Evolving roles of magnifying endoscopy and endoscopic resection for neoplasia in inflammatory bowel diseases
lNTRODUCTlON
Patients with inflammatory bowel disease(IBD)have an increased risk of developing colorectal cancer(CRC)compared with general population[1,2].Surveillance colonoscopy is recommended 8-10 years after the diagnosis of IBD[3].A forty-year analysis of colonoscopy surveillance program demonstrated that the incidence rate of advanced CRC have decreased while the incidences of early CRC and dysplasia have increased in patients with ulcerative colitis(UC),suggesting that the implementation of surveillance colonoscopy plays a significant role in reducing the likelihood of CRC in IBD patients[4].During the surveillance colonoscopy for IBD patients,an early detection and an accurate diagnosis of neoplastic lesions are crucial components of the exam that impact further therapeutic plans and might improve patients’ prognosis.
High-definition chromoendoscopy or white-light endoscopy with narrow-band imaging(NBI)are the preferred procedures and modalities for the detection of neoplastic lesions in patients with IBD[3].Magnifying chromoendoscopy with indigo carmine or methylene blue is a useful procedure to assess pit patterns,opening shapes of tumor crypts.Pit patterns can be predictive of the presence of neoplastic lesions and their invasion depth.The classification proposed by Kudo
[5]includes 8 pit pattern types(types I,II,III
,III
,IV,V
low-irregularity,V
high-irregularity,and V
)and can predict pathological diagnosis of colorectal tumors and tumor depths.In general,pit pattern types III-V are considered as neoplastic lesions in non-IBD patients[5].Furthermore,recent advances in endoscopy now enable narrow-band-imaging(NBI)technology,and magnifying endoscopy with NBI has been widely used to diagnose colorectal neoplasia in non-IBD patients.The Japan NBI expert team(JNET)proposed a classification system based on the vessel and surface patterns of tumors.This JNET classification includes 4 types(Types 1,2A,2B,and 3)and types 2-3 are considered as neoplasia[6].These classifications are essential in determining the indication of endoscopic mucosal resection(EMR)or endoscopic submucosal dissection(ESD)to achieve a curative resection of colorectal tumors.
A voice came from within and asked her, Where do you come from, and where do you want to go? She answered, I have lost my way to my father s kingdom, and I shall never get home again
Many investigations have been undertaken to understand the utility of pit patterns or use of NBI to diagnose neoplastic lesions,as well as the feasibility and outcomes of endoscopic resection to remove these lesions in IBD patients.In this review,we summarized updated data regarding these important topics.
UTlLlTY OF MAGNlFlNG CHROMOENDOSCOPY FOR lBD
Magnifying chromoendoscopy is a well-validated tool to assess neoplastic lesions in non-IBD patients and data regarding its utility for IBD patients has been emerging.The pit patterns of UC-associated neoplasm are characterized by spare distribution,loss of polarity,irregular pits,size variation,and wide-open or fused pits[7].Similar to non-IBD patients,neoplasia in IBD patients also show pit pattern types III
,III
,IV or V[7,8].A randomized controlled study including 165 UC patients randomized in a 1:1 ratio to undergo conventional colonoscopy or chromoendoscopy using 0.1% methylene blue demonstrated that pit pattern classification has high sensitivity(93%)and specificity(93%)to differentiate neoplastic lesions from non-neoplastic lesions[9].A recent multicenter prospective study which assessed the detection rate of dysplasia in IBD patients showed that endoscopic findings of Kudo pit pattern types III-V were predictive of dysplasia(Table 1)[10].
A randomized trial comparing the detection rate of neoplasia between dye spraying colonoscopy and high-definition or electronic virtual chromoendoscopy revealed that Kudo pit pattern type IIo or types III-V were significantly predictive of neoplastic lesions during IBD surveillance colonoscopy(Odds ratio 21.5,95% confidence interval 8.7-60.1)[11].Another study using 769 stereomicroscopic pictures(509 neoplastic and 260 non-neoplastic)obtained from surgically resected specimens showed that pit pattern types III-V were significantly associated with the presence of neoplasia(sensitivity 77.4% and specificity89.5%)[12].Further,previous studies also demonstrated that pit pattern types I-II had a high negative predictive value to rule out the diagnosis of neoplasia(Table 1)[13,14].The findings in all of these studies suggest that magnifying endoscopy assessing Kudo pit patterns may be useful to differentiate between neoplastic lesions and non-neoplastic lesions in IBD patients.
Each study assessing the utility of magnifying endoscopy with NBI showed similar sensitivity and specificity for neoplastic lesions in UC,although the sensitivity of JNET type 3 was low.Hence,it is still unclear which of vascular or surface patterns of tumors are important to differentiate neoplasia and non-neoplastic lesions in UC.Given that each study only assessed the small number of UC-associated neoplasia,further investigations with larger sample sizes are warranted to better understand the characteristics of NBI findings of IBD-associated neoplasia and diagnostic accuracy of JNET classification as well as its limitations.
