Gut dysbiosis and small intestinal bacterial overgrowth as independent forms of gut microbiota disorders in cirrhosis
Roman Maslennikov,Vladimir Ivashkin,Irina Efremova,Elena Poluektova, Anna Kudryavtseva,GeorgeKrasnov
Abstract
Key Words: Dysbiosis; Gut-liver axis; Microbiome; Small intestinal bacterial overgrowth; Cirrhosis;Microbiota
lNTRODUCTlON
Cirrhosis is one of the most serious problems in hepatology and is not only a disease of the liver, but it also affects other organs. The damage that occurs in these other organs can contribute to further progression of cirrhosis, forming a vicious circle. The examples of such organs are the gut and the community of microorganisms that reside within it (the gut microbiota). Two forms of disorders of gut microbiota have been described in cirrhosis: changes in its composition[1 -5 ] (gut dysbiosis[6 -10 ]) and an increase of bacterial number in the small intestine (small intestinal bacterial overgrowth, SIBO)[11 -14 ]. It is believed that these disorders, in combination with increased intestinal permeability, lead to increased penetration of bacteria and their components into the intestinal wall, mesenteric lymph nodes, ascitic fluid, and portal and systemic bloodstreams[15 ]. This, in turn, leads to systemic inflammation,vasodilation, compensatory fluid retention, and worsening portal hypertension[16 ,17 ].
Despite the substantial number of articles describing the relations between disorders of gut microbiota and various manifestations of cirrhosis, dysbiosis and SIBO were always studied separately.Our research aims to study the relationship of gut dysbiosis and SIBO in cirrhosis.
MATERlALS AND METHODS
Patients
In this observational study, 95 consecutive patients with cirrhosis were admitted to the Department of Hepatology of Clinic for Internal Diseases, Gastroenterology and Hepatology at Sechenov University(Moscow, Russia) and screened for inclusion. The study procedures were explained to potential participants, and written informed consent was obtained before enrollment. The present study was approved by the Ethics Committee of Sechenov University.
The inclusion criteria were as follows: diagnosis of cirrhosis verified by histology or clinical,biochemical, and ultrasound findings; and age between 18 and 70 years. The exclusion criteria were as follows: use of lactulose, lactitol, or other prebiotics, probiotics, antibiotics, or metformin in the past 6 wk; alcohol consumption in the past 6 wk; or inflammatory bowel disease, cancer, or any other serious disease. We used these criteria to exclude the influence of these factors on the composition of the gut microbiota. Of the original 95 patients screened for inclusion, 47 were enrolled in the study while 48 were excluded (Figure 1 ).
SIBO assessment
The participants of this study were 47 in-patients who were diagnosed with cirrhosis at admission. The morning after admission, SIBO was assessed using a lactulose hydrogen breath test (Gastrolyzer,Bedfont, The United Kingdom), following the manufacturer’s instructions and in accordance with the North American Consensus[18 ]. Participants were considered to have SIBO if breath hydrogen concentration increased by at least 20 ppm above the baseline value within 90 min[18 ].
Gut microbiome analysis
The morning after admission, a stool sample was taken into a sterile disposable container and immediately frozen at -80 °C[19 ].
DNA from the stool was isolated using the MagNa Pure Compact Nucleic Acid Isolation Kit I (Roche,Basel, Switzerland) according to the manufacturer’s instructions. Libraries for sequencing were prepared by two rounds of polymerase chain reaction (PCR) amplification. In the first round, specific primers for the v3 -v4 region of the 16 S ribosomal RNA gene were used: 16 S-F TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and 16 S-R GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC.
After amplification, the PCR product was purified using AMPure XP magnetic beads (Beckman Coulter, Brea, CA, United States). Then, a second round of PCR was performed to attach specific adapters and enable multiplexing of the samples. To begin, 5 μL of the first PCR product was added to the reaction after ball cleaning with primers containing Illumina indices (Nextera XT Index v2 Primers;San Diego, CA, United States) and adapter sequences as well as 2 × KAPA HiFi HotStart ReadyMix. The amplification products were also purified using AMPure XP beads (Beckman Coulter). The concentrations of the prepared libraries were then measured using a Qubit 2 .0 fluorimeter (London, United Kingdom) and quantitative PCR. The quality of the libraries was assessed using the Agilent 2100 Bioanalyzer (Santa Clara, CA, United States). The libraries were mixed in equal proportions and diluted to the required concentration to be run on a MiSeq (Illumina) device. Pair-end readings of 300 + 300 nucleotides were obtained. Reads were trimmed from the 3 ’-tail with Trimmomatic (Illumina) and then merged into a single amplicon with the MeFiT tool[20 ,21 ]. We did not perform operational taxonomic unit picking; instead, we classified amplicon sequences with the Ribosomal Database Project (RDP)classifier and RDP database[22 ].
