Lipid-lowering effect of Oroxylum indicum (L.) Kurz extract in hyperlipidemic mice
Tanaporn Hengpratom, Sajeera Kupittayanant, Seekaow Churproong, Griangsak Eumkeb✉
1Division of Health and Applied Sciences, Faculty of Science, Prince of Songkla University, Thailand
2School of Preclinical Sciences, Institute of Science, Suranaree University of Technology, Nakhon Ratchasima, Thailand
3Department of Family Medicine and Community Medicine, Institute of Medicine, Suranaree University of Technology, Nakhon Ratchasima, Thailand
ABSTRACT
Objective:To investigate the effect of Oroxylum indicum fruit extract on high-fat diet-induced hyperlipidemic mice.
Methods:The phytochemical composition of Oroxylum indicum fruit extract was determined by liquid chromatographymass spectrometry/mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry. Forty-two male mice were used.The mice were divided into six groups: normal control, high-fat diet control, simvastatin treatment (20 mg/kg BW/day), and Oroxylum indicum fruit extract (100, 200, 300 mg/kg BW/day) treatment groups. Food intake, body weight, serum parameters, lipid profile,and histopathological lesions of the kidney, liver, and epididymal fat were observed.
Results:LC-MS/MS results revealed four major components of Oroxylum indicum fruit extract: luteolin, apigenin, baicalein,and oroxylin A. Twenty-seven volatile oils were identified from Oroxylum indicum fruit extract. Daily oral administration of Oroxylum indicum fruit extract at 100 to 300 mg/kg BW/day significantly reduced the body weight, total cholesterol, triglyceride,and low-density lipoprotein cholesterol level (P<0.05), whereas high-density lipoprotein cholesterol was higher than the high-fat diet control group. Treatment with 300 mg/kg BW/day Oroxylum indicum fruit extract reduced the pathological lesion and prevented fat accumulation in the kidney and liver.
Conclusions:Oroxylum indicum fruit extract has hypolipidemic effect in hyperlipidemic mice, and the active ingredients of Oroxylum indicum fruit extract, both flavonoids and volatile oils, should be further explored as an antihyperlipidemic agent.
KEYWORDS: Oroxylum indicum; Hyperlipidemia; Lipid-lowering effect; Extract; Mice; Anti-adipogenesis; Antihyperlipidemic;Flavonoids; Volatile oils
Significance
Our previous study reported that Oroxylum indicum fruit extract has in vitro anti-adipogenesis activity. In this study,the hypolipidemic effect of this extract was determined and studied in a hyperlipidemic mouse model. This study reveals that Oroxylum indicum fruit extract significantly prevented the increase in body weight and lipid levels in high-fat diet-induced hyperlipidemic mice.
1. Introduction
Hyperlipidemia is one of the major risk factors of atherosclerosis and the main cause of cardiovascular diseases (CVDs)[1].Approximately 17.9 million people worldwide died from CVDs in 2019, representing 32% of all global deaths[1]. Hyperlipidemia is a condition in which the levels of lipids or lipoproteins in the blood abnormally increase[2]. Generally, the treatment modalities focus on diet and lifestyle modification with lipid-lowering medications.However, the current lipid-lowering drugs such as statins have many serious side effects such as constipation, myopathy, elevated creatine kinase level, and liver damage (elevation of aminotransferase levels)[3]. Currently, various medicinal plant extracts and their phytochemical compounds at non-toxic doses have been studied and used to reduce plasma lipid levels, including total cholesterol (TC),triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C)[4].
Oroxylum indicum (O. indicum) is a herbal medicine found in Southeast Asia and Asia countries. The previous study reported that the chemical constitutions in the O. indicum fruit extract (OIE)included flavonoids (baicalein, chrysin, oroxylin A, and oroxylin B)[5,6]. It was claimed that hyperlipidemia rats treated with O.indicum stem bark extract at 400 mg/kg BW/day for 12 weeks could decrease TC, TG, and LDL levels significantly, and no mortality was observed[7]. In addition, the OIE had prevented the generation of new fat cells in in vitro studies[8,9]. It showed promising results against adipogenesis. Therefore, this study hypothesized that OIE might also prevent increasing lipid profiles in hyperlipidemia mice. Thus, this study aimed to investigate the effect of the OIE on a high-fat diet(HFD)-induced hyperlipidemia mice.