These findings suggested that Kudo’s pit patterns may have a high sensitivity to rule out neoplasia,but limited utility to accurately diagnose neoplasia in IBD patients due to its low specificity.Given that the regenerative mucosa can present pit patterns type III
and IV and may decrease its specificity to diagnose neoplasia in IBD,it is suggested that providers must achieve mucosal healing prior to the scopes to overcome this disadvantage.
Despite these promising findings,there are several studies that have suggested some limitations of pit patterns in diagnosing colitis-associated neoplasia.A case study assessing histopathological findings of 35 Lesions from UC patients found that pit pattern types III
and IV can be observed in the neoplastic lesions as well as the surrounding flat mucosa,resulting in the “low specificity”(57%)of pit patterns type III-V to diagnose neoplasia.They suggested that pit patterns type III
and IV can be observed not only in the neoplastic lesions but also in the “regenerative mucosa” in UC patients[15].Indeed,histopathological findings of regenerative mucosa can masquerade as dysplastic findings and make the diagnosis of dysplasia equivocal[16].Several studies have also suggested that the correlation between dysplasia and pit pattern types III-V was low(Table 1)[13,14].Moreover,Kudo’s pit patterns cannot necessarily classify all of the findings which are observed in the neoplastic lesions in UC patients.For instance,a previous observational study showed that “pinecone and villi patterns” were endoscopic signs suggestive of neoplastic lesions in UC patients[12].
UTlLlTY OF MAGNlFYlNG ENDOSCOPY WlTH NBl FOR lBD
With regard to the utility of NBI in IBD patients,a case report initially found that magnifying colonoscopy with NBI can differentiate dysplastic and non-dysplastic lesions,and enabled the detection of dysplastic lesions in a patient with UC[17].This study revealed that dysplastic lesions have “a stronger capillary vascular pattern” in comparison to normal mucosa[17].A retrospective single-center study examining 10 flat-type predominant dysplasia in UC patients demonstrated that all lesions can be recognized as “demarcated,red-colored areas with increased vascular densities” on NBI,and such lesions were histologically diagnosed with low- or high-grade dysplasia.This study performed CD34 immunohistostaining to assess intramucosal vessels and found that low-grade dysplasia displayed an increased number of vessels,whereas high-grade dysplasia contained increased/enlarged vessels[18].Kinoshita
[19]assessed the utility of the Sano magnifying NBI classification(capillary pattern classification)for neoplasia in UC and demonstrated that capillary patterns IIIA(high microvessel density with a lack of uniformity)or IIIB(the presence of an area with nearly avascular or sparse microvascular findings)had a sensitivity of 72.2% and a specificity of 85.7% to diagnose high-grade dysplasia or submucosal deep invasive carcinoma in patients with UC.These data suggest that intramucosal vessels are proliferated in dysplastic lesions in UC patients,and an increased vascular pattern may be a practical sign in detecting such lesions during magnifying colonoscopy with NBI.
A cross-sectional study including 46 UC patients classified the surface structure of neoplasia into honeycomb-like,villous,or tortuous pattern;the detection rate of dysplasia was higher in “tortuous surface pattern” than honeycomb-like or villous patterns[20].Another observational study assessing both surface and vascular patterns of neoplasia in IBD patients by magnifying colonoscopy with NBI found that “irregular/amorphous surface patterns” were significantly associated with neoplastic lesions(sensitivity 81.3% and specificity 82.4%),whereas irregular/amorphous vascular patterns showed lower sensitivity(75.0%)and specificity(58.8%)(Table 2)[21].A systematic review and meta-analysis revealed that the sensitivity and specificity of NBI to discriminate neoplasia from non-neoplastic lesions were 0.64(95%CI 0.50-0.77)and 0.74(95%CI 0.69-0.79),respectively[22].
As soon as they were cooked the old dame39 took a pair of scales and a morsel40 of bread from the cupboard, and was just about to divide it when Prince Vivien, who really could wait no longer, seized the whole piece and ate it up, saying in his turn, Patience
The JNET established a NBI magnifying endoscopic classification based on the vessel and surface patterns of tumors in non-IBD patients[6].Data regarding its diagnostic utility for neoplastic lesions in IBD patients remain limited.A recent case study including 17 patients with UC-associated neoplasia demonstrated that JNET types 2A,2B,and 3 were correlated with the pathological diagnosis of lowgrade dysplasia/high-grade dysplasia,high-grade dysplasia,and massive submucosal invasion of cancer,respectively[23].The sensitivity and specificity of JNET type 3 in diagnosing massively invading CRC were 25% and 100%,respectively(Table 2)[23].