Statistical analysis
Statistical analysis was performed with STATISTICA 10 (StatSoft Inc., Tulsa, OK, United States). The data are presented as medians (interquartile ranges). The abundance of taxa of the gut microbiome is presented as a percentage. Differences between continuous variables were assessed with the Mann-Whitney test. Fisher’s exact test was used to assess the differences between categorical variables.Pvalues ≤ 0 .05 were considered as statistically significant.
RESULTS
Out of the 47 participants in our study, the results from the lactulose hydrogen breath test revealed that 24 (51 .1 %) of patients met the criteria for SIBO. No significant differences in etiology (Figure 2 ),demographics, drugs used before inclusion, and the main manifestations of cirrhosis were observed between patients with and without SIBO, other than the value of C-reactive protein, which was higher in patients with SIBO. Patients with SIBO also displayed a trend toward having a larger spleen and higher incidence of ascites (Table 1 ).
In terms of phyla, samples from patients with SIBO revealed a higher abundance of Firmicutes and Fusobacteria, and lower abundance of Bacteroidetes compared to those from patients without SIBO.There were no significant differences in the numbers of Proteobacteria and Actinobacteria as well as the main Firmicutes classes (Clostridia, Bacilli and Negativicutes, Table 2 ).
Our results reveal that the increase in the abundance of Firmicutes observed in patients with SIBO occurs mainly due to a larger population of bacterial species from theLachnospiraceaefamily. The number of bacteria from the other major families of this phylum does not differ significantly between the samples taken from the two groups of patients (Table 3 ). After the exclusion ofLachnospiraceaefrom Firmicutes, the difference in the abundance of bacteria under this phylum was no longer significant[39 .8 (32 .3 -53 .3 ) vs 42 .6 (25 .1 -54 .3 ); P = 0 .773 ].

Table 1 Descriptive statistics of patients with and without small intestinal bacterial overgrowth
The reduced population of Bacteroidetes in patients with SIBO results from a reduction in all major families of this phylum, with the exception ofPrevotellaceae. Although there was no significant difference in the overall levels of Proteobacteria between the samples from the two patient groups,differences were detected in the minor families of the phylum. Specifically, samples from patients with SIBO revealed that the proportion ofPasteurellaceaeandMoraxellaceaewere higher, andDesulfovibrionaceaewas lower, compared to those from patients without SIBO. Similarly, there was also no significant difference in the overall levels of bacteria in the Actinobacteria phylum, but the proportion of bacteria from theActinomycetaceaeandMicrococcaceaeminor families were higher in the samples taken from patients with SIBO than in samples from patients without (Table 3 ).
At the genus level, analysis of samples from patients with SIBO revealed increased the abundance of
Intestinimonaslevels were lower (Table 4 ).
Further analysis demonstrated thatBrautiawas the main contributor to the increase in the abundances of theLachnospiraceaefamily and the Firmicutes phylum in patients with SIBO. After subtracting the contribution of these bacteria, the statistical differences between the patient groups were no longer significant [Lachnospiraceae: 28 .6 (19 .6 -35 .0 ) vs 23 .2 (10 .7 -33 .2 ), P = 0 .177 ; Firmicutes: 74 .0 (62 .2 -79 .5 ) vs 65 .7 (55 .9 -78 .6 ); P = 0 .425 ].
The Firmicutes/Bacteroides ratio was significantly higher in patients with SIBO than in patients without SIBO, while the modified dysbiosis ratio that determines the prognosis in cirrhosis[10 ] did not differ significantly between the two groups of patients (Figure 3 ).

