Epoxiconazole Improved Photosynthesis, Yield Formation, Grain Quality and 2-Acetyl-1-Pyrroline Biosynthesis of Fragrant Rice
Luo Haowen, He Longxin, Du Bin, Pan Shenggang, Mo Zhaowen, Yang Shuying, Zou Yingbin, Tang Xiangru
Research Paper
Epoxiconazole Improved Photosynthesis, Yield Formation, Grain Quality and 2-Acetyl-1-Pyrroline Biosynthesis of Fragrant Rice
Luo Haowen1, 2, 3, He Longxin1, 2, 3, Du Bin1, 2, 3, Pan Shenggang1, 2, 3, Mo Zhaowen1, 2, 3, Yang Shuying4, Zou Yingbin4, Tang Xiangru1, 2, 3
(State Key Laboratory for Conservation and Utilization of Subtropical Agricultural Bioresources, South China Agricultural University, Guangzhou 510642, China; Scientific Observing and Experimental Station of Crop Cultivation in South China, Ministry of Agriculture and Rural Affairs, Guangzhou 510642, China; Guangzhou Key Laboratory for Science and Technology of Aromatic Rice, Guangzhou 510642, China; College of Agronomy, Hunan Agricultural University, Changsha 410128, China)
Epoxiconazole is a triazole compound. However, the effects of epoxiconazole on crop productivity and quality were rarely reported. In this study, we investigated the effects of epoxiconazole application on yield formation, grain quality attributes, and 2-acetyl-1-pyrroline (2-AP) content in fragrant rice. A three-year field experiment was carried out with a fragrant rice variety, Meixiangzhan 2. At the heading stage, 0, 0.02, 0.04, 0.08, 0.16 and 0.32 g/L epoxiconazole solutions were foliar applied to fragrant rice plants, respectively. The results showed that epoxiconazole application significantly increased grain yield, seed-setting rate and 1000-grain weight. Chlorophyll content and net photosynthetic rate of fragrant rice during the grain-filling stage significantly increased due to epoxiconazole application. Foliar application of epoxiconazole at 0.08 g/L increased grain protein content and decreased both chalky rice rate and chalkiness area ratio of fragrant rice. Epoxiconazole also substantially increased grain 2-AP content by inducing the regulation in contents of related synthetic precursors, including proline, pyrroline-5- carboxylic acid, ∆1-pyrroline and methylglyoxal. Overall, foliar application of epoxiconazole could be used for the improvement in grain yield, grain quality and 2-AP content in fragrant rice production when applied concentration at 0.08–0.32 g/L. Our findings provided the new roles of epoxiconazole in crop production.
epoxiconazole; fragrant rice; 2-acetyl-1-pyrroline; yield formation; grain quality
Epoxiconazole is a triazole that belongs to the group of azole compounds. Like other azole compounds, epoxiconazole is able to inhibit the synthesis of steroidergosterol, an important membrane component in fungi(van den Bossche et al, 1983). The application of epoxiconazole provides more than 75% control efficacy to rice blast (Chen et al, 2013). Epoxiconazole also has substantial effects on controlling wheat fusarium head blight (Liu S M et al, 2020; Zhao et al, 2021). Moreover, epoxiconazole is a pesticide with low environmental and health risk because more than 95% of applied epoxiconazole residues in straw, paddy water and soil would be degraded, and epoxiconazole residues in grain and straw would be undetectable (less than 0.01 mg/kg) at 28 d after application with the applied rate within 112.5 g/hm2(Yan et al, 2015; Zhao et al, 2021). Therefore, epoxiconazole is widely used as fungicides in cereals, grapes and other crops.
Besides the benefits of controlling fungal diseases, epoxiconazole can improve the growth and developmentof crops. Ajigboye et al (2014) showed that applicationof epoxiconazole enhances PSII efficiency and dry matteraccumulation of winter wheat in the environment without disease pressure, but Bertelsen et al (2001) showed that epoxiconazole has no significant effect on senescence, above-ground biomass, or yield of winter wheat without inoculating the saprophytic fungi. In addition, there are few studies on the effects of epoxiconazole on rice productivity, yield formation and rice quality.
Fragrant rice is well-known and popular for its goodquality and special aroma around the world (Wakte et al, 2017; Bao et al, 2018). 2-acetyl-1-pyrroline (2-AP) is the characteristic component of fragrant rice aroma. In the last decade, scientists found many ways to improveyield and quality of fragrant rice (Feng et al, 2019). For example, Mo et al (2019) indicated that alternate wetting and drying management combined with nitrogen fertilizer application substantially increases grain yield and 2-AP content of fragrant rice. Luo et al (2020) showed that foliar application of selenium not only enhances yield formation and 2-AP biosynthesis, but also improves the grain quality of fragrant rice. Xie et al (2020) revealed that exogenous γ-aminobutyric acid increases dry matter accumulation and grain 2-AP concentration of fragrant rice. Li et al (2016) also demonstrated higher grain yield and 2-AP content, as well as better grain quality of fragrant rice, are received after application of manganese fertilizer. Despite numerous studies about fragrant rice, it is unknown whether epoxiconazole couldaffect the productivity, 2-AP biosynthesis or grain quality attributes of fragrant rice without disease pressure.