My husband and I had been together for six years, and with him I had watched as his young children became young teenagers. Although they lived primarily with their mother, they spent a lot of time with us as well. Over the years, we all learned to adjust, to become more comfortable with each other, and to adapt to our new family arrangement. We enjoyed vacations together, ate family meals, worked on homework, played baseball, rented videos. However, I continued to feel somewhat like an outsider, infringing1 upon foreign territory. There was a definite boundary line that could not be crossed, an inner family circle which excluded me. Since I had no children of my own, my experience of parenting was limited to my husband s four, and often I lamented2 that I would never know the special bond that exists between a parent and a child.
UTlLlTY OF ENDOCYTOSCOPY FOR lBD
Given the diagnostic limitations of Kudo’s pit patterns for IBD-associated neoplasia,Kudo
[5]retrospectively compared the diagnostic utility between pit patterns alone and its combination with an assessment using endocytoscopy,an ultra-magnifying contact microscope which has proven to enable visualization of cell nuclei or microvasculature,and detection of neoplasia in non-IBD patients[24,25].Their data found that both pit patterns and its combination with endocytoscopy had high sensitivities to diagnose UC-associated neoplasia(97.8%
100%).In addition,the authors also demonstrated that the specificity was higher in the combined assessment of pit patterns with endocytoscopy compared to pit patterns alone(84.4%
57.5%)(Table 1)[26].This study demonstrated that endocytoscopy can enhance the specificity of pit patterns for the diagnosis of neoplasia in IBD patients,suggesting an endocytoscopy-assisted pit pattern assessment may be a novel approach to overcome the limitation of pit patterns alone.However,they also found that the specificity can be affected by active inflammation(Mayo endoscopic subscore 2-3)and that false positive rates were high(16.2% in type II-V pits with positive irregularly-formed nuclei and 10.3% in type V)[26].Therefore,achieving endoscopic remission is essential for an accurate evaluation of pit-patterns or cellular nuclei using magnifying scopes.
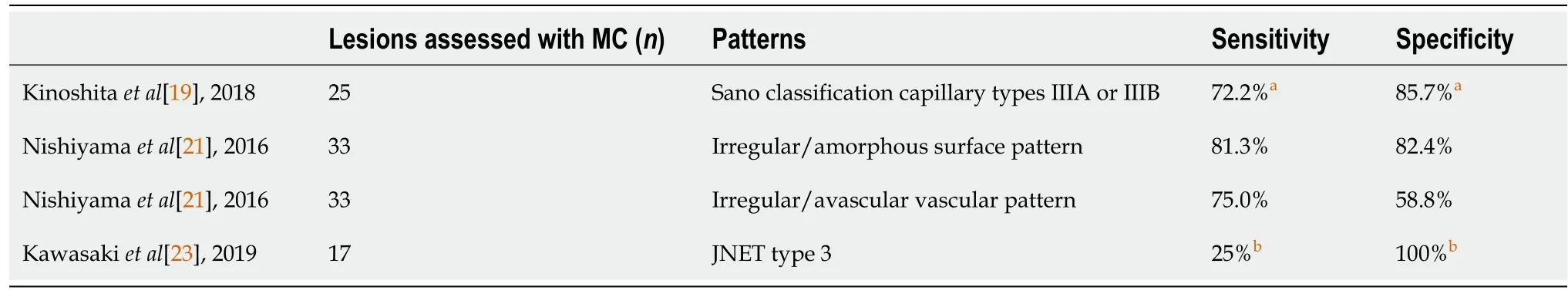
Figures 1 and 2 show representative findings of a neoplastic lesion in a patient with UC.
With regard to magnifying colonoscopy with NBI,one case study assessed the utility of an endocytoscopy with NBI to diagnose dysplasia in a patient with UC.The authors demonstrated that an endocytoscopy with NBI identified “surface microvessels of uniform caliber and arrangement” in a reddish lesion(5 mm)in the lower rectum.An artificial intelligence-based system diagnosed it as a neoplasm.Consequently,its pathological diagnosis was high-grade dysplasia[27].Given that endocytoscopy can increase the specificity of pit patterns for neoplasia in IBD[26],this technique may be useful to improve the diagnostic utility of JNET classification for IBD-associated neoplasia as well.