Table 2 Abundance of bacteria at the phylum and class levels in gut microbiome of patients with and without small intestinal bacterial overgrowth
DlSCUSSlON
We detect SIBO in half of the patients with cirrhosis, which is consistent with the data of previous studies[13 ]. SIBO is thought be caused by a slowdown in the motility of the small intestine, use of proton pump inhibitors, exocrine pancreatic insufficiency, immune and other disorders[23 ,24 ].Furthermore, an association between delayed orocecal transit and SIBO has been reported in patients with cirrhosis[25 ]. Despite the presence of immune disorders[26 ], there are no studies on the association of them with the development of SIBO in cirrhosis. There was no difference in the frequency of using proton pump inhibitors between the patients with and without SIBO in our study.
Cirrhosis-associated gut dysbiosis is represented by an increase in the abundance of Proteobactria that produce active endoctoxin[1 -5 ], and the facultative anaerobic bacteria Bacilli[4 ,6 -8 ] that is capable of bacterial translocation[27 ]. This is accompanied by the decrease in the levels of beneficial bacteria from the major families under the Clostridia class[4 ,7 ,8 ,10 ]. Since the number of Clostridia and Bacilli bacteria can change in opposite directions, the abundance of Firmicutes, which include Clostridia and Bacilli, has been demonstrated to be either increased[9 ] or decreased[5 ] in cirrhosis. The change in levels of Bacteriodetes in cirrhosis has been reported to decrease[1 ,9 ], increase[2 ], not change[10 ], and be increased in compensated cirrhosis, while reduced to normal in decompensated cirrhosis[7 ].
The mechanisms that lead to gut dysbiosis in cirrhosis are not clear; it is suggested that they may be similar to those for SIBO. If SIBO and gut dysbiosis result from the same factors, it would be logical to assume that dysbiosis is more pronounced in patients with SIBO. However, the abundance of the main biomarkers of cirrhosis-associated dysbiosis (Proteobacteria, Bacilli and Clostridia) was not significantly different between patients with and without SIBO in our study. Moreover, the abundance ofLachnospiraceae, a decrease in which is also one of the biomarkers of cirrhosis-associated dysbiosis[6 -8 ,10 ], on the contrary, was significantly higher in SIBO.
The gut microbiome has been evaluated depending on the presence of SIBO in patients who do not have cirrhosis in a small number of studies. It was shown that the composition of the small intestine[28 ,29 ] and fecal[30 ] microbiome does not depend significantly on the presence of SIBO. However, among patients with diarrheal variant of irritable bowel syndrome, the abundance ofPrevotellawere higher,while the abundance ofBacteroideswere reduced in persons with SIBO compared with persons without SIBO[31 ]. Another study revealed that Firmicutes was increased in fecal microbiota of patients with SIBO[32 ]. Our research also showed an increase in the abundance of Firmicutes and a decrease in the abundance ofBacteroidesbut without a significant change in the abundance ofPrevotellain fecal microbiota of patients with SIBO. Thus, changes in the gut microbiome of SIBO patients with cirrhosis are partly consistent with changes in it in SIBO patients with other diseases and do not coincide with the changes that are characteristic of cirrhosis-associated dysbiosis.
陆军越是现代化、越是信息化,越要法治化。建设强大现代化新型陆军,离不开健全的法律、严明的纪律、正规的秩序,必须充分发挥法治的引导、推动、规范、保障作用,为建强陆军夯实法治根基。
An increase in Firmicutes/Bacteroides ratio which was observed in our study in patients with SIBO is reported in patients with a significant slowdown in orosecal transit in cirrhosis[9 ]. Nevertheless, there were no differences in the abundance of Proteobacteria and Bacilli between cirrhotic patients with normal and delayed orocecal in that study. It can therefore be assumed that the slowing down of orocecal transit is a main factor in the development of SIBO, but it has a minimal effect on the development of cirrhosis-associated gut dysbiosis.

Table 3 Abundance of bacteria at the family level in gut microbiome of patients with and without small intestinal bacterial overgrowth
In our study, bacterial species that mainly increase in patients with SIBO belong to theBlautiagenus.These bacteria have the ability to convert primary bile acids into secondary bile acids[33 ], for example,chenodeoxycholic acid to lithocholic acid. The ratio of lithocholic to chenodeoxycholic acid in feces strongly correlates with the content ofBlautiain the gut microbiome of cirrhosis patients[8 ].Furthermore, an increase in the content of bile acids in the feces is accompanied by an increase in the abundance ofBlautiain persons on a cholerectic diet[34 ].