2. Materials and methods
2.1. Reagent and drugs
Scutellarin, daidzein, luteolin, apigenin, naringenin, genistein,baicalein, and oroxylin A (purity > 98%) were purchased from Sigma-Aldrich (St. Louis, USA). High-fat diet was purchased from Bio-Serv (Auckland, New Zealand). Hematoxylin and eosin solution were purchased from Bio-optic (Milano, Italy). Formaldehyde,DMSO, and simvastatin (as an internal control) were obtained from Sigma-Aldrich (St. Louis, USA).
2.2. Plant collection and preparation
The fruit of O. indicum was collected from the Wang Nam Khiao District, Nakhon Ratchasima Province, Thailand. The plant samples were identified by Dr. Santi Watthana, a botanist from the School of Biology, Institute of Science at Suranaree University of Technology,Thailand. The voucher specimens were deposited at Suranaree University of Technology Herbarium (SOI0808U). The extraction procedure was performed as described in a previous study[9]. Briefly,fresh fruits of O. indicum were put in the oven at 40 ℃ until dried.The dried pieces were pulverised using a mechanical grinder until they became powder. A total of 500 g of the dried powder was extracted with 95% ethanol by a soxhlation for 8 h. The extract was filtered to discard any solid material using filter paper, and the filtrate extract was then concentrated using a rotary evaporator at 50 ℃ under vacuum to remove the ethanol. Subsequently, the sample was lyophilized in a freeze dryer (LABCONCO), automatic mode,vacuum 240×10-3mBar, and collector -55 ℃. The extract was finally collected and stored at -20 ℃ for later treatment.
2.3. Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC-MS) quantification
The method was conducted as previously described with some modifications[10]. The phytochemical composition of the OIE was analyzed on the Dionex Ultimate 3000 UHPLC system(Dionex, USA) coupled with an electrospray ionization tandem mass spectrometer (micro-TOF-Q Ⅱ). The target phenolic and flavonoid compounds were identified and quantified with Bruker Quant analysis software (Ver 2.0 SP 5). The calibration curves were constructed from peak areas of different concentrations (from 0.5 μg/mL to 250 μg/mL) of the reference standard scutellarin, daidzein,luteolin, apigenin, naringenin, genistein, baicalein, and oroxylin A (purity > 98%) using the equation for linear regression obtained from the calibration curves.
GC/MS (Bruker 450-GC/Bruker 320-MS equipped with Rtx-5MS fused silica capillary column) was used to determine volatiles and performed as previously described with some modifications[11].GC/MS analyses were carried out on a column 30 m×0.25 mm with a film 0.25 μm thickness. Analytical condition: the injector temperature was 250 ℃, the oven temperature was programmed from 110 ℃ for 2 min, 200 ℃ for 3 min, and 280 ℃ for 20 min with rates of 0, 10, and 5 ℃/min, respectively. MS conditions: source temperature: 200 ℃, ionization mode: electron impact (70 eV), scan rate: 0.2 u/s, mass range: 45-500 m/z. The spectrum was analyzed by using NIST mass spectral library 2008.
2.4. Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures involving animals were in accordance with guidelines for the care and use of laboratory animals by the Animal Care and Use Committee, Suranaree University of Technology. The study was approved by the Animal Care and Use Committee, Suranaree University of Technology (4/2561).
2.5. Animal and treatment
Adult male Albino mice were purchased from the National Laboratory Animal Center, Mahidol University. The animals were acclimatized for 7 d, a photoperiod of 12 h-light and 12 h-dark in an individual cage with controlled temperature [(25 ± 0.5) ℃]. They were free to access food and water. Forty-two male Albino mice were divided into six groups, each group containing 7 mice.Group 1 (NC): mice received a normal diet and fed with 5% tween 80; Group 2 (HFD): mice received a high-fat diet and provided with 5% tween 80; Group 3 (HFD+SIM 20): mice received a highfat diet and treated with 20 mg/kg BW/day simvastatin; Group 4(HFD+OIE 300): mice received a high-fat diet and treated with 300 mg/kg BW/day O. indicum fruit extract; Group 5 (HFD+OIE 200):mice received a high-fat diet and treated with 200 mg/kg BW/day O. indicum fruit extract; Group 6 (HFD+OIE 100): mice received a high-fat diet and treated with 100 mg/kg BW/day O. indicum fruit extract. The experiment was carried out for 12 weeks. All the treatment regimens were administered orally. HFD was purchased from Bio-Serv (S3282, Auckland, New Zealand) and contained fat(36%), carbohydrate (35.7%), protein (20.5%), ash (3.5%), and moisture (< 10%).