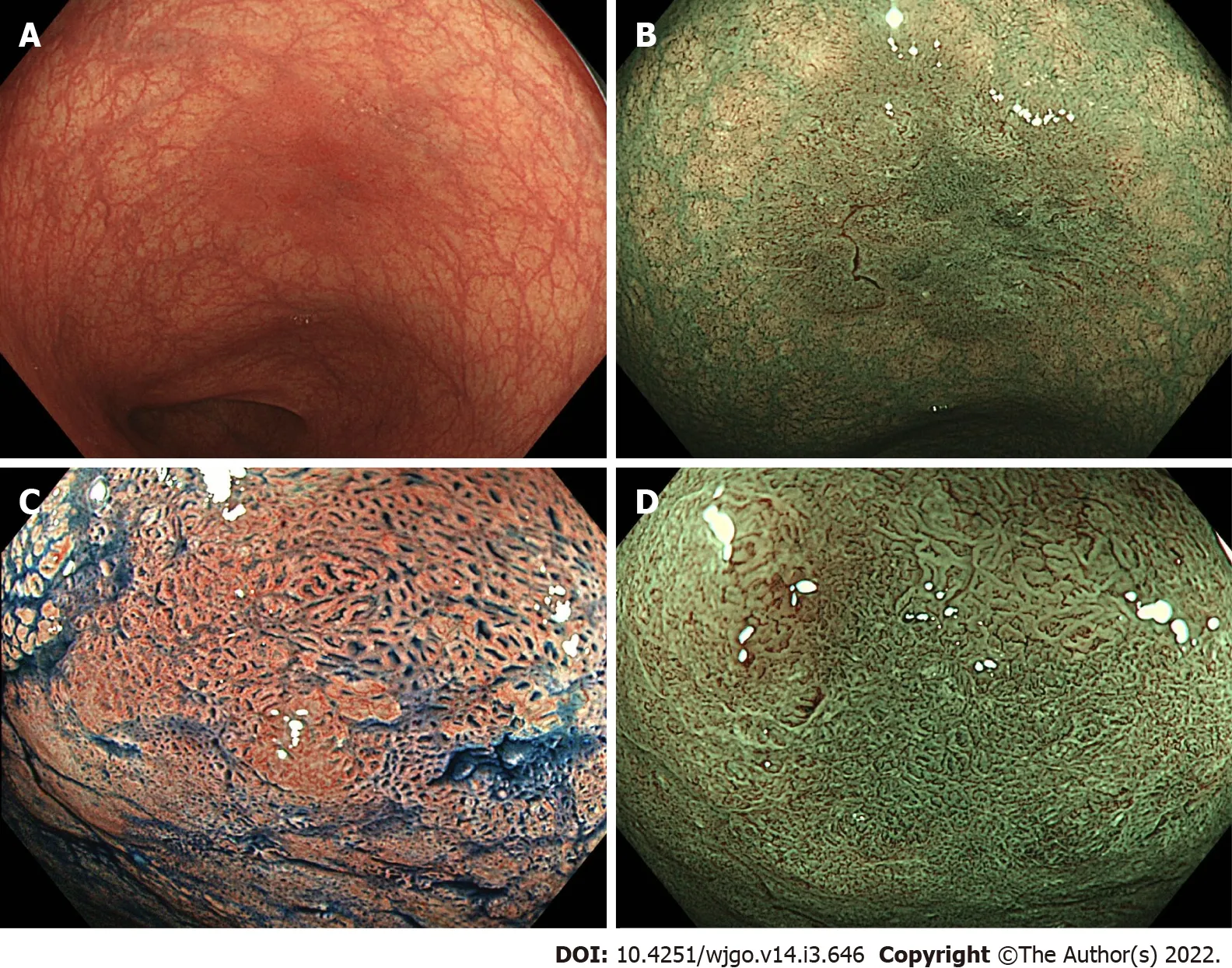
ENDOSCOPlC RESECTlON FOR NEOPLASTlC LESlONS lN lBD
As previously described,magnifying endoscopy is useful to discriminate between dysplasia and nondysplastic lesions in IBD patients,and determine whether such lesions are completely resectable or not.An endoscopic resection of neoplasia is safe and feasible in most IBD patients.A systematic review and meta-analysis including 1,428 resected colonic lesions in IBD patients showed the pooled incidences of bleeding and perforation after endoscopic resection were 0.022(95%CI 0.011-0.044)and 0.020(95%CI 0.009-0.044),respectively[28].Another meta-analysis revealed that the pooled rates of margin-negative(R0)and
resection rates of non-polypoid dysplasia in IBD patients were 0.70(95%CI 0.55-0.81)and 0.86(95%CI 0.65-0.95),respectively[29].
The SCENIC statement,an international consensus on surveillance and management of neoplasia in IBD patients,recommended a surveillance colonoscopy rather than colectomy after the complete endoscopic resection of “polypoid dysplasia”[36].On the other hand,given that “non-polypoid lesions”have a greater risk of metachronous dysplasia after the endoscopic resection compared with polypoid lesions,providers must understand the importance of follow-up colonoscopy to survey for other neoplastic lesions.A recent systematic review and meta-analysis showed the pooled risks of CRC and any dysplasia after the endoscopic resection of neoplastic lesions in IBD patients was 2 and 43 per 1,000 person-year of follow-up,respectively,suggesting the requirement of surveillance colonoscopy after the endoscopic resection[28].Another meta-analysis including 202 IBD patients with non-polypoid dysplasia demonstrated that the pooled incidences of CRC and metachronous dysplasia after the endoscopic resection were 33 and 90 per 1,000 person-year of follow-up,respectively[29],suggesting that the likelihood of metachronous lesions would be higher in non-polypoid lesions compared with other types.These findings emphasize the critical importance of having a discussion regarding the risks and benefits of surveillance colonoscopy and colectomy with patients following endoscopic resection.
I am going to break it, answered the little hare; but of course he had done it on purpose, as he wanted to fix Big Lion s tail so firmly to the hut that he would not be able to move
Many years ago, I read that James A. Michener, who did not publish until he was forty years of age, advised young writers to do extensive research before trying to write a novel. He visited the countries and areas he was interested in writing about, interviewing countless1 people as well as reading more than two hundred books for back-ground material for each of his books -- Hawaii, Iberia, The Source, Texas, Poland, Alaska, Caribbean -- and for some forty other book projects, spanning2 a fifty-year writing career.
As for an endoscopic technique to remove neoplasia in IBD patients,endoscopic mucosal resection(EMR)has been widely used.However,endoscopic submucosal dissection(ESD)may be a preferred technique in order to remove non-polypoid or larger lesions.Many observational studies published in 2021 have demonstrated the feasibility and safety of ESD for neoplasia in IBD patients[30-35].Due to the technical difficulty of ESD for IBD-associated neoplasia which are often complicated with submucosal fibrosis,each study subsequently had a small sample size.Thus,further studies with larger sample sizes or analyses with integrated data may be necessary to investigate the feasibility,safety,and long-term outcomes of ESD for neoplasia in IBD patients.
CONCLUSlON
Magnifying colonoscopies assessing Kudo’s pit patterns or surface/vascular patterns with NBI are practical tools that can be used to aid in the diagnosis of neoplasia in IBD patients.Endoscopic characteristics of neoplasia in IBD patients include Kudo’s pit pattern types III-V in magnifying chromoendoscopy or JNET types 2A,2B,and 3 in magnifying colonoscopy with NBI.Endocytoscopy is a supportive tool which can assist providers in confirming these diagnoses.As compared to non-IBD patients,patients with IBD can have mucosa with active colonic inflammation that may alter the findings on magnifying endoscopy,thus increasing the risk of misdiagnosis of neoplasia.Accordingly,providers should control active mucosal inflammation and achieve mucosal healing in advance to ensure the quality of surveillance colonoscopy with magnifying scopes.Although an endoscopic resection is feasible and safe for IBD-associated neoplasia,it is critically important to take into account the subsequent risk of metachronous neoplasia development,even after the complete endoscopic resection of neoplasia in IBD patients.
FOOTNOTES
Akiyama S designed and performed the research;Akiyama S,Sakamoto T and Saito Y analyzed the data;Akiyama S,Sakamoto T,Steinberg JM and Tsuchiya K wrote the paper.
SA and TS have no relevant disclosures.JMS was on Advisory Board for Pfizer.YS is on Advisory Board for Boston Scientific KT has received grant supports from Takeda Pharmaceutical Co.,Ltd.,Mitsubishi Tanabe Pharmaceutical Corp.,and Hitachi Ltd.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Japan
Shintaro Akiyama 0000-0003-0727-7638;Taku Sakamoto 0000-0002-0239-4977;Joshua Steinberg 0000-0002-8809-3437;Yutaka Saito 0000-0003-2296-8373;Kiichiro Tsuchiya 0000-0002-1977-8707.
The wretch80 not long after managed to approach me under another form, and one day, when I was in the garden, and asked for some refreshment81, he brought me--in the disguise of a slave--a draught82 which changed me at once to this horrid83 shape
Wang LL
A
I met Harry before Tuesday s game, took him aside and worked with him on keeping his eyes open. He tried, but it s tough to overcome the habit of fear. We were about to play a team that had beat us 22-1 the last time. It didn t seem a fortunate moment for a breakthrough. Then I thought, Why not?
Wang LL
1 Fumery M,Dulai PS,Gupta S,Prokop LJ,Ramamoorthy S,Sandborn WJ,Singh S.Incidence,Risk Factors,and Outcomes of Colorectal Cancer in Patients With Ulcerative Colitis With Low-Grade Dysplasia:A Systematic Review and Meta-analysis.