The changes in bile metabolism may explain the differences in the gut microbiome of cirrhosis patients with SIBO. The increase in the number of bacteria leads to the increased deconjugation of primary bile acids in the small intestine in patients with SIBO[35 ]. As deconjugated primary bile acids have a lower affinity for the proteins that carry them through the epithelium of the terminal ileum[36 ],more of these acids enter the large intestine. These bile acids may be a growth factor forBlautiaand similar bacteria. The reduction in the abundance of Bacteroidetes may be the result of suppression their growth by bile acids or due to antagonism withBlautia. A larger study examining the amount and composition of bile acids in feces, the time of orocecal transit, the gut microbiome, and SIBO in cirrhosis should be performed to test this hypothesis.

Table 4 Abundance of bacteria at the genus level in gut microbiome of patients with and without small intestinal bacterial overgrowth
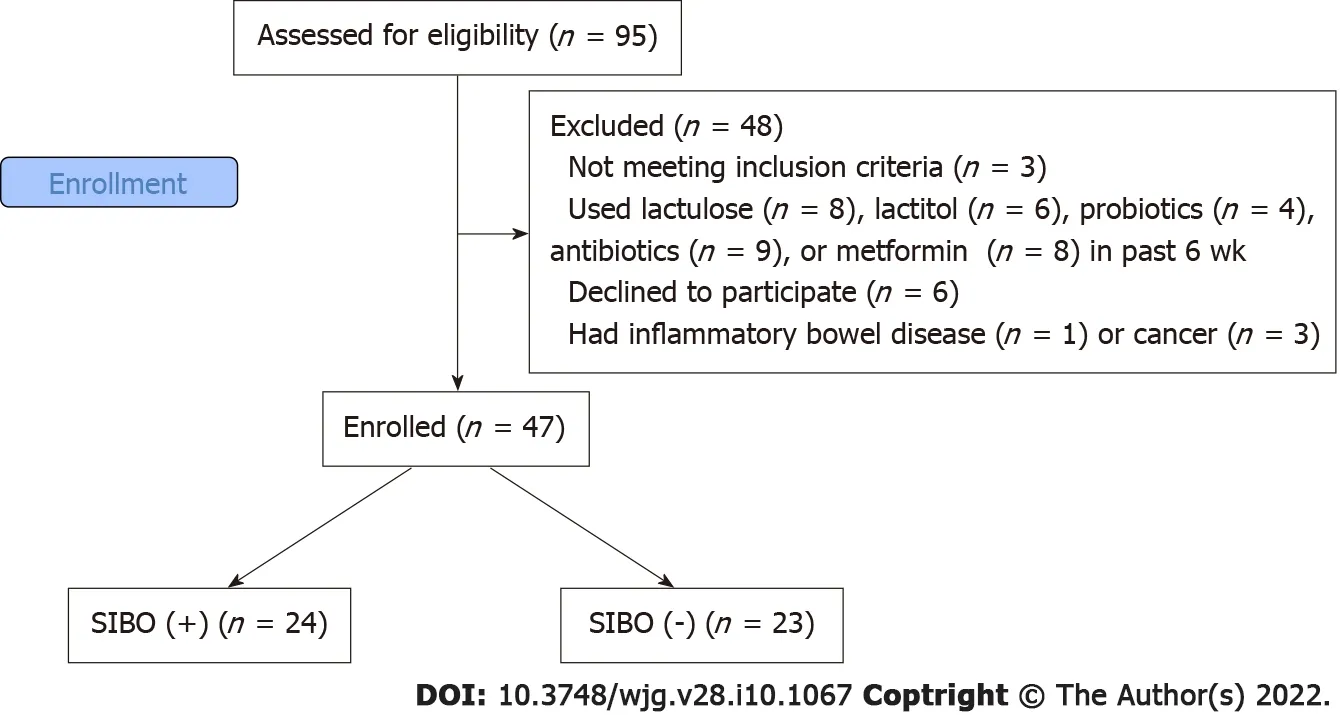
Figure 1 CONSORT 2010 flow diagram. SIBO: Small intestinal bacterial overgrowth.