2.6. Food intake, body weight, and relative organ weight
The food intake of mice was measured daily, while the body weight was measured weekly. The relative organ weights of mice, including the epididymal fat pad, kidney, lung, heart, spleen, and liver, were calculated following the previous study[12].
2.7. Biochemical analysis
On day 0 (pre-treatment), the mice were fasted overnight. Then, the blood sample (0.3 mL) was taken from the tail-vein to measure the level of high-density lipoprotein cholesterol (HDL-C), LDL-C, total cholesterol (TC), triglyceride (TG), creatinine, blood urea nitrogen(BUN), alanine transaminase (ALT), alkaline phosphatase (ALP), and complete blood count (CBC). Blood samples were centrifuged at 3 000 r/min for 10 min[13]. The serum separated was analyzed at a veterinary diagnostic laboratory in Thailand. After 12 weeks (post-treatment), all of the serum parameters were measured again. Mice fasted overnight were sacrificed with anesthetic CO2, and the blood sample was taken from the heart.
2.8. Histological examination
The kidney, liver, and epididymal fat tissues were collected and fixed in 10% formalin for 24 h. It was then dehydrated by a graded series of ethyl alcohol (70% to 100%), cleared by toluene, and embedded in paraffin wax. Sections of paraffin blocks were cut with a rotary microtome (5 μm thickness), Leica 2235 scanner (Germany),and stained with hematoxylin and eosin (H&E). The samples were examined under a light microscope (Zeiss Axio Scope A1,Germany), and images were taken at 100× and 400× magnification.
2.9. Statistical analysis
All data were expressed as mean ± standard deviation (mean ±SD). The differences in body weight, food intake, relative organ weight, and epididymal fat pads were analyzed by one-way analysis of variance (ANOVA) with a Tukey's HSD post hoc test. Paired Student's t-test was used to compare the serum lipid profile (TC,LDL-C, HDL-C, and TG) and blood toxicity (creatinine, BUN, ALP,ALT, CBC) between pre- and post-treatment groups. P<0.05 was considered a statistically significant difference.
3. Results
3.1. Identification of main compounds in OIE
The result of LC-MS/MS presented 24 compounds in OIE including flavonoids: baicalein (509.96 μg/mL), oroxylin A (5.33 μg/mL), luteolin (4.20 μg/mL), apigenin (1.55 μg/mL), and other unknown compounds (Figure 1, Supplementary Table 1). In addition, the GC-MS results revealed that γ-sitosterol (17.19%),2-cyclohexen-1-one (15.28%), 2-methyl-benzeneethanol (13.33%),and 4-hydroxy-3-hydroxy-2-methylbenzaldehyde (11.18%) were the most abundant volatiles of all 27 detected volatiles in the OIE(Figure 2, Supplementary Table 2).