2017;15:665-674.e5[PMID:27916678 DOI:10.1016/j.cgh.2016.11.025]
2 Friedman S,Rubin PH,Bodian C,Harpaz N,Present DH.Screening and surveillance colonoscopy in chronic Crohn's colitis:results of a surveillance program spanning 25 years.
2008;6:993-8;quiz 953[PMID:18585966 DOI:10.1016/j.cgh.2008.03.019]
3 Rubin DT,Ananthakrishnan AN,Siegel CA,Sauer BG,Long MD.ACG Clinical Guideline:Ulcerative Colitis in Adults.
2019;114:384-413[PMID:30840605 DOI:10.14309/ajg.0000000000000152]
4 Choi CH,Rutter MD,Askari A,Lee GH,Warusavitarne J,Moorghen M,Thomas-Gibson S,Saunders BP,Graham TA,Hart AL.Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis:An Updated Overview.
2015;110:1022-1034[PMID:25823771 DOI:10.1038/ajg.2015.65]
5 Kudo S,Tamura S,Nakajima T,Yamano H,Kusaka H,Watanabe H.Diagnosis of colorectal tumorous lesions by magnifying endoscopy.
1996;44:8-14[PMID:8836710 DOI:10.1016/s0016-5107(96)70222-5]
6 Sano Y,Tanaka S,Kudo SE,Saito S,Matsuda T,Wada Y,Fujii T,Ikematsu H,Uraoka T,Kobayashi N,Nakamura H,Hotta K,Horimatsu T,Sakamoto N,Fu KI,Tsuruta O,Kawano H,Kashida H,Takeuchi Y,Machida H,Kusaka T,Yoshida N,Hirata I,Terai T,Yamano HO,Kaneko K,Nakajima T,Sakamoto T,Yamaguchi Y,Tamai N,Nakano N,Hayashi N,Oka S,Iwatate M,Ishikawa H,Murakami Y,Yoshida S,Saito Y.Narrow-band imaging(NBI)magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team.
2016;28:526-533[PMID:26927367 DOI:10.1111/den.12644]
7 Kudo SE.New frontiers of endoscopy from the large intestine to the small intestine.
2007;66:S3-S6[PMID:17709026 DOI:10.1016/j.gie.2007.03.1083]
8 Sada M,Igarashi M,Yoshizawa S,Kobayashi K,Katsumata T,Saigenji K,Otani Y,Okayasu I,Mitomi H.Dye spraying and magnifying endoscopy for dysplasia and cancer surveillance in ulcerative colitis.
2004;47:1816-1823[PMID:15622573 DOI:10.1007/s10350-004-0682-0]
9 Kiesslich R,Fritsch J,Holtmann M,Koehler HH,Stolte M,Kanzler S,Nafe B,Jung M,Galle PR,Neurath MF.Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis.
2003;124:880-888[PMID:12671882 DOI:10.1053/gast.2003.50146]
10 Carballal S,Maisterra S,López-Serrano A,Gimeno-García AZ,Vera MI,Marín-Garbriel JC,Díaz-Tasende J,Márquez L,Álvarez MA,Hernández L,De Castro L,Gordillo J,Puig I,Vega P,Bustamante-Balén M,Acevedo J,Peñas B,López-Cerón M,Ricart E,Cuatrecasas M,Jimeno M,Pellisé M;EndoCAR group of the Spanish Gastroenterological Association and Spanish Digestive Endoscopy Society.Real-life chromoendoscopy for neoplasia detection and characterisation in longstanding IBD.
2018;67:70-78[PMID:27612488 DOI:10.1136/gutjnl-2016-312332]
11 Iacucci M,Kaplan GG,Panaccione R,Akinola O,Lethebe BC,Lowerison M,Leung Y,Novak KL,Seow CH,Urbanski S,Minoo P,Gui X,Ghosh S.A Randomized Trial Comparing High Definition Colonoscopy Alone With High Definition Dye Spraying and Electronic Virtual Chromoendoscopy for Detection of Colonic Neoplastic Lesions During IBD Surveillance Colonoscopy.
2018;113:225-234[PMID:29134964 DOI:10.1038/ajg.2017.417]
12 Shinagawa T,Hata K,Morikawa T,Takiyama H,Emoto S,Murono K,Kaneko M,Sasaki K,Nishikawa T,Tanaka T,Kawai K,Fukayama M,Nozawa H.Pine-cone and villi patterns are endoscopic signs suggestive of ulcerative colitisassociated colorectal cancer and dysplasia.
2019;89:565-575.e3[PMID:30326231 DOI:10.1016/j.gie.2018.09.037]
13 Aladrén BS,González-Lama Y,García-Alvarado M,Sierra M,Barrio JA,Vicente VP,Hernández L,Velayos B,Garcia LA,Relea L,Suarez P,Atienza R,Vásquez M,Fernández-Salazar L,Muñoz F;GEICYL(group of inflammatory bowel disease of Castilla y León).Even non-experts identify non-dysplastic lesions in inflammatory bowel disease
chromoendoscopy:results of a screening program in real-life.
