Diagnostic performance of endoscopic classifications for neoplastic lesions in patients with ulcerative colitis: A retrospective case-control study
Yuichi Kida,Takeshi Yamamura, Keiko Maeda,Tsunaki Sawada,Eri Ishikawa,Yasuyuki Mizutani,NaomiKakushima, Kazuhiro Furukawa,Takuya Ishikawa,Eizaburo Ohno, Hiroki Kawashima,Masanao Nakamura,Masatoshi Ishigami,Mitsuhiro Fujishiro
Abstract
Key Words: Diagnostic performance; Japan Narrow-Band Imaging Expert Team classification; Pit pattern classification; Sporadic neoplasms; Ulcerative colitis; Ulcerative colitis-associated neoplasms
lNTRODUCTlON
Patients with long-standing ulcerative colitis (UC) are at risk for colorectal tumors due to chronic inflammation. The cumulative risk of colorectal cancer at 10 , 20 , and 30 years after UC onset are reportedly 1 .6 %, 8 .3 %, and 18 .4 %, respectively[1 ]. Consequent to improvements in UC treatment, longstanding UC cases have gradually increased, and surveillance colonoscopy has become more important.UC patients are exposed to the risk of not only UC-associated neoplasms (UCAN) but also sporadic neoplasms (SN). As the treatment strategy for UCAN greatly differs from that for SN, distinguishing UCAN from SN is important[2 ]. In line with the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations (SCENIC) consensus statement, endoscopic resection now tends to be accepted as a treatment for endoscopically visible dysplasia[3 ]. With the support from the SCENIC consensus statement, endoscopic treatments for visible dysplasia have gradually increased and have attracted attention recently[4 -6 ].
UCAN differentiation by endoscopic findings had been described previously. Prior studies revealed that features of surface structure and vascular pattern obtained by magnifying Narrow-Band Imaging(NBI) and chromoendoscopy are useful in diagnosing UCAN[7 -11 ]. Additionally, multimodal endo-scopic classification without the use of magnifying endoscopy has been reported[12 ]. Most of these studies focused on differentiating neoplastic from non-neoplastic lesions or on detecting these lesions,not on qualitatively diagnosing neoplastic lesions in UC patients. While several studies have focused on differentiating UCAN from non-neoplastic lesions, few reports have explored the differentiation of UCAN from SN[2 ].