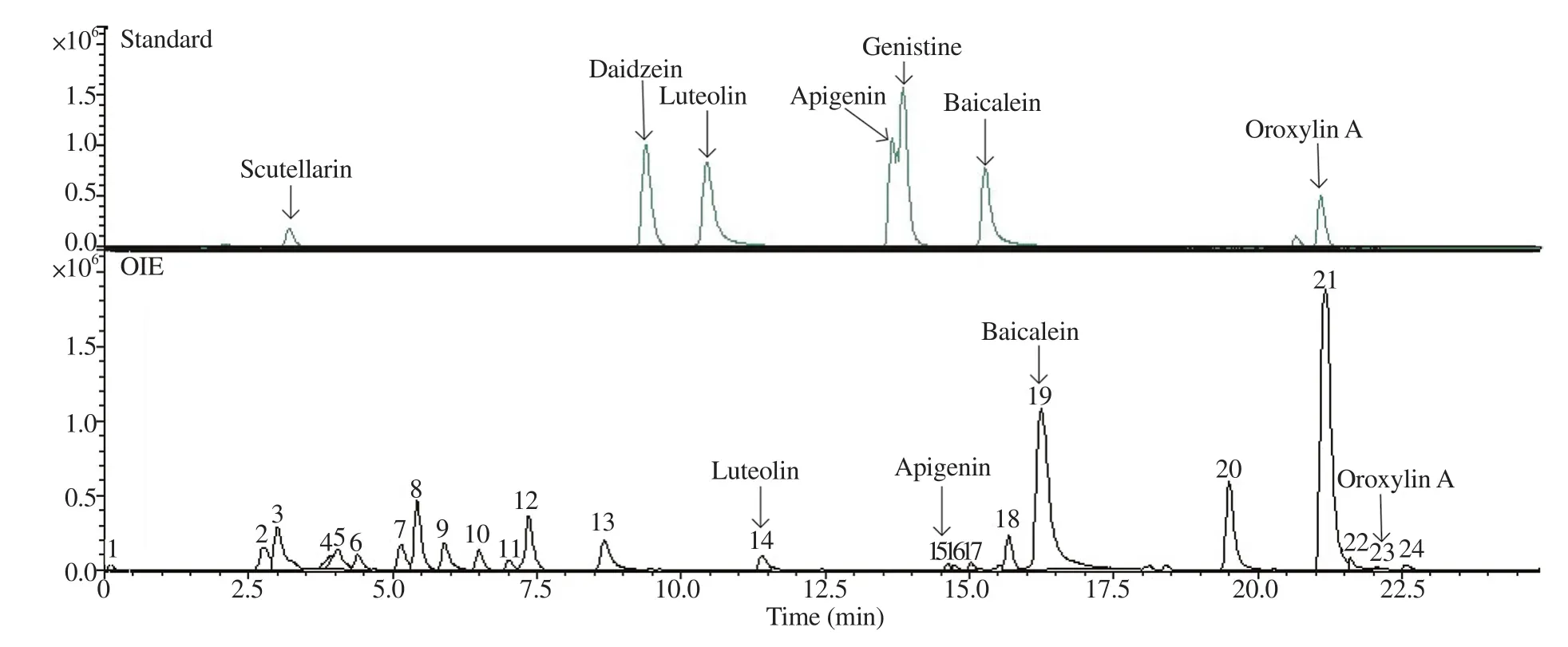
Figure 1. LC-MS/MS chromatogram of the Oroxylum indicum fruit extract (OIE) at different times (min).

Figure 2. GC-MS chromatogram of the OIE at different times (min).
3.2. Effect of OIE on food intake, body weight, and relative organ weight
The food intake showed no significant difference in all groups(P>0.05; Figure 3A). Whilst, the body weight of the HFD group was significantly higher than other groups at weeks 6 to 12 (P<0.05;Figure 3B), which was corresponded with the epididymal fat weight(Supplementary Table 3). However, treatment with OIE at 100 to 300 mg/kg BW/day significantly reduced body weight compared to HFD (P<0.05). These results indicate that HFD does not affect the amount of food intake but could induce weight gain in mice, while OIE has no effect on the food intake but could significantly reduce weight gain.
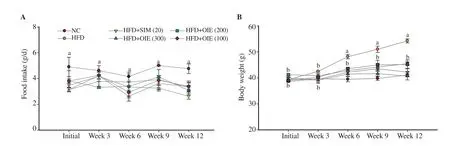
Figure 3. Effect of the OIE on food intake (A) and body weight (B) in high fat diet-induced hyperlipidemic mice. Normal control (NC), high-fat diet control(HFD), high-fat diet + simvastatin 20 mg/kg BW/day (HFD+SIM 20), high fat-diet + OIE 300, 200, 100 mg/kg BW/day (HFD+OIE 300, 200, 100). Data are expressed as mean ± SD (n=7). The different letters a, b at simultaneous week show statistically significant differences at P<0.05 (ANOVA and Tukey’s post hoc test).
3.3. Effect of OIE on lipid profiles
The impact of the OIE on TC, TG, HDL-C, and LDL-C is shown in Figure 4. The results revealed that the simvastatin and OIE-treated groups significantly lowered TC, TG, and LDL-C levels compared with the HFD group (P<0.05; Figure 4A, B, and D, respectively). At the same time, the HDL-C level in mice treated with OIE at 200 and 300 mg/kg BW/day was significantly higher than in the HFD group(P<0.05) (Figure 4C).

Figure 4. Effect of OIE on total cholesterol (A), triglyceride (B), high-density lipoprotein cholesterol (C), and low-density lipoprotein cholesterol (D) in highfat diet-induced hyperlipidemic mice. Data are expressed as mean ± SD (n=7). *P<0.05, **P<0.01: significant difference between pre- and post-test in each group were compared using paired Student’s t-test. The different letters a, b, c, d show statistically significant differences at P<0.05 (ANCOVA with Tukey’s HSD post hoc test).