To study the effects of epoxiconazole on yield formation, grain quality attributes and 2-AP biosynthesis,we conducted a three-year field experiment in Guangdong Province, China with the hypothesis that grain yield, grain quality or 2-AP content of fragrant rice could be regulated by foliar application of epoxiconazole.
Results
Grain yield and yield-related traits
The fragrant rice yield significantly increased due to the application of epoxiconazole during all the three experimental years (Table 1). In 2018, 9.59%, 10.62% and 9.76% higher grain yields were recorded in EP3 (foliar spray with 0.08 g/L epoxiconazole), EP4 (foliar spray with 0.16 g/L epoxiconazole) and EP5 (foliar spray with 0.32 g/L epoxiconazole) treatments than in CK (foliar spray with distilled water). In 2019, compared with CK, EP3, EP4 and EP5 treatments significantly increased grain yield by 7.95%, 6.77%, and 10.66%. In 2020, 11.61%, 13.00% and 12.65% higher grain yields were recorded in EP3, EP4 and EP5 treatments than in CK. Additionally, 4.73%–8.90% higher seed-setting rate and 2.46%–7.20% higher 1000-grain weight were recorded in EP3, EP4 and EP5 treatments related to CK during all the three experimental years. There was no significant difference among all the treatments in number of effective panicles per square meter and number of grains per panicle.

Table 1. Effects of epoxiconazole on grain yield and yield related traits of fragrant rice.
CK, Foliar spray with distilled water; EP1, Foliar spray with 0.02 g/L epoxiconazole; EP2, Foliar spray with 0.04 g/L epoxiconazole; EP3, Foliar spray with 0.08 g/L epoxiconazole; EP4, Foliar spray with 0.16 g/L epoxiconazole; EP5, Foliar spray with 0.32 g/L epoxiconazole.
Data are Mean ± SE (= 3). Values with different lowercase letters within a column in each year indicate significant difference (< 0.05) according to the least significant difference test.
Chlorophyll content
The application of epoxiconazole significantly increased the chlorophyll contents at the grain-filling stage of fragrant rice leaves in the three experimental years (Fig. 1-A to -C). In comparison with CK, EP3, EP4 and EP5 treatments significantly (< 0.05) increased soil and plant analyzer development (SPAD) values by 8.78%–10.15%, 7.74%–10.27% and 7.65%– 12.87%, respectively, at 7 d of receiving treatments, while by 11.82%–13.00%, 9.84%–12.88% and 10.15%– 15.55% at 14 d of receiving treatments, 16.31%–18.24%,13.78%–17.66% and 14.32%–20.73% at 21 d of receiving treatments, and 16.40%–21.47%, 14.57%–23.13% and 14.55%–28.15% at 28 d of receiving treatments.
Net photosynthetic rate
The application of epoxiconazole significantly enhanced the net photosynthetic rates of fragrant rice in three experimental years (Fig. 1-D to -F). In comparison with CK, EP3, EP4 and EP5 treatments significantly improved net photosynthetic rates by 9.15%–11.83%, 10.33%–12.29% and 9.52%–14.66%, respectively, at 7 d of receiving treatments, while by 12.51%–13.87%, 10.34%–16.41% and 10.30%–19.22% at 14 d of receiving treatments, 12.84%–13.00%, 10.43%–14.30% and 13.25%–16.68% at 21 d of receiving treatments, and 22.05%–28.37%, 18.28%–28.09% and 19.94%– 28.64% at 28 d of receiving treatments.
Grain 2-AP content
Foliar application of epoxiconazole significantly increased grain 2-AP contents at the harvest stage of fragrant rice in all the three experimental years (Fig. 2). In 2018, 8.18%, 13.29%, 16.40% and 18.08% higher 2-AP contents were recorded in EP2 (foliar spray with 0.04 g/L epoxiconazole), EP3, EP4 and EP5 treatments than in CK. In 2019, 5.44%, 12.12%, 14.49% and 18.58% higher 2-AP contents were recorded in EP2, EP3, EP4 and EP5 treatments than in CK. In 2020, 7.96%, 11.82%, 10.71% and 12.49% higher 2-AP contents were recorded in EP2, EP3, EP4 and EP5 treatments than in CK.
Contents of precursors related to 2-AP biosynthesis
The contents of synthetic precursors [proline, pyrroline-5-carboxylic acid (P5C), ∆1-pyrroline and methylglyoxal (MG)] of 2-AP in fragrant rice were significantly regulated by epoxiconazole application in the three experimental years (Table 2). In comparison with CK, EP3, EP4 and EP5 treatments significantly increased grain proline contents by 44.19%–48.89%, 35.43%–50.81% and 45.79%–57.59%, respectively, P5C contents by 22.73%–37.27%, 20.00%–29.09% and 16.36%–21.90%, respectively, and grain ∆1-pyrroline contents by 10.42%–16.30%, 21.88%–23.91% and 19.79%–24.47%, while decreased grain MG contents by 17.52%–21.80%, 21.10%– 26.13% and 22.41%–26.09%, respectively.