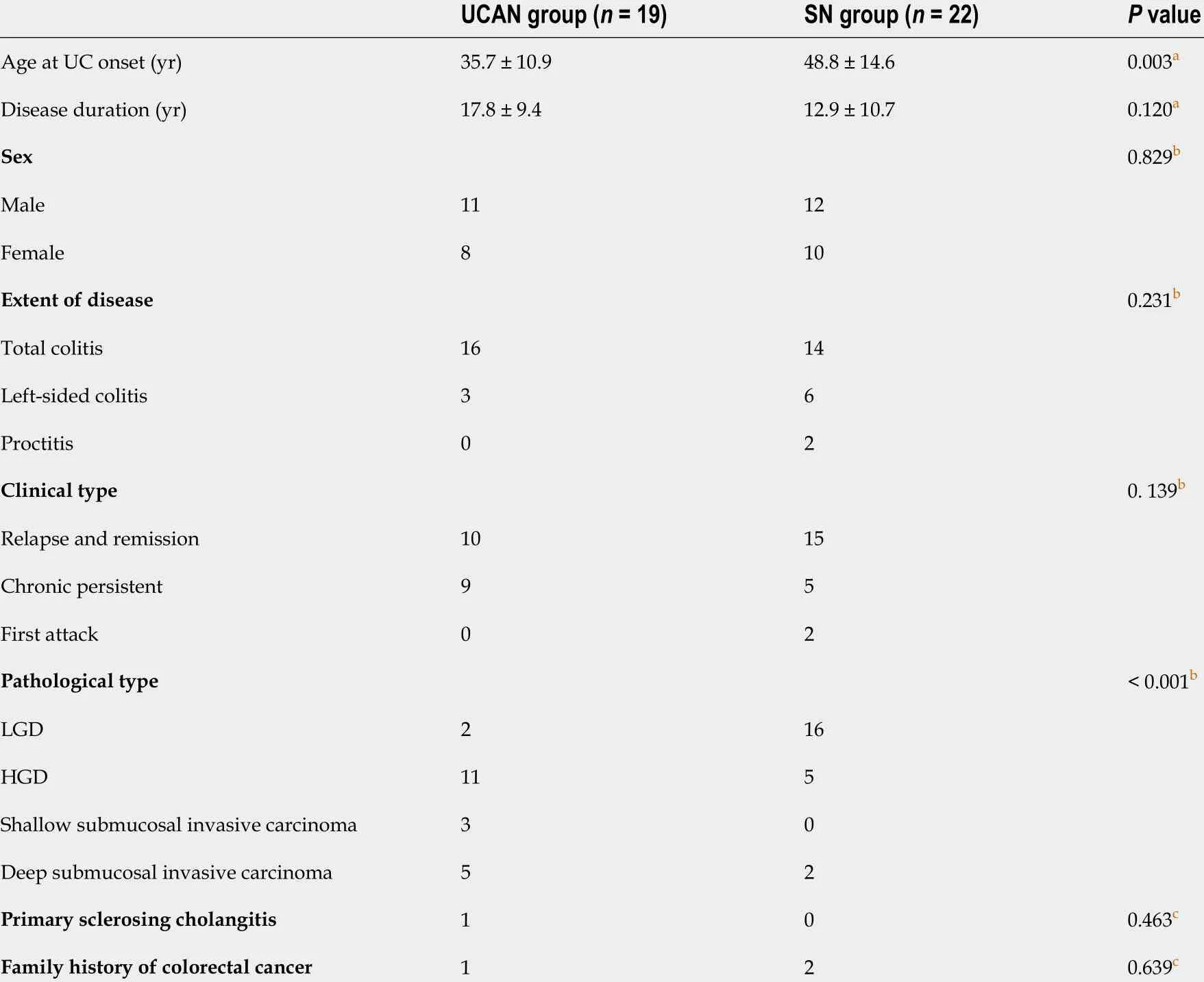
Table 1 Clinical characteristics of ulcerative colitis patients with neoplastic lesions (mean ± SD)
The Japan NBI Expert Team (JNET) and pit pattern classifications are useful for determining the pathology and invasion depth of colorectal tumors[13 ,14 ]. Both classifications have high reproducibility and good diagnostic accuracy in terms of pathology and invasion depth[15 -19 ], as well as good intraand inter-observer agreement rates for diagnosing colorectal tumors[18 ,20 ]. Dysplastic pit patterns are sometimes observed even in non-dysplastic lesions due to inflammation and regenerative changes in UC patients[21 ]. Surface and vascular patterns are modified by inflammation in UCAN[22 ]. These patterns are likely to be modified by inflammation not only in UCAN but also in SN located in the inflamed mucosa. Therefore, whether these endoscopic classifications apply to the diagnosis of neoplastic lesions in UC patients remains unclear. Only a few reports have described the usefulness of both classifications in diagnosing UCAN[22 ], and there have been no reports on their use for classifying SN in UC patients. Hence, the present retrospective case-control study aimed to evaluate the diagnostic performance of the JNET and pit pattern classifications for neoplastic lesions in UC patients.
MATERlALS AND METHODS
Patients
A total of 89 UC patients who had neoplastic lesions that could be pathologically evaluated by biopsy,endoscopic resection, or surgery and who underwent colonoscopy at Nagoya University Hospital fromAugust 2005 to April 2020 were consecutively registered. Neoplastic lesions located in the colonic mucosa outside the previously or currently inflamed mucosa were excluded. Additionally, lesions magnified using both NBI or Blue LASER imaging (BLI) and chromoendoscopy with indigo carmine or crystal violet were included. The present study ultimately enrolled 41 UC patients with 44 lesions that could be assessed using both the JNET and pit pattern classifications. According to pathological findings, these patients were divided into two groups—namely, the UCAN group, which comprised 19 patients with 21 lesions, and the SN group, which consisted of 22 UC patients with 23 lesions.