2019;7:E743-E750[PMID:31157291 DOI:10.1055/a-0839-4514]
14 Bisschops R,Bessissow T,Dekker E,East JE,Para-Blanco A,Ragunath K,Bhandari P,Rutter M,Schoon E,Wilson A,John JM,Van Steen K,Baert F,Ferrante M.Pit pattern analysis with high-definition chromoendoscopy and narrow-band imaging for optical diagnosis of dysplasia in patients with ulcerative colitis.
2017;86:1100-1106.e1[PMID:28986266 DOI:10.1016/j.gie.2017.09.024]
15 Hata K,Watanabe T,Motoi T,Nagawa H.Pitfalls of pit pattern diagnosis in ulcerative colitis-associated dysplasia.
2004;126:374-376[PMID:14753219 DOI:10.1053/j.gastro.2003.05.020]
16 Riddell RH,Goldman H,Ransohoff DF,Appelman HD,Fenoglio CM,Haggitt RC,Ahren C,Correa P,Hamilton SR,Morson BC.Dysplasia in inflammatory bowel disease:standardized classification with provisional clinical applications.
1983;14:931-968[PMID:6629368 DOI:10.1016/s0046-8177(83)80175-0]
17 East JE,Suzuki N,von Herbay A,Saunders BP.Narrow band imaging with magnification for dysplasia detection and pit pattern assessment in ulcerative colitis surveillance:a case with multiple dysplasia associated lesions or masses.
2006;55:1432-1435[PMID:16966701 DOI:10.1136/gut.2005.087171]
18 Ikebata A,Shimoda M,Okabayashi K,Uraoka T,Maehata T,Sugimoto S,Mutaguchi M,Naganuma M,Kameyama K,Yahagi N,Kanai T,Kitagawa Y,Kanai Y,Iwao Y.Demarcated redness associated with increased vascular density/size:a useful marker of flat-type dysplasia in patients with ulcerative colitis.
2021;9:E552-E561[PMID:33860072 DOI:10.1055/a-1352-2709]
19 Kinoshita S,Uraoka T,Nishizawa T,Naganuma M,Iwao Y,Ochiai Y,Fujimoto A,Goto O,Shimoda M,Ogata H,Kanai T,Yahagi N.The role of colorectal endoscopic submucosal dissection in patients with ulcerative colitis.
2018;87:1079-1084[PMID:29122603 DOI:10.1016/j.gie.2017.10.035]
20 Matsumoto T,Kudo T,Jo Y,Esaki M,Yao T,Iida M.Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis:a pilot study.
2007;66:957-965[PMID:17826773 DOI:10.1016/j.gie.2007.04.014]
21 Nishiyama S,Oka S,Tanaka S,Sagami S,Hayashi R,Ueno Y,Arihiro K,Chayama K.Clinical usefulness of narrow band imaging magnifying colonoscopy for assessing ulcerative colitis-associated cancer/dysplasia.
2016;4:E1183-E1187[PMID:27853744 DOI:10.1055/s-0042-116488]
22 Lv XH,Wang BL,Cao GW.Narrow Band Imaging for Surveillance in Inflammatory Bowel Disease:A Systematic Review and Meta-Analysis.
2019;53:607-615[PMID:30096061 DOI:10.1097/MCG.0000000000001101]
23 Kawasaki K,Nakamura S,Esaki M,Kurahara K,Eizuka M,Nuki Y,Kochi S,Fujiwara M,Oshiro Y,Sugai T,Matsumoto T.Clinical usefulness of magnifying colonoscopy for the diagnosis of ulcerative colitis-associated neoplasia.
2019;31 Suppl 1:36-42[PMID:30994234 DOI:10.1111/den.13382]
24 Kudo SE,Wakamura K,Ikehara N,Mori Y,Inoue H,Hamatani S.Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study.
2011;43:869-875[PMID:21837586 DOI:10.1055/s-0030-1256663]
25 Kudo SE,Misawa M,Wada Y,Nakamura H,Kataoka S,Maeda Y,Toyoshima N,Hayashi S,Kutsukawa M,Oikawa H,Mori Y,Ogata N,Kudo T,Hisayuki T,Hayashi T,Wakamura K,Miyachi H,Ishida F,Inoue H.Endocytoscopic microvasculature evaluation is a reliable new diagnostic method for colorectal lesions(with video).