Fig. 1. Effects of epoxiconazole on soil and plant analyzer development (SPAD) value (A–C) and net photosynthetic rate (D–F) at grain-filling stage in fragrant rice.
CK, Foliar spray with distilled water; EP1, Foliar spray with 0.02 g/L epoxiconazole; EP2, Foliar spray with 0.04 g/L epoxiconazole; EP3, Foliar spray with 0.08 g/L epoxiconazole; EP4, Foliar spray with 0.16 g/L epoxiconazole; EP5, Foliar spray with 0.32 g/L epoxiconazole.
Data are Mean ± SE (= 3). Different lowercase letters above the error bars indicate significant difference (< 0.05) among different treatments.
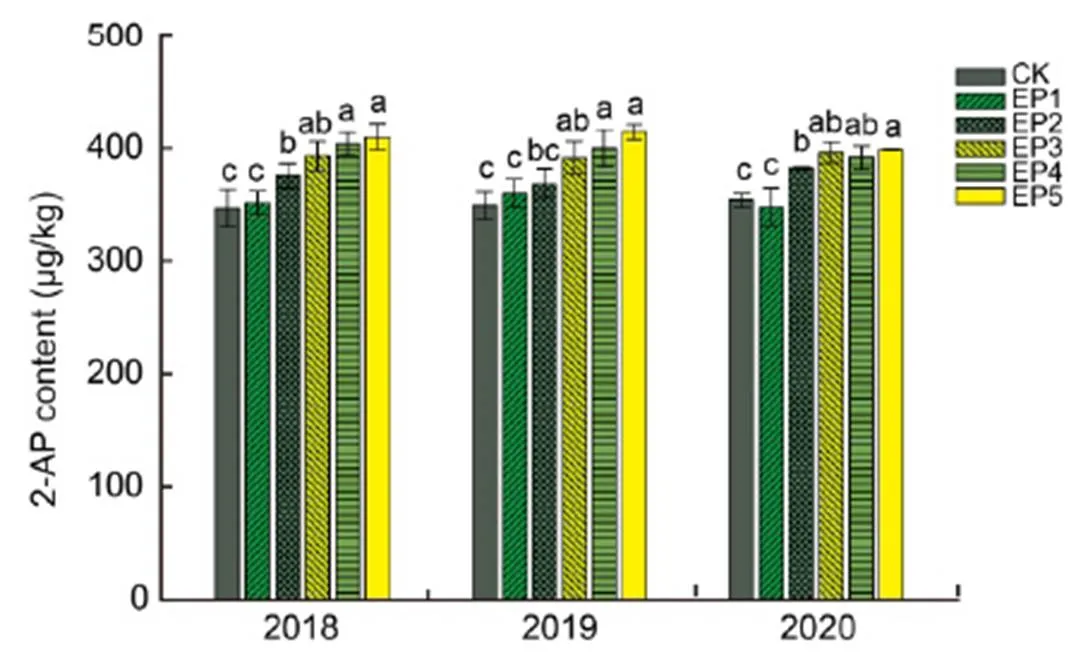
Fig. 2. Effects of epoxiconazole on 2-acetyl-1-pyrroline (2-AP) content at harvest stage of fragrant rice.
CK, Foliar spray with distilled water; EP1, Foliar spray with 0.02 g/L epoxiconazole; EP2, Foliar spray with 0.04 g/L epoxiconazole; EP3, Foliar spray with 0.08 g/L epoxiconazole; EP4, Foliar spray with 0.16 g/L epoxiconazole; EP5, Foliar spray with 0.32 g/L epoxiconazole.
Data are Mean ± SE (= 3). Different lowercase letters above the error bars indicate significant difference (< 0.05) among different treatments.
Grain quality attributes
Some grain quality attributes of fragrant rice were substantially influenced by epoxiconazole application. There was no significant difference between CK with all epoxiconazole treatments in brown rice rate, milled rice rate and head rice rate (Table 3). Compared with CK, EP3 treatment significantly increased grain protein contents by 5.44%–6.11%. Compared with CK, EP5 treatment significantly increased grain amylose contents by 2.51%–4.52%, and decreased grain protein content by 5.07%–6.14% in the three experimental years. Compared with CK, EP3 treatment significantly decreased the chalky rice rate and chalkiness area by 27.21%–38.59% and 21.55%–27.66%, respectively, in the three experimental years, while EP5 treatment significantly increased the chalky rice rate by 15.89% and 17.79% in 2018 and 2019, respectively. Moreover, compared with CK, EP5 treatment significantly increased chalkiness degree in 2020.