Endoscopic evaluation
Endoscopists conducted routine white-light imaging observation. When neoplastic lesions were identified, magnifying NBI or BLI and magnifying chromoendoscopy using indigo carmine or crystal violet were performed. All lesions were endoscopically detectable, visually identified, and subsequently diagnosed using target biopsy. The morphological type of neoplasms was categorized in accordance with the SCENIC consensus statement[3 ]. The severity of inflammation in the mucosa surrounding neoplasms was assessed using the Ulcerative Colitis Endoscopic Index of Severity (UCEIS)[23 ]. The JNET and pit pattern classifications were employed to evaluate the pathology and invasion depth of neoplasms by endoscopy. With the JNET classification, lesions were categorized based on surface and vascular patterns into types 1 , 2 A, 2 B, and 3 . The pit pattern classification was used under indigo carmine or crystal violet observation; lesions were categorized based on form of crypt orifices into types I, II, III, IV, VIlow irregularity, VIhigh irregularity, and VN. This study used JNET type 2 A and pit pattern type III/IV as indicators of low-grade dysplasia (LGD), based on previous reports[22 ].Furthermore, in the same manner, as LGD, JNET type 2 B and pit pattern type VIlow irregularity were utilized as indicators of high-grade dysplasia (HGD) to shallow submucosal invasive carcinoma (sSM),whereas JNET type 3 and pit pattern type VIhigh irregularity/VNwere used as indicators of deep submucosal invasive carcinoma (dSM). Endoscopic images corresponding to the part that could be evaluated pathologically were extracted, and six endoscopists (three experts, three non-experts) each evaluated the endoscopic findings. Experts were defined as those with ≥ 5 -year experience in magnifying image-enhanced endoscopy and who had managed more than 1000 cases[18 ]. Endoscopists independently evaluated the images obtained from 44 lesions; when individual diagnostic interpretations differed, they discussed the case until a consensus was reached. Diagnostic performance was assessed by consensus of the first diagnosis of three endoscopists. Inter- and intra-observer agreements were calculated for the diagnostic results of each endoscopist. The second diagnosis was performed by randomly switching the order of images at ≥ 1 mo after the first round of diagnosis to calculate for intraobserver agreement.
Pathological assessment
Two pathologists specializing in the gastrointestinal tract conducted pathological diagnosis of UCAN and SN according to the Riddellet al[24 ]’s pathological system. UCAN and SN were differentiated based on pathological results. If necessary, p53 and Ki-67 immunostaining were performed. UCAN was diagnosed for cases with diffuse and strong expression or complete absence of p53 immunostaining[25 ]. Differentiation of Ki-67 -positive cells from the basal mucosal side toward the superficial mucosal side[25 ,26 ], called “bottom-up,” was also useful in diagnosing UCAN. Contrary to the UCAN,expression of p53 is low in SN. Moreover, Ki-67 -positive cells are mainly distributed at the superficial zone of the mucosal layer, and tumor cells differentiate towards the basal side of the mucosa in the SN[26 ], also known as “top-down”. Dysplasia was classified into LGD and HGD according to the degree of cellular and nuclear dysplastic change. Submucosal invasive carcinoma was divided into dSM and sSM depending on whether the vertical invasion depth exceeded 1000 μm. When two pathologists had different diagnoses, they discussed the case until a conclusion was reached.
Data collection
Clinical data, including age at UC onset, disease duration, sex, disease distribution (total colitis, leftsided colitis, proctitis), clinical type (relapse and remission, chronic persistent, first attack), primary sclerosing cholangitis (PSC), and family history of colorectal cancer, were retrospectively collected from medical records and investigated. Endoscopic findings, including location, color, lesion border,morphology, and UCEIS, were obtained from medical reports and evaluated.
Statistical analysis
Continuous variables were expressed as mean ± SD or as median with range and were compared using Student’st-test or Mann-WhitneyUtest, depending on the normality of data distribution, as determined by the Shapiro-Wilk test. Categorial variables were compared using Fisher’s exact test or chi-square test. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV),and accuracy were calculated for both the JNET and pit pattern classifications. Inter- and intra-observer agreements were calculated using κ coefficient and arbitrarily interpreted as follows: 0 -0 .20 , poor;0 .21 -0 .40 , fair; 0 .41 -0 .60 , moderate; 0 .61 -0 .80 , substantial; and 0 .81 -1 .00 , excellent. Statistical analysis was conducted using SPSS Statistics version 27 (IBM Corp., Armonk, NY, United States) and R3 .6 .3 (CRAN, freeware, https://personal.hs. hirosaki-u.ac.jp/pteiki/reserch/stat/R/), withP< 0 .05 being indicative of statistical significance.