2015;82:912-923[PMID:26071058 DOI:10.1016/j.gie.2015.04.039]
26 Kudo SE,Maeda Y,Ogata N,Misawa M,Ogawa Y,Takishima K,Ishiyama M,Mochizuki K,Minegishi Y,Ogura Y,Abe M,Okumura T,Matsudaira S,Ishigaki T,Sasanuma S,Mori Y,Kudo T,Hayashi T,Wakamura K,Miyachi H,Baba T,Ishida F,Nemoto T,Hamatani S,Ohtsuka K.Combined endocytoscopy with pit pattern diagnosis in ulcerative colitisassociated neoplasia:Pilot study.
2021[PMID:33641190 DOI:10.1111/den.13964]
27 Fukunaga S,Kusaba Y,Ohuchi A,Nagata T,Mitsuyama K,Tsuruta O,Torimura T.Is artificial intelligence a superior diagnostician in ulcerative colitis?
2021;53:E75-E76[PMID:32590852 DOI:10.1055/a-1195-1986]
28 Mohan BP,Khan SR,Chandan S,Kassab LL,Ponnada S,Asokkumar R,Shen B,Iacucci M,Navaneethan U.Endoscopic resection of colon dysplasia in patients with inflammatory bowel disease:a systematic review and meta-analysis.
2021;93:59-67.e10[PMID:32592777 DOI:10.1016/j.gie.2020.06.048]
29 Chen W,Zhang YL,Zhao Y,Yang AM,Qian JM,Wu D.Endoscopic resection for non-polypoid dysplasia in inflammatory bowel disease:a systematic review and meta-analysis.
2021;35:1534-1543[PMID:33523273 DOI:10.1007/s00464-020-08225-9]
30 Manta R,Zullo A,Telesca DA,Castellani D,Germani U,Reggiani Bonetti L,Conigliaro R,Galloro G.Endoscopic Submucosal Dissection for Visible Dysplasia Treatment in Ulcerative Colitis Patients:Cases Series and Systematic Review of Literature.
2021;15:165-168[PMID:32710744 DOI:10.1093/ecco-jcc/jjaa158]
31 Matsui A,Hoteya S,Hayasaka J,Yamashita S,Ochiai Y,Suzuki Y,Fukuma Y,Okamura T,Mitsunaga Y,Tanaka M,Nomura K,Dan N,Odagiri H,Kikuchi D.Real-World Experience of Endoscopic Submucosal Dissection for Ulcerative Colitis-Associated Neoplasia.
2021;6:70-77[DOI:10.1159/000512292]
32 Matsumoto K,Oka S,Tanaka S,Tanaka H,Boda K,Yamashita K,Sumimoto K,Ninomiya Y,Arihiro K,Shimamoto F,Chayama K.Long-Term Outcomes after Endoscopic Submucosal Dissection for Ulcerative Colitis-Associated Dysplasia.
2021;102:205-215[DOI:10.1159/000503341]
33 Kasuga K,Yamada M,Shida D,Tagawa T,Takamaru H,Sekiguchi M,Sakamoto T,Uraoka T,Sekine S,Kanemitsu Y,Saito Y.Treatment outcomes of endoscopic submucosal dissection and surgery for colorectal neoplasms in patients with ulcerative colitis.
2021[DOI:10.1002/ueg2.12118]
34 Nishio M,Hirasawa K,Ozeki Y,Sawada A,Ikeda R,Fukuchi T,Kobayashi R,Makazu M,Sato C,Kunisaki R,Maeda S.An endoscopic treatment strategy for superficial tumors in patients with ulcerative colitis.
2021;36:498-506[DOI:10.1111/jgh.15207]
35 Lightner AL,Vaidya P,Allende D,Gorgun E.Endoscopic submucosal dissection is safe and feasible,allowing for ongoing surveillance and organ preservation in patients with inflammatory bowel disease.
2021;23:2100-2107[PMID:34021968 DOI:10.1111/codi.15746]
36 Laine L,Kaltenbach T,Barkun A,McQuaid KR,Subramanian V,Soetikno R;SCENIC Guideline Development Panel.SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease.
2015;148:639-651.e28[PMID:25702852 DOI:10.1053/j.gastro.2015.01.031]
 World Journal of Gastrointestinal Oncology2022年3期
World Journal of Gastrointestinal Oncology2022年3期
- World Journal of Gastrointestinal Oncology的其它文章
- lnflammatory bowel disease-related colorectal cancer:Past,present and future perspectives
- Barrett’s esophagus:Review of natural history and comparative efficacy of endoscopic and surgical therapies
- Gut and liver involvement in pediatric hematolymphoid malignancies
- Pathological,molecular,and clinical characteristics of cholangiocarcinoma:A comprehensive review
- Clinical significance of molecular subtypes of gastrointestinal tract adenocarcinoma
- Colorectal cancer carcinogenesis:From bench to bedside