3.4. Toxic effect of OIE on serum parameters
The results of serum creatinine, BUN, ALT, and ALP showed no significant differences in all groups between pre- and post-treatment in each group and among post-treatment groups (P>0.05; Figure 5).Furthermore, the RBC, WBC, and platelet count results revealed no significant differences between pre- and post-treated in each group and between post-treatment groups (P>0.05; Figure 6). In addition,no behavioral changes were observed, and no toxic reactions were found in any group. These findings indicated that simvastatin and OIE at test dosages might not be harmful to the kidney, liver, or blood function after treatment for 12 weeks.
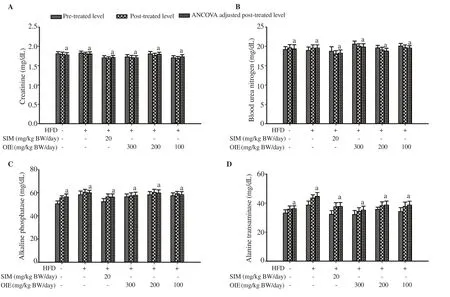
Figure 5. Effect of OIE on serum creatinine (A), blood urea nitrogen (B), alkaline phosphatase (C), and alanine transaminase (D) in high-fat diet-induced hyperlipidemic mice. Data are expressed as mean ± SD (n=7). Bars annotated with the same letter a mean no significant differences at P<0.05 (ANCOVA with Tukey’s HSD post hoc test).

Figure 6. Effect of OIE on red blood cell (A), white blood cell (B), and platelet count (C) in high-fat diet-induced hyperlipidemic mice. Data are expressed as mean ± SD (n=7). Bars annotated with the same letter a mean no significant differences at P<0.05 (ANCOVA with Tukey’s HSD post hoc test).
3.5. Histological changes
The kidney results showed normal histological structure in all groups (Figure 7A). The morphology of the liver in the HFD group prominently exhibited micro (arrows) and macro lipid droplets (dot arrows) in their cytoplasm (Figure 7B). Conversely, the lipid droplets of the simvastatin and OIE treated groups at 200 and 300 mg/kg BW/day were less than those of the HFD group. Histological results of epididymis fat, size and number of adipocytes are presented in Figures 7C and Supplementary Figure 1, respectively. Cells treated with OIE at a higher dose showed a smaller size and a higher number of lipid cells in a dose-dependent manner.

Figure 7. Histological examination of kidney (A), liver (B), epididymal fat (C). Normal control (NC), high-fat diet control (HFD), high-fat diet + simvastatin 20 mg/kg BW/day (HFD+SIM 20), high fat-diet + OIE 300, 200, 100 mg/kg BW/day (HFD+OIE 300, 200, 100). Original magnification ×100 (scale bar, 100 μm), inserted image magnification ×400 (scale bar, 20 μm).
4. Discussion
Hyperlipidemia is characterized by increased levels of TC, TG,LDL-C, and decreased HDL-C[14]. It is an important cause of CVDs,which is considered a severe public health concern globally[1].Early prevention and treatment of hyperlipidemia are essential to reduce the occurrence of CVDs[14,15]. Prolonged hyperlipidemia may escalate into plaque formation, leading to a reduced blood flow and an increased risk of thrombosis. Nowadays, pharmaceuticals or functional foods that would improve blood lipid profiles and blood flow may reduce the risk of stroke or other thrombotic diseases.
O. indicum is an important herbal medicine. It is found in South and Southeast Asia. The fruit of the plant is prevalent in Thailand[16]. A previous study indicated that the stem bark and root extracts from O.indicum significantly reduced TC, TG, LDL-C, VLDL-C levels but remarkably increased the levels of HDL-C in hyperlipidemic rats[17].Although, the stem bark and root extract showed antihyperlipidemic activity. To date, the antihyperlipidemic activity from the fruit part of O. indicum extract is still unknown. Thus, this study investigated the potential effects of the fruit of O. indicum extract on HFD mice.HFD mice showed significantly higher final body weight than the control group fed on a regular diet (P<0.05). These results could be explained that the content of nutrients such as fat in HFD is higher than the regular diet. HFD mice supplemented with OIE exhibited significantly lower body weight than the HFD group (P<0.05). In addition, our result showed that the serum TG, TC, and LDL-C levels of the HFD group were significantly higher than the regular diet group (P<0.05), confirming the establishment of the model.After administration of OIE at 100-300 mg/kg BW/day, the serum TC, TG, and LDL-C dropped significantly (P<0.05), while HDL-C rose. The HDL-C levels of OIE groups at 200 and 300 mg/kg BW/day were increased considerably compared to the HFD control group (P<0.05). Raised HDL-C might be because HDL-C transports lipids to hepatic cells and eliminates bile acids[18]. It could also be due to a decrease in the activity of cholesteryl ester transfer protein and lipoprotein lipase, which is associated with increased HDL concentrations[19]. Interestingly, increased HDL-C reduces the risk of CVDs[20]. These findings lead us to believe that the fruit of O.indicum extract could have a preventative effect on hyperlipidemic mice and bring benefits to CVDs.