RESULTS
Clinical characteristics
All patients were divided into two groups according to pathological findings. Inter-observer agreement in the diagnosis between UCAN and SN by the two pathologists was 0 .531 . The clinical characteristics of both groups are summarized in Table 1 . The UCAN group had a significantly lower mean age at UC onset than the SN group (35 .7 vs 48 .8 years, P = 0 .003 ). Pathological findings indicated 2 LGD lesions, 11 HGD lesions, 3 sSM lesions, and 5 dSM lesions in the UCAN group and 16 LGD lesions, 5 HGD lesions,and 2 dSM lesions in the SN group. No significant differences in the disease duration, sex, extent of disease, disease distribution, presence of PSC, and family history of colorectal cancer were identified between the two groups.
Endoscopic and clinical findings
The endoscopic findings for both groups are presented in Table 2 . A total of 17 (81 .0 %) and 12 (52 .2 %)lesions were detected in the proctosigmoid colon in the UCAN and SN groups, respectively (P= 0 .044 ).The UCAN group had a higher percentage of reddish lesions than the SN group (71 .4 % vs 39 .1 %,P=0 .032 ). All lesions in the SN group exhibited a clear border, whereas 16 lesions (76 .2 %) in the UCAN group showed an unclear border (P< 0 .001 ). The UCAN group had a higher proportion of flat or depressed lesions than the SN group (28 .6 % vs 8 .7 %, P = 0 .094 ). Inflammation in the mucosa surrounding neoplasms was more severe in the UCAN group than in the SN group [UCEIS (median): 2vs0 , P < 0 .001 ].
Diagnostic performance of the JNET and pit pattern classifications
Diagnostic performance for each type in the JNET and pit pattern classifications is shown in Tables 3 and 4 , respectively. Sensitivity, specificity, PPV, NPV, and accuracy were calculated for experts and non-experts separately.
