weight of obese mice by 35.11%. The fat contents in faeces of FEG animals were 10.13% which was proximal to orlistat-treated obese mice. Fat contents in faeces of HFD mice were comparatively lower than in the FEG and HEG. Differences in dietary intake and water consumption were not significant among treatment groups, however, these values were different from NDG to some extent. The highest dietary intake of the HFD was slightly greater than other groups and extracts slightly increased the water intake, however, this increase was not so remarkable.
3.9.2. Lipid profile and blood biochemistry
Figure 2C shows the changes in lipid profile (TC, HDL, LDL,and TG) and blood biochemistry (Hb, ALT, and AST). The results revealed that extract at 300 mg/kg body weight significantly reduced the TC by altering HDL, LDL, and TG levels of obese mice in comparison to HFD mice (P<0.05). The extract at 300 mg/kg body weight reduced the TC of obese mice by 60.73% compared with HFD mice. The Hb level of the mice treated with 300 mg/kg extract was also significantly improved by 25.90% in comparison to HFD mice (P<0.05). The ALT and AST levels of obese mice were also significantly reduced upon administrating the extract at 300 mg/kg(P<0.05).
3.9.3. Organ weights
The changes in organ weight are shown in Figure 2D. Extract at 300 mg/kg body weight reduced the weight of the liver, kidney,and heart and the difference was significant (P<0.05), comparable to HFD group mice. The reduction in the kidney weight was also comparable to the NDG and orlistat groups (P>0.05), while the heart weight of the FEG group was quite comparable with the orlistat group (P>0.05).
4. Discussion
The efficacy of plants in treating chronic ailments is well established and depends upon the biologically active secondary metabolites.Usually, plant extracts have a very low concentration of secondary metabolites of pharmacological importance. The extraction process is a very important step before evaluating biological activities of plants. The extraction process should be optimized for better extract yield to evaluate the medicinal potential of a plant at maximum. The solvent system usually operates based on its constituents that control the solubility of a particular compound or class of compounds.Therefore, the selection of solvent and extraction methodology is of great significance to enhance extract yield. Studies have used hydroethanolic solvent systems, freeze-drying, and ultrasonication as novel and effective strategies for improved green extraction[10,15].
In the current study, 80% ethanolic extract exhibited the highest extract yields, TPC, and TFC, which were most probably due to the polarity of the solvent. TPC and TFC obtained in the current work were comparatively higher than the previous report of the same plant.It may be due to extraction technique and solvent composition. The polarity of solvent governed by solvent composition is imperative for maximum extraction of likely substances[23].
The 80% hydroethanolic extract of S. nigrum exhibited the highest TPC, TFC and strongest antioxidant activities. Higher TPC and TFC of 80% ethanolic extract can be related to high extract yield. TPC and TFC are very important compounds due to their antioxidant and other pharmacological activities. Phenolic compounds are considered an essential component of medicinally important diets[24]. It is well established that high TPC and TFC contribute to antioxidant activities of plant extracts. Antioxidant activities in current work were assessed by DPPH radical scavenging and total antioxidant power assay, which are mostly used. Antioxidants are of great interest as they play a critical role in metabolic disorders including obesity[25].
The link between obesity and oxidative stress is obvious and a matter of great concern. Obesity is a metabolic disorder that is influenced by multiple factors and could generate reactive oxygen species (ROS) leading to abnormal metabolic activities. The ROS generation may be accompanied by various metabolic pathways like adipokines production from adipose tissues, and mitochondrial oxidation of fatty acids. Consequently, the function of antioxidant enzymes in the body is reduced which leads to the development of oxidative stress[26]. The living system has a well-developed antioxidant system that acts as a defense line to scavenge free radicals and maintains normal functions. However, overcrowded concentration of ROS leads to development of oxidative stress which stimulates disease pathogenesis and related complications. Dietary intake of sources rich in natural antioxidants may help to mitigate or reduce the extent of oxidative stress. Plants are known to possess antiradical and antioxidant properties. The antioxidant activities of plants are due to phytochemicals, mainly phenolics and flavonoids which may be utilized to improve the antioxidant status of the body[27]. The excellent antioxidant activities of S. nigrum shown in this study were most probably due to polyphenolic components present in the extract. Reasonable inhibition of pancreatic lipase was also observed in 80% ethanolic extract of S. nigrum. The inhibition of pancreatic lipase is considered an important target for obesity management and therapy. Metabolite profiling revealed the presence of pharmacologically important compounds which were reported for potential medicinal activities. The antioxidant,antiobesity and other therapeutic roles of cannabiscitrin, ellagic acid, hibiscetin-3-O-glucoside, and inositol are well recorded and these compounds were identified in the current study of S.nigrum[28]. The molecular docking study also predicted the possible structural interactions between metabolites and pancreatic lipase,which is mainly responsible for the enzyme inhibitory properties of the extract. Several compounds identified in S. nigrum extract showed thermodynamically feasible bindings and structural conformations with pancreatic lipase, which are even better than standard compound orlistat. The molecular docking analysis provided valuable information on the mode of attachment adopted by secondary metabolites, which might be a helping tool to look for novel and effective enzyme inhibitors. Molecular docking has already been reported as an excellent tool to study the interaction of molecules with active sites of enzymes to explore a probable mode of action[29]. The computational determination of IP of active sites of isolated compounds added valuable information regarding electron transfer potential for antioxidant activity. The compounds having low IP values were considered as good antioxidants due to high electrondonating potential. The mapping of active sites by molecular electrostatic potential surfaces, frontier molecular orbitals, spatial distribution of charge density, and ionization potential computation predicted that these compounds would have noteworthy reactivity to show antioxidant and anti-obesity behavior.
The results of faecal fat contents elaborated the probable inhibitory action of active ingredients of S. nigrum extract against pancreatic lipase. The dietary intake of fat-rich diet was responsible for the high lipid profile of untreated mice (HFD) due to disturbed lipid metabolism. The HFD increased the body weight of mice due to fat accumulation. HFD was reported to increase the acylation of fatty acids and reduction in satiety signal leads to weight gain. Moreover,HFD intake was also known to increase the TC, HDL, and LDL levels in serum. S. nigrum extract administration (300 mg/kg) to obese mice probably inhibited the pancreatic lipase activity which resulted in high faecal fat contents. The reduction in lipid absorption also improved the body weights of obese mice upon consuming plant extract. Excretion of fats in faeces from the body is believed to be an effective way to improve lipid profile and reduce body weight. Improvement in Hb level of obese mice was also observed in obese mice having plant extract treatment. The AST and ALT are indicators of normal liver functions and their concentrations are increased as a result of abnormal liver function. HFD was reported to damage the liver working leading to high AST and ALT levels in the serum of mice. The obesity-induced oxidative damage to tissues might be a significant cause for high AST and ALT levels in serum.The up-regulation or down-regulation of certain genetic responses is pivotal in adipogenesis and obesity development. A study reported that olive leaf extract reduced the PPAR γ and C/EBPα expression and substantially increased PGC-1α and UPC-1 levels in HFD obese mice[30]. A study examined the antiobesity impact of Nelumbo nucifera, Morusv alba, and Raphanus sativus mixture on 3T3-L1 adipocytes and C57BL/6J obese mice. The results showed that the plant extract mixture significantly mitigated obesity propagation by suppressing the expression of FAS, DGAT1, SCD-1, leptin, and SREBP1c[31]. The secondary metabolites of the plant can improve the lipid profile by modulating the lipid metabolism and energy homeostasis, most probably due to their antioxidant potential[10].Cosmos caudatus Kunth leaf was reported to exhibit antiobesity properties in obese rats by inhibiting the intestinal absorption of lipids and adipocyte biomarker inflections[32]. The reduced weights of liver, heart, and kidney of obese mice treated with 300 mg/kg of S.nigrum extract were most probably due to its antiobesity attributes.The reduction in body weight of obese mice influenced the organ weights. Most probably, the low-fat uptake and regulation of lipid metabolism were the major factors influencing the weights of the liver, heart, and kidney. The antioxidant role of plant extracts due to polyphenolic constituents improves the antiradical defense system of the body, which helps to regulate various metabolic functions and helps recovery from severe pathological situations.Interestingly, the results of current work were very encouraging for naturopathic management of obesity using S. nigrum as a potent source of pharmacologically important agents. The results of in vitro antioxidant assays, pancreatic lipase inhibition, molecular docking, and in vivo hypolipidemic investigations supported the ethnopharmacological use of S. nigrum in treating and managing obesity. The metabolite profiling provided valuable information on the secondary metabolites of S. nigrum extract, which most probably contribute to antioxidant and antiobesity properties.
Conclusively, the hydroethanolic extract of S. nigrum is proved as an effective, viable, and low-cost treatment of obesity. Its secondary metabolites synergically regulate the lipid profile of HFD obese mice via their antioxidant and enzyme inhibitory properties. The molecular docking analysis and binding energy data along with structural interactions provide valuable information on the possible mode of action adopted by secondary metabolites, mainly polyphenols.S. nigrum extract could be used as an innovative naturopathic antiobesity approach and be further processed for functional food.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
A. Irfan has extended his obligation to the Deanship of Scientific Research at KKU, Saudi Arabia through research group programs(R.G.P.1/42/42).
Authors’ contributions
This scientific study was designed by ZULA, MWM and MTA.Experiments were performed by ZULA, and data validation and interpretation was done by ZULA, HM, SAR and MN. Computation work and interpretation was conducted by AI and YSL. Draft was written by ZULA, MWM and MTA. The draft was proofread by YSL and MAR. All the authors read and approved the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2022年4期
Asian Pacific Journal of Tropical Biomedicine2022年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Methyl gallate isolated from Mangifera pajang kernel induces proliferation inhibition and apoptosis in MCF-7 breast cancer cells via oxidative stress
- Analgesic-like activity of perillyl acetate: In vivo and in silico studies
- Lipid-lowering effect of Oroxylum indicum (L.) Kurz extract in hyperlipidemic mice
- Cardiovascular protective properties of gastrodin