The toxic effect of OIE on serum parameters was measured,including serum ALT, which is used as a representative biomarker of liver pathology[21]. Histological studies and biochemical markers indicated no toxic damage to the rat liver. There was no increase in either ALT or ALP, and the pathological change of the liver was unremarkable. In the normal control and treatment groups, liver tissue appeared normal hepatocytes with a preserved cytoplasm,a prominent nucleus, and a central vein. These results provide evidence that the regular diet and OIE treatment do not have side effects on lipid metabolism in mice. Whist, the HFD control group showed more fat deposited in the hepatocytes. These findings could imply that OIE has a beneficial effect on the fatty liver at the safety dose (100-300 mg/kg BW/day). The antihyperlipidemic action could be possibly attributed to the phytochemicals found in the fruit of O.indicum extract. In vitro experiment demonstrated that the fruit of O.indicum extract at 200 μg/mL significantly decreased the intracellular lipid accumulation by approximately 52% in 3T3-L1 adipocytes[9].Our results are consistent with the in vitro result that OIE prevents fat cells' accumulation, leading to no significant change in adipocytes size compared to the regular diet group. In addition, a previous study showed that the fruit of O. indicum extract could suppress the expression of fatty acid synthetase, sterol regulatory elementbinding proteins-1c, and proliferator-activated receptor-γ2[9], which potentiate the regulation of lipid metabolism[22]. These parameters are also expressed in the liver, specifically in hepatocytes, positive expressions of which are correlated with fat accumulation[23]. These findings may prove that OIE may decrease proliferator-activated receptor-γ2, leading to reduced fat accumulation in hepatocytes.A previous study presented that baicalein, one of the chemical compounds found in the fruit of O. indicum extract, inhibited pancreatic lipase activity[24]. This enzyme hydrolyzes dietary fats,especially triglyceride hydrolysis resulting in dietary triglycerides absorption by enterocytes[25]. In another experiment, baicalein also has an anti-adipogenic effect by altering adipogenic genes' expression,including cyclooxygenase (COX-2). During adipogenesis, COX-2 mRNA expression was decreased[26]. Mice that are heterozygous for the COX-2 +/- gene showed a 3.5 fold increase in their fat pad compared with wild-type mice (Cox-2 +/+)[27]. Moreover, other compounds identified in this study also have antihyperlipidemic effects leading to a decrease in the serum lipid levels, including naringenin[28],oroxylin A[29], and γ-sitosterol[30]. However, the mechanisms of antihyperlipidemic action of the fruit of O. indicum extract need to be further investigated.
In conclusion, the OIE exhibited antihyperlipidemic activity in HFD-induced mice. This effect could be mediated by their phytochemical compounds, which react to lipid metabolic pathways.Therefore, OIE could be developed as a health food supplement for subjects suffering from hyperlipidemia.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This work was supported by a Full-time Doctoral Researcher grant from Suranaree University of Technology (Full-time 61/24/2563)and by Thailand Science Research and Innovation.
Authors’ contributions
TH, SK, and GE planned and designed the research work. TH, SK,SC, and GE conducted the experiments and collected the data. SK and GE were involved in data analysis and interpretation. TH and SC wrote the manuscript. TH, SK, and GE revised the manuscript. TH,SK, SC, and GE finally approved the version to be published.
 Asian Pacific Journal of Tropical Biomedicine2022年4期
Asian Pacific Journal of Tropical Biomedicine2022年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Methyl gallate isolated from Mangifera pajang kernel induces proliferation inhibition and apoptosis in MCF-7 breast cancer cells via oxidative stress
- Anti-obesity effect and UHPLC-QTOF-MS/MS based metabolite profiling of Solanum nigrum leaf extract
- Analgesic-like activity of perillyl acetate: In vivo and in silico studies
- Cardiovascular protective properties of gastrodin