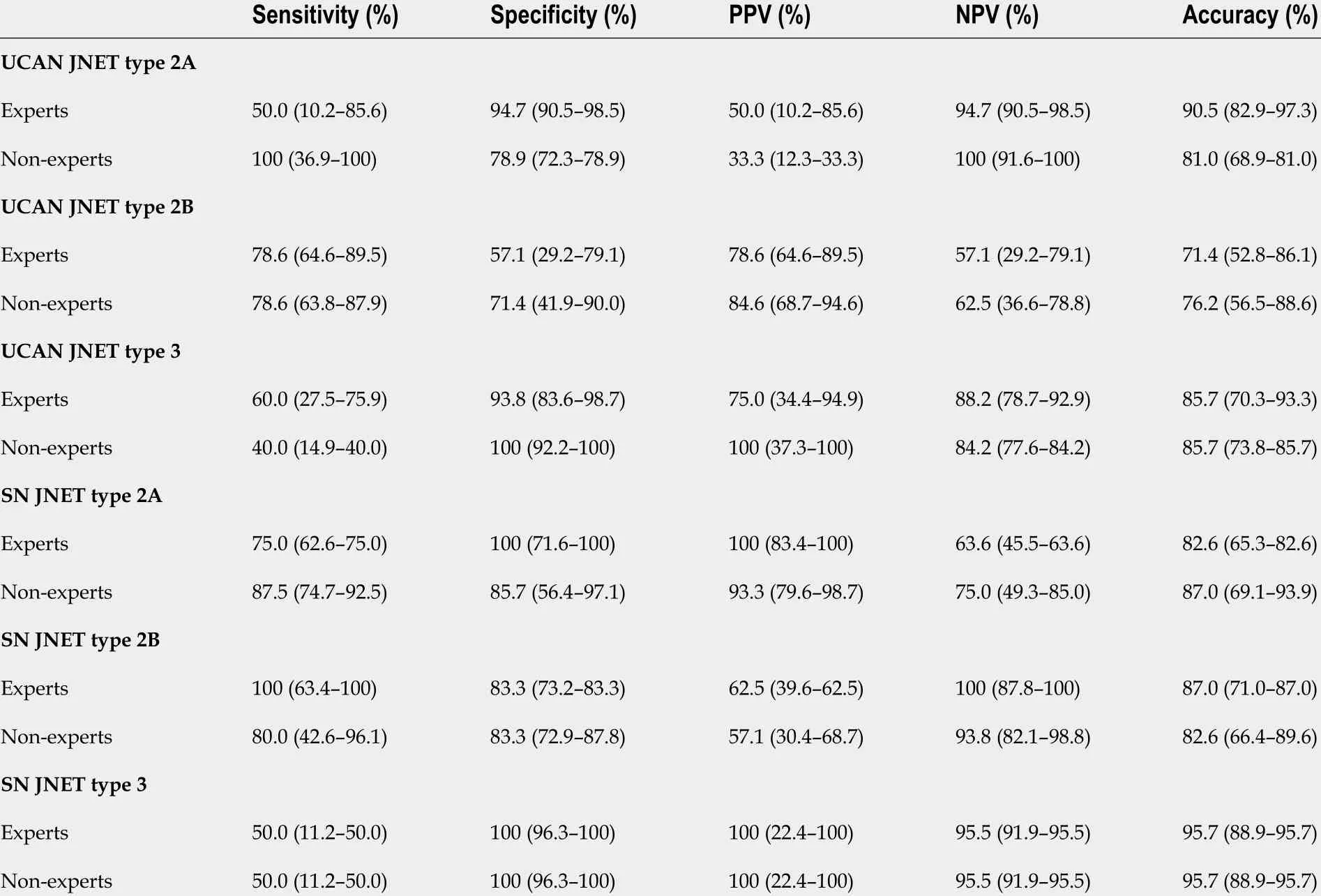
Table 3 Diagnostic performance (95 % confidence interval) for each type in the Japan Narrow-Band lmaging Expert Team classification
In the UCAN group, JNET type 2 A had a low PPV [experts vs non-experts: 50 .0 % (10 .2 -85 .6 ) vs 33 .3 %(12 .3 -33 .3 )] and a high NPV [experts vs non-experts: 94 .7 % (90 .5 -98 .5 ) vs 100 % (91 .6 -100 )] for both experts and non-experts. Conversely, in the SN group, JNET type 2 A had a high PPV [expertsvsnonexperts: 100 % (83 .4 -100 ) vs 93 .3 % (79 .6 -98 .7 )] and a low NPV [experts vs non-experts: 63 .6 % (45 .5 -63 .6 )vs75 .0 % (49 .3 -85 .0 )]. In the UCAN group, the accuracy of diagnosis for JNET types 2 A, 2 B, and 3 by experts was 90 .5 %, 71 .4 %, and 85 .7 %, respectively, and that by non-experts was 81 .0 %, 76 .2 %, and 85 .7 %, respectively.
In the UCAN group, pit pattern type III/IV had a low PPV [expertsvsnon-experts: 40 .0 % (14 .9 -40 .0 )vs20 .0 % (7 .3 -20 .0 )] and a high NPV [experts vs non-experts: 100 % (92 .2 -100 ) vs 100 % (88 .4 -100 )] for both experts and non-experts. Conversely, in the SN group, pit pattern type III/IV had a high PPV[expertsvsnon-experts: 100 % (86 .4 -100 ) vs 92 .9 % (77 .8 -98 .6 )] and a low NPV [77 .8 % (56 .6 -77 .8 ) vs 66 .7 %(43 .3 -75 .6 )]. In the UCAN group, the accuracy of diagnosis for pit pattern type III/IV, type VIlow irregularity, and type VIhigh irregularity/VNby experts was 85 .7 %, 57 .1 %, and 76 .2 %, respectively, and that by non-experts was 61 .9 %, 57 .1 %, and 85 .7 %, respectively. The accuracy of diagnosis for JNET type 3 and pit pattern type VIhigh irregularity/VNby both experts and non-experts was higher in the SN group than in the UCAN group. Figure 1 shows a representative case of UCAN misdiagnosed by all endoscopists.