The strength of our study is represented by the facts that it is the first that assesses the relationship between SIBO and gut dysbiosis in cirrhosis, as well as that we suggested the mechanism of development of changes in the gut microbiota in cirrhosis patients with SIBO and the idea for the next studies that can confirm or refute this hypothesis.

Figure 2 Etiology of cirrhosis in patients with and without small intestinal bacterial overgrowth. SIBO: Small intestinal bacterial overgrowth.

Figure 3 Complex indices of gut microbiome in patients with and without small intestinal bacterial overgrowth. SIBO: Small intestinal bacterial overgrowth.
The limitation of our study is its small size, but this did not prevent significant results from being obtained.
CONCLUSlON
In conclusion, we showed that despite the difference in the gut microbiome between patients with and without SIBO, gut dysbiosis and SIBO are most likely relatively independent forms of disorders of the gut microbiota in cirrhosis.
ARTlCLE HlGHLlGHTS

Research methods
This observational study included 47 in-patients with cirrhosis. Stool microbiome was assessed using 16 S rRNA gene sequencing. SIBO was assessed using the lactulose hydrogen breath test.
Research results
Patients with SIBO had a higher abundance of Firmicutes and Fusobacteria, and a lower abundance of Bacteroidetes than patients without SIBO. This increase in the abundance of Firmicutes occurred mainly due to an increase in the abundance of bacteria from the genusBlautiaof theLachnospiraceaefamily,while the abundance of other major families of this phylum did not differ significantly between the patients with and without SIBO. There were no significant differences in the abundance of taxa that were the main biomarkers of cirrhosis-associated gut dysbiosis between patients with and without SIBO.
Research conclusions
Despite the differences observed in the gut microbiome between patients with and without SIBO, gut dysbiosis and SIBO are most likely independent disorders of gut microbiota in cirrhosis.
Research perspectives
Research perspectives are to study the mechanisms of development of SIBO and gut dysbiosis in patients with cirrhosis.
ACKNOWLEDGEMENTS
The authors are grateful to the staff of the Department of Hepatology: Zharkova M, Lapshin A, Ondos S,Fedosina E, Tkachenko P, Tikhonov I, and others.
FOOTNOTES
Author contributions:Ivashkin V contributed research idea; Ivashkin V and Maslennikov R contributed study design;all authors contributed research and data analysis; Kudryavtseva A performed gut microbiota sequencing; Krasnov G performed bioinfomatics analysis of gut microbiota sequencing data; Maslennikov R contributed draft writing; all authors contributed draft editing; Maslennikov R is the guarantor.
Supported byBiocodex Microbiota Foundation: National Research Grant Russia 2019 .
lnstitutional review board statement:The present study was approved by the Ethics Committee of Sechenov University.
lnformed consent statement:The study procedures were explained to potential participants, and written informed consent was obtained before enrollment.
Conflict-of-interest statement:No conflicts of interest.
Data sharing statement:The data can be provided upon request to the corresponding author.
STROBE statement:The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:Russia
ORClD number:Roman Maslennikov 0000 -0001 -7513 -1636 ; Vladimir Ivashkin 0000 -0002 -6815 -6015 ; Irina Efremova 0000 -0002 -4112 -0426 ; Elena Poluektova 0000 -0002 -9038 -3732 ; Anna Kudryavtseva 0000 -0002 -3722 -8207 ; George Krasnov 0000 -0002 -6493 -8378 .
S-Editor:Gao CC
L-Editor:A
P-Editor:Gao CC
 World Journal of Gastroenterology2022年10期
World Journal of Gastroenterology2022年10期
- World Journal of Gastroenterology的其它文章
- Comment “Asymptomatic small intestinal ulcerative lesions: Obesity and Helicobacter pylori are likely to be risk factors”
- Diagnostic performance of endoscopic classifications for neoplastic lesions in patients with ulcerative colitis: A retrospective case-control study
- Loss of LAT1 sex-dependently delays recovery after caerulein-induced acute pancreatitis
- Malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer
- Feasibility of therapeutic endoscopic ultrasound in the bridge-to-surgery scenario: The example of pancreatic adenocarcinoma
- An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms