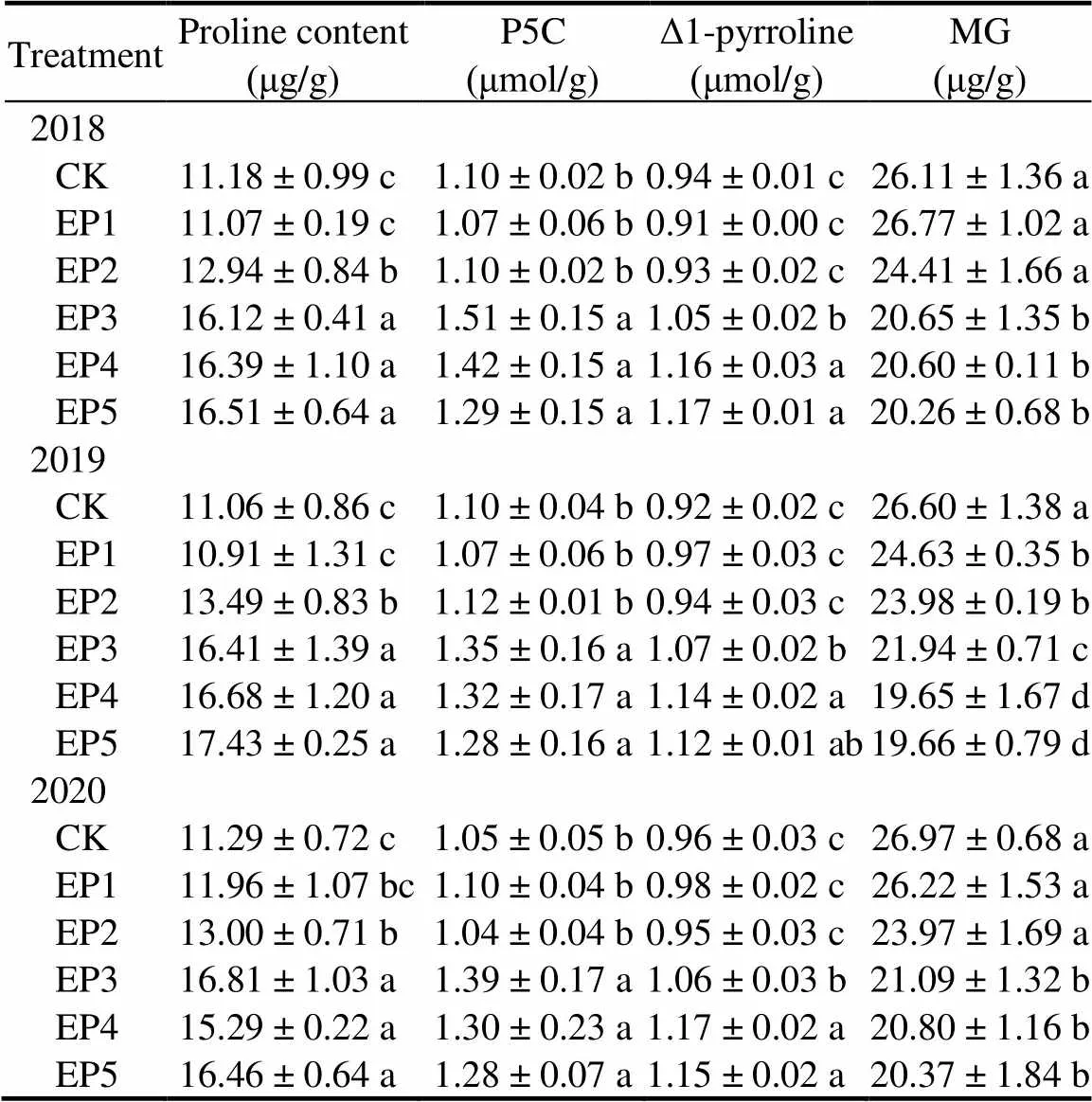
Table 2. Effects of epoxiconazole on contents of proline, pyrroline- 5-carboxylic acid (P5C), ∆1-pyrroline andmethylglyoxal (MG) in fragrant rice.
CK, Foliar spray with distilled water; EP1, Foliar spray with 0.02 g/L epoxiconazole; EP2, Foliar spray with 0.04 g/L epoxiconazole; EP3, Foliar spray with 0.08 g/L epoxiconazole; EP4, Foliar spray with 0.16 g/L epoxiconazole; EP5, Foliar spray with 0.32 g/L epoxiconazole.
Data are Mean ± SE (= 3). Values with different lowercase letter within a column in each year indicate significant difference (< 0.05) according to the least significant difference test.
Discussion
In the past, almost all studies on epoxiconazole focused on the controlling effects of fungal disease, toxicity or degradation (Kaziem et al, 2020, 2021; Liu S M et al, 2020). The current study firstly discovered the beneficial effects of epoxiconazole application on yield formation, grain quality attributes, and aroma biosynthesis in fragrant rice without disease pressure. During all the three experimental years, the application of epoxiconazole increased grain yield of fragrant rice (Table 1), which indicated that foliar application of epoxiconazole at the heading stage can increase fragrant rice yield by about 10%. Our results are similar to the results of Ajigboye et al (2014), who demonstrated that foliar application of epoxiconazole increases winter wheat yield without disease pressure. The increments in grain yield were attributed to the increased seed-setting rate and 1000-grain weight because compared with control, epoxiconazole treatmentssignificantly increased seed-setting rate and 1000-grain weight in all the three experimental years. On the other hand, epoxiconazole application did not significantly influence number of effective panicles per square meter and number of grains per panicle, which might due to epoxiconazole was foliar applied at the heading stage when the processes of effective panicle and panicle differentiation have been completed (Alam et al, 2015; Pan et al, 2017; Zhang et al, 2017).

Table 3. Effects of epoxiconazole on grain quality attributes of fragrant rice.
CK, Foliar spray with distilled water; EP1, Foliar spray with 0.02 g/L epoxiconazole; EP2, Foliar spray with 0.04 g/L epoxiconazole; EP3, Foliar spray with 0.08 g/L epoxiconazole; EP4, Foliar spray with 0.16 g/L epoxiconazole; EP5, Foliar spray with 0.32 g/L epoxiconazole.
Data are Mean ± SE (= 3). Values with different lowercase letters within a column in each year indicate significant difference (< 0.05) according to the least significant difference test.
As far as photosynthesis was concerned, foliar application of epoxiconazole at the heading stage substantially increased chlorophyll content and enhanced net photosynthetic rate at the grain-filling stage, which might be the reason for the improvements in fragrant rice yield under epoxiconazole treatments. From 14 to 28 d of receiving the treatments, the chlorophyll content and net photosynthetic rate were gradually declined but they were still at a higher level under EP3, EP4 and EP5 treatments compared with CK at the late phase of the grain-filling stage (21 and 28 d after heading stage). Luo et al (2019) showed that the photosynthesis during the grain-filling stage has substantial effects on seed-setting rate, 1000-grain weight and grain yield of fragrant rice. Kong et al (2017) showed that declines in chlorophyll content and photosynthetic rate cause the decrement in rice yield. Huang et al (2015) indicated that carbon assimilation is the main way for the biomass accumulation of rice. Duan et al (2019) also revealed that delaying the leaf senescence at the grain-filling stage is an effective way to increase the grain yield of fragrant rice. In this study, we concluded that exogenous epoxiconazole enhanced the yield formation of fragrant rice by increasing chlorophyll content and enhancing the net photosynthetic rate at the grain-filling stage, especially the late phase. The higher chlorophyll content at the late grain-filling stage under epoxiconazole treatments also indicated that epoxiconazole delayed the leaf senescence or chlorophyll degradation. But more studies should be conducted at the physiological or molecular level to investigate the effects of epoxiconazole on photosynthesis, chlorophyll biosynthesis and degradation.
2-AP is the key and main component of fragrant rice aroma. The results of this study showed that foliar application of epoxiconazole remarkably increased the grain 2-AP content and induced regulation in related precursor (proline, P5C, MG and ∆1-pyrroline) contents in fragrant rice. The biosynthesis of 2-AP in fragrant rice is a complicated phenomenon that involves many substances (Wakte et al, 2017; Pan et al, 2021). Yoshihashi et al (2002) found that 2-AP are formed in the aboveground tissue of fragrant rice from proline as the nitrogen precursor. Poonlaphdecha et al (2016) revealed that ∆1-pyrroline is a limiting substrate in 2-AP biosynthesis in fragrant rice. Many previous studies indicated that in fragrant rice, proline is firstly converted to P5C, and then P5C is converted to pyrroline, and finally, pyrroline reacts with acetone aldehyde to produce 2-AP (Wakte et al, 2011; Li et al, 2016; Bao et al, 2018; Mo et al, 2019). In this study, epoxiconazole application increased the contents of proline, P5C and ∆1-pyrroline, and decreased the MG contents in fragrant rice, which indicated that the foliar application of epoxiconazole enhanced the biosynthesis of proline and led to the increments in P5C content and ∆1-pyrroline content, and then the consumption of MG was accelerated due to the improvement of 2-AP synthesis. Besides the aroma, the rice quality includes cooking, appearance, eating and nutrient qualities (Zhang et al, 2008). The results of this studyshowed that foliar application of 0.08 g/L epoxiconazolesignificantly increased grain protein contentand decreased chalky rice rate and chalkiness area ratio, while epoxiconazole application at 0.32 g/L decreased grain protein content and increased the grain amylose content. Zhao et al (2020) indicated that high amylose content increases hardness and reduces stickiness and thus influences the texture of rice.