Intra-observer and inter-observer agreements for the JNET and pit pattern classifications
Intra-observer agreement was separately calculated for experts and non-experts (Table 5 ). The intraobserver agreement among experts for the JNET classification of UCAN, pit pattern classification of UCAN, JNET classification of SN, and pit pattern classification of SN was 0 .387 , 0 .454 , 0 .803 , and 0 .567 ,respectively. The corresponding values for non-experts were 0 .640 , 0 .569 , 0 .828 , and 0 .628 , respectively.The intra-observer agreement for SN was higher than that for UCAN. Among non-experts, the intraobserver agreement for both UCAN and SN was higher with the JNET classification than with the pit pattern classification.
Inter-observer agreement was calculated similarly (Table 6 ). The inter-observer agreement among experts for the JNET classification of UCAN, pit pattern classification of UCAN, JNET classification of SN, and pit pattern classification of SN was 0 .401 , 0 .364 , 0 .666 , and 0 .597 , respectively. The corresponding values for non-experts were 0 .237 , 0 .378 , 0 .503 , and 0 .437 , respectively. Overall, the inter-observer agreement for SN was higher than that for UCAN among both experts and non-experts,irrespective of the classification system used.

Table 4 Diagnostic performance (95 % confidence interval) for each type in the pit pattern classification

Table 5 lntra-observer agreement
DlSCUSSlON
In the present study, we evaluated the performance of the JNET and pit pattern classifications in patients with UC. The JNET classification evaluates the tumors’ surface and vascular patterns, whereas the pit pattern classification assesses the form of pits on the tumor surface. Colonic mucosal inflammation in UC patients modifies the tumors’ surface and vascular patterns and is considered to reduce the diagnostic accuracy of both classifications. Here, we revealed that the accuracy of diagnosing colorectal tumors using JNET and pit pattern classifications was lower in UC patients, particularly those with UCAN, than in non-UC patients[15 -18 ]. The agreement rates were lower for both UCAN and SNpatients than for non-UC patients. The diagnostic performance of both classifications in UC patients is substantially lower than their previously reported diagnostic performance in non-UC patients[15 -18 ].
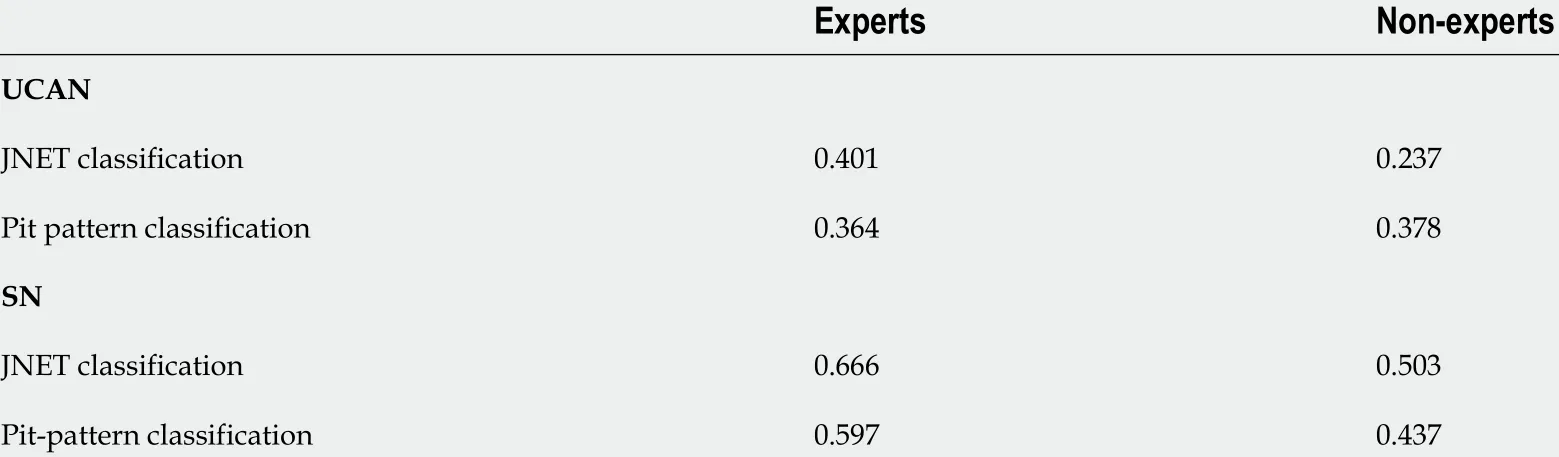
Table 6 lnter-observer agreement

Figure 1 Endoscopic features of ulcerative colitis-associated neoplasms misdiagnosed by all endoscopists. A: White-light imaging reveals a flat elevated lesion in the rectum; B: Chromoendoscopy with indigo carmine shows a clear lesion border; C: Magnifying endoscopy with narrow-band imaging of box in (B) shows regular surface and vascular patterns, which were classified by all endoscopists as Japan Narrow-Band Imaging Expert Team classification type 2 A; D:Magnifying endoscopy with crystal violet chromoendoscopy of box in (B) reveals relatively uniform villous structures, which were classified by all endoscopists as pit pattern type IV; E: Pathological examination of the resected specimen by endoscopic submucosal dissection shows architectural atypia. This lesion was pathologically diagnosed as high-grade dysplasia (hematoxylin and eosin staining, original magnification × 50 ); F: Immunohistochemistry for p53 on serial section of(E).
Previous reports revealed that, compared to SN, UCAN is more common in the proctosigmoid colon and features more redness, unclear border, flat and depressed lesions, and a higher degree of surrounding inflammation[2 ]. On magnifying chromoendoscopy, pit pattern types III, IV, and V, which are also caused by regenerative changes, are useful in diagnosing UCAN[9 ,21 ]. In the present study, the UCAN group had a significantly higher proportion of lesions with endoscopically unclear border and severe inflammation in the mucosa surrounding neoplasms than the SN group. As colorectal tumors can considerably impact the quality of life of UC patients, it is essential for endoscopists to understand these endoscopic features of UCAN.
In the UCAN group, JNET type 2 A and pit pattern type III/IV had a low PPV but with a high NPV in LGD diagnosis, and JNET type 2 B and pit pattern type VIlow irregularity had a low NPV in the diagnosis of HGD to sSM. This was because several lesions in UCAN were diagnosed as JNET type 2 A or pit pattern type III/IV, even though they were actually HGD to sSM. Additional detailed analysis revealed that most endoscopists diagnosed about one-quarter of HGD to sSM lesions as JNET type 2 A and one-third of HGD to sSM lesions as pit pattern type III/IV. Furthermore, a small number of dSM lesions were diagnosed as JNET type 2 A or pit pattern type III/IV (Table 7 ).
In the SN group, JNET type 2 A and pit pattern type III/IV had a high PPV but with a low NPV in LGD diagnosis, and JNET type 2 B and pit pattern type VIlow irregularity had a low PPV in the diagnosis of HGD to sSM. Because JNET type 2 B and pit pattern type VIlow irregularity include lesions from LGD to dSM, these types have low PPV even in non-UC patients[17 ,19 ,27 ]. Several LGD and dSM lesions in the SN group were diagnosed as JNET type 2 B and pit pattern type VIlow irregularity. JNET type 3 and pit pattern type VIhigh irregularity/VNin both UCAN and SN groups showed low sensitivity but with high specificity and accuracy. Previous studies showed that JNET type 3 and pit pattern type VIhigh irregularity/VNhave high specificity for dSM diagnosis in both UC and non-UC patients[17 -19 ,22 ]. Regardless of whether the surface and vascular patterns are modified by inflammation, JNET type 3 and pit pattern type VIhigh irregularity/VNhave been confirmed to be useful in diagnosing dSM among UC patients. In UCAN, the tumors’ surface structure sometimes could not represent the dysplastic change due to the bottom-up growth pattern; hence, it is considered that several lesions are underestimated by endoscopic classifications. Additionally, SN located in the inflamed mucosa, especially SN in the severely inflamed mucosa, tends to be misdiagnosed due to the influence of inflammation.
Intra-observer agreement was higher among non-experts than among experts; however, the difference was not statistically significant. Inter-observer agreement did not also significantly differ but was higher in experts than in non-experts. Irrespective of the endoscopists’ experience, a consistent endoscopic diagnosis of neoplastic lesions in UC patients was difficult to achieve. In particular, the intra- and inter-observer agreements were lower for UCAN than for SN.
The present study has some limitations. First, our study was conducted on a small number of cases;however, given its retrospective nature, the same size could not be set a priori. We believe that the small number of typical LGDs in this study was responsible for the unsatisfactory diagnostic accuracy.Second, only neoplastic lesions evaluated using both the JNET and pit pattern classifications were included. While inflammation and regenerative changes might be evaluated as neoplastic patterns by both JNET and pit pattern classifications, our study could not include non-neoplastic lesions. Nonneoplastic lesions should be included in future studies. Third, differentiation between UCAN and SN was based on pathology; nevertheless, even in pathology, distinguishing UCAN from SN can be difficult.
CONCLUSlON
The JNET and pit pattern classifications did not show high accuracy in diagnosing the pathology and invasion depth of neoplastic lesions in UC patients. Overall, the endoscopic diagnosis of UCAN tended to be underestimated as compared to the pathological results. Endoscopic diagnosis of neoplastic lesions in UC patients is still difficult, and treatment strategies need to be carefully determined.
ARTlCLE HlGHLlGHTS

ACKNOWLEDGEMENTS
We would like to thank Go Kajikawa, Masaya Esaki, Issei Hasegawa, Kentaro Yamada, and Shuji Ikegami for evaluating the endoscopic data.
FOOTNOTES
Author contributions:Kida Y and Yamamura T contributed to the conception and design; Kida Y, Yamamura T,Kawashima H, Ishikawa E, and Kakushima N contributed to the analysis and interpretation of the data; Kida Y drafted the article; Nakamura M, Ohno E, Sawada T, Maeda K, Ishikawa T, Ishigami M, and Furukawa K contributed to the critical revision of the article for important intellectual content; Mizutani Y, Yamamura T and Nakamura M contributed to statistical analysis; Fujishiro M made the final approval of the article; all authors have read and approved the final manuscript.
lnstitutional review board statement:The use of patient data for this study was approved by the Ethics Committee of Nagoya University Hospital, No. 2015 -0485 .
lnformed consent statement:The need for patient consent was waived due to the retrospective nature of the study.
Conflict-of-interest statement:The authors declare no conflict of interests for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:Japan
ORClD number:Yuichi Kida 0000 -0002 -1345 -1922 ; Takeshi Yamamura 0000 -0003 -4994 -016 X; Keiko Maeda 0000 -0001 -7615 -0476 ; Tsunaki Sawada 0000 -0002 -4779 -9708 ; Eri Ishikawa 0000 -0003 -1623 -7996 ; Yasuyuki Mizutani 0000 -0002 -4363 -3161 ; Naomi Kakushima 0000 -0002 -9635 -2099 ; Kazuhiro Furukawa 0000 -0003 -0980 -9095 ; Takuya Ishikawa 0000 -0001 -5814 -3555 ; Eizaburo Ohno 0000 -0002 -7730 -4630 ; Hiroki Kawashima 0000 -0002 -3720 -781 X; Masanao Nakamura 0000 -0002 -5444 -143 X; Masatoshi Ishigami 0000 -0003 -0938 -631 X; Mitsuhiro Fujishiro 0000 -0002 -4074 -1140 .
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年10期
World Journal of Gastroenterology2022年10期
- World Journal of Gastroenterology的其它文章
- Comment “Asymptomatic small intestinal ulcerative lesions: Obesity and Helicobacter pylori are likely to be risk factors”
- Gut dysbiosis and small intestinal bacterial overgrowth as independent forms of gut microbiota disorders in cirrhosis
- Loss of LAT1 sex-dependently delays recovery after caerulein-induced acute pancreatitis
- Malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer
- Feasibility of therapeutic endoscopic ultrasound in the bridge-to-surgery scenario: The example of pancreatic adenocarcinoma
- An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms