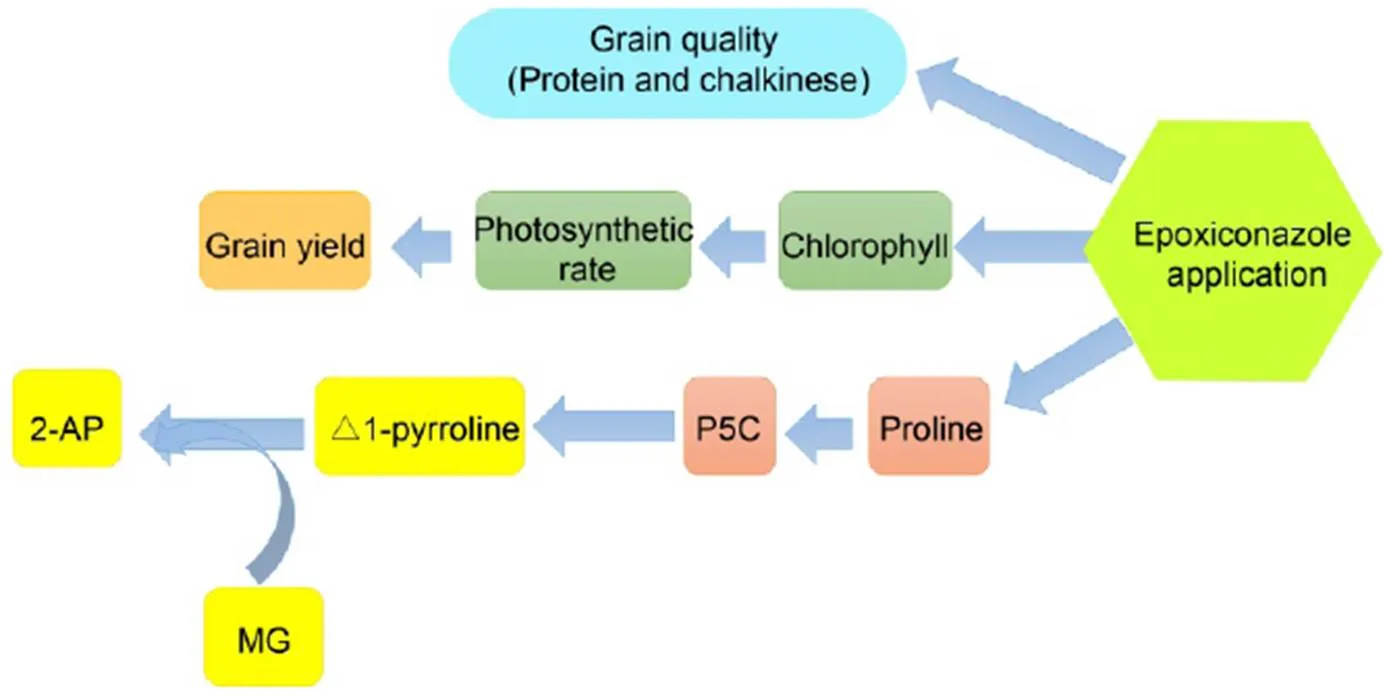
Fig. 3. Multiple roles of epoxiconazole in fragrant rice.
2-AP, 2-acetyl-1-pyrroline; P5C, Pyrroline-5-carboxylic acid; MG, Methylglyoxal.
In conclusion, foliar application of epoxiconazole at 0.08–0.32 g/L substantially increased grain yield and 2-AP content of fragrant rice. The multiple roles of epoxiconazole in fragrant rice performances, including photosynthesis, yield formation, grain quality attributes and 2-AP biosynthesis are shown in Fig. 3. Moreover, no epoxiconazole residue was detectedin paddy water, soil and grains in the present experiment. Therefore, epoxiconazole could be considered as a plant growth regulator to regulate the yield, grain quality and 2-AP content of fragrant rice.
methods
Plant growth conditions and experimental design
A field study was conducted from July to November 2018 and repeated in 2019 and 2020 at the Experimental Research Farm, South China Agricultural University, at Ningxi county (23º16′ N, 113º22′ E and 11 m from the mean sea level), Guangdong Province, China. The place enjoys a subtropical monsoon climate. The rice grown in this season is regarded as ‘late-season rice’ in the double rice cropping system of South China. The experimental paddy soil was sandy loam consisting of 16.39 g/kg organic matter, 58.96 mg/kg available nitrogen, 18.07 mg/kg available phosphorus, and 133.65 mg/kg available potassium with a soil pH of 6.63.
Seeds of fragrant rice variety, Meixiangzhan 2 (Lemont × Fengaozhan), widely planted in South China, were used as the rice material. After the nursery in the greenhouse, 14-day-old seedlings were mechanically transplanted to a paddy field with a distance of 15 cm × 30 cm. At the heading stage, 0, 0.02, 0.04, 0.08, 0.16 and 0.32 g/L epoxiconazole solutions were foliar applied to fragrant rice plants at the dose of 225 L/hm2, respectively. Those treatments were named as CK, EP1, EP2, EP3, EP4 and EP5 and arranged in a randomized complete block design in triplicate with a net plot size of 8.5 m × 3.0 m. The epoxiconazole was first dissolved in a small amount of ethanol (7 mL/L) and then diluted to the corresponding volume with distilled water before application in each treatment. Ten days after treatment, fresh grains from each treatment were collected and stored at -80 ºC for physio-biochemical analysis. At harvest, fresh and mature grains were also collected and stored at -80 ºC for the determination of 2-AP content. All agronomic practices i.e., pest and disease management and weed control, were the same in all treatments by following the guidelines and standards recommended by the province, and no other chemical pesticides or fertilizers were applied at the heading stage. There was no obvious disease during the whole experiment process.
Determination of chlorophyll content and net photosynthetic rate
After 7, 14, 21 and 28 d of receiving treatment, the net photosynthetic rate was determined according to Kong et al (2017) with the portable photosynthesis system (LI-6400, LI-COR, USA). Meanwhile, leaf chlorophyll content was determined using a SPAD meter ‘SPAD-502’ (Konica Minolta, Japan).
Determination of grain yield and yield-related traits
Fragrant rice plants were harvested at 30 d after treatment, and the determinations of grain yield and yield-related traits were carried out according to Luo et al (2020). The rice grains were harvested from three sampling areas (1 m2) in each plot then threshed by machine, and the harvested grains were sun-dried and weighed to determine the grain yield. Another three sampling areas from each plot were selected to estimate the average effective panicle number per square meter. Nine representative hills from each plot were sampled to measure the number of grains per panicle and seed-setting rate using a Rice Digital Seed Testing machine (YTS-5D, Red Star Yang Technology, China). The filled grains were weighted and 1000-grain weights were calculated.
Determination of grain 2-AP and related precursor contents
The grain 2-AP content was determined using the method of Liu X W et al (2020) with the simultaneous distillation-extraction method and analyzed by a GCMS-QP 2010 Plus (Shimadzu Corporation, Japan). The proline content of grains was determined after boiling in the water bath with ninhydrin in acid condition according to Mo et al (2015). The P5C content and Δ1-pyrroline content were determined using the methods of Bao et al (2018), and both were expressed as μmol/g. The MG content was determined according to Banu et al (2010) after reacting with 1,2-diaminobenzene and perchloric acid. The absorbance was read at 336 nm. The content was calculated according to the standard curve and expressed as μg/g.
Determination of grain quality
The determination of grain quality of fragrant rice was carried out according to Kong et al (2017). After sun-drying, grains were stored at room temperature for at least three months. Then, rice grains from each treatment were taken from storage and brown rice rate was estimated using a rice huller (Beijing, China) while milled rice and head rice rates were calculated by using a Jingmi testing rice grader (Hangzhou, China). Grains with chalkiness area ratio and chalkiness degree were estimated by using an SDE-A light box (Guangzhou, China) while the grain amylose and protein contents were determined by an Infratec-1241 grain analyzer (FOSS-TECATOR, Shanghai, China).
Determination of epoxiconazole residues
At the harvest stage, the paddy water and soil, as well as grains, were collected to determine the epoxiconazole content using HPLC-MS/MS analysis which was performed on a 12000SL HPLC system with an Agilent G6410A triple quadrupole mass spectrometer (Agilent Technologies, CA, USA) according to Yan et al (2015). The contents of epoxiconazole in paddy water, soil and grains were too low to be detected, so data regarding epoxiconazole residues have not been presented.
Statistical analysis
Experimental data were analyzed using statistical software ‘Statistix 8.1’ (Analytical Software, FL, USA) to test the effects of epoxiconazole application on fragrant rice performances. The means of three repetitions were separated using the Tukey’s least significant difference (LSD) test at the 0.05 level.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 31971843), the Technology System of Modern Agricultural Industry in Guangdong (Grant No. 2020KJ105) and Guangzhou Science and Technology Project in China (Grant No. 202103000075).
Ajigboye O O, Murchie E, Ray R V. 2014. Foliar application of isopyrazam and epoxiconazole improves photosystem II efficiency, biomass and yield in winter wheat., 114: 52‒60.
Alam M M, Tanaka T, Nakamura H, Ichikawa H, Kobayashi K, Yaeno T, Yamaoka N, Shimomoto K, Takayama K, Nishina H, Nishiguchi M. 2015. Overexpression of a rice heme activator protein gene () confers resistance to pathogens, salinity and drought, and increases photosynthesis and tiller number., 13(1): 85‒96.
Banu M N A, Hoque M A, Watanabe-Sugimoto M, Islam M M, Uraji M, Matsuoka K, Nakamura Y, Murata Y. 2010. Proline and glycinebetaine ameliorated NaCl stressscavenging of hydrogen peroxide and methylglyoxal but not superoxide or nitric oxide in tobacco cultured cells., 74(10): 2043‒2049.
Bao G G, Ashraf U, Wang C L, He L X, Wei X S, Zheng A X, Mo Z W, Tang X R. 2018. Molecular basis for increased 2-acetyl- 1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice., 133: 149‒157.
Bertelsen J R, de Neergaard E, Smedegaard-Petersen V. 2001. Fungicidal effects of azoxystrobin and epoxiconazole on phyllosphere fungi, senescence and yield of winter wheat., 50(2): 190‒205.
Chen Y, Yao J, Wang W X, Gao T C, Yang X, Zhang A F. 2013. Effect of epoxiconazole on rice blast and rice grain yield in China., 135(4): 675‒682.
Duan M Y, Cheng S R, Lu R H, Lai R F, Zheng A X, Ashraf U, Fan P S, Du B, Luo H W, Tang X R. 2019. Effect of foliar sodium selenate on leaf senescence of fragrant rice in South China., 17(2): 3343‒3351.
Feng H Y, Jiang H L, Wang M, Tang X R, Duan M Y, Pan S G, Tian H, Wang S L, Mo Z W. 2019. Morphophysiological responses of different scented rice varieties to high temperature at seedling stage., 33(1): 68‒74. (in Chinese with English abstract)
Huang L C, Dai L P, Wang L, Leng Y J, Yang Y L, Xu J, Hu J, Rao Y C, Zhang G H, Zhu L, Dong G J, Guo L B, Qian Q, Zeng D L. 2015. Genetic dissection for chlorophyll content of the top three leaves during grain filling in rice (L.)., 34(2): 381‒391.
Kaziem A E, Gao B B, Li L S, Zhang Z X, He Z Z, Wen Y, Wang M H. 2020. Enantioselective bioactivity, toxicity, and degradation in different environmental mediums of chiral fungicide epoxiconazole., 386: 121951.
Kaziem A E, He Z Z, Li L S, Wen Y, Wang Z, Gao Y Y, Wang M H. 2021. Changes in soil and rat gut microbial diversity after long-term exposure to the chiral fungicide epoxiconazole., 272: 129618.
Kong L L, Ashraf U, Cheng S R, Rao G S, Mo Z W, Tian H, Pan S G, Tang X R. 2017. Short-term water management at early filling stage improves early-season rice performance under high temperature stress in South China., 90: 117‒126.
Li M J, Ashraf U, Tian H, Mo Z W, Pan S G, Anjum S A, Duan M Y, Tang X R. 2016. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice., 103: 167‒175.
Liu S M, Fu L Y, Chen J P, Wang S, Liu J L, Jiang J, Che Z P, Tian Y E, Chen G Q. 2020. Baseline sensitivity and control efficacy of epoxiconazole againstin Henan Province, China., 157: 825‒833.
Liu X W, Huang Z L, Li Y Z, Xie W J, Li W, Tang X R, Ashraf U, Kong L L, Wu L M, Wang S L, Mo Z W. 2020. Selenium- silicon (Se-Si) induced modulations in physio-biochemical responses, grain yield, quality, aroma formation and lodging in fragrant rice., 196: 110525.
Luo H W, He L X, Du B, Wang Z M, Zheng A X, Lai R F, Tang X R. 2019. Foliar application of selenium (Se) at heading stage induces regulation of photosynthesis, yield formation, and qualitycharacteristics in fragrant rice., 57(4): 1007‒1014.
Luo H W, He L X, Du B, Pan S G, Mo Z W, Duan M Y, Tian H, Tang X R. 2020. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation., 255: 107909.
Mo Z W, Li W, Pan S G, Fitzgerald T L, Xiao F, Tang Y J, Wang Y L, Duan M Y, Tian H, Tang X R. 2015. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice., 8: 9.
Mo Z W, Li Y H, Nie J, He L X, Pan S G, Duan M Y, Tian H, Xiao L Z, Zhong K Y, Tang X R. 2019. Nitrogen application and different water regimes at booting stage improved yield and 2-acetyl- 1-pyrroline (2AP) formation in fragrant rice., 12(1): 74.
Pan S G, Wen X C, Wang Z M, Ashraf U, Tian H, Duan M Y, Mo Z W, Fan P S, Tang X R. 2017. Benefits of mechanized deep placement of nitrogen fertilizer in direct-seeded rice in South China., 203: 139‒149.
Pan Y Y, Chen Y B, Wang C R, Li H, Huang D Q, Zhou D G, Wang Z D, Zhao L, Gong R, Zhou S C. 2015. Metabolism of γ-aminobutyrate and 2-acetyl-1-pyrroline analyses at various grain developmental stages in rice (L.)., 35(2): 121‒129. (in Chinese with English abstract)
Poonlaphdecha J, Gantet P, Maraval I, Sauvage F X, Menut C, Morère A, Boulanger R, Wüst M, Gunata Z. 2016. Biosynthesis of 2-acetyl-1-pyrroline in rice calli cultures: Demonstration of 1-pyrroline as a limiting substrate., 197: 965‒971.
van den Bossche H, Willemsens G, Cools W, Marichal P, Lauwers W. 1983. Hypothesis on the molecular basis of the antifungal activity of-substituted imidazoles and triazoles., 11(6): 665‒667.
Wakte K, Zanan R, Hinge V, Khandagale K, Nadaf A, Henry R. 2017. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (L.): A status review., 97(2): 384‒395.
Wakte K V, Kad T D, Zanan R L, Nadaf A B. 2011. Mechanism of 2-acetyl-1-pyrroline biosynthesis inRoxb. flowers., 17(3): 231‒237.
Xie W J, Kong L L, Ma L, Ashraf U, Pan S G, Duan M Y, Tian H, Wu L M, Tang X R, Mo Z W. 2020. Enhancement of 2-acetyl-1-pyrroline (2AP) concentration, total yield, and quality in fragrant rice through exogenous γ-aminobutyric acid (GABA) application., 91: 102900.
Yan B P, Ye F, Gao D P. 2015. Residues of the fungicide epoxiconazole in rice and paddy in the Chinese field ecosystem., 71(1): 65‒71.
Yoshihashi T, Huong N T T, Inatomi H. 2002. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety., 50(7): 2001‒2004.
Zhang W Y, Chen Y J, Wang Z Q, Yang J C. 2017. Polyamines and ethylene in rice young panicles in response to soil drought during panicle differentiation., 82: 491‒503.
Zhang Z C, Zhang S F, Yang J C, Zhang J H. 2008. Yield, grain quality and water use efficiency of rice under non-flooded mulching cultivation., 108(1): 71‒81.
Zhao R M, Luo H W, Wang Z M, Hu L. 2020. Benefits of continuous plow tillage to fragrant rice performance., 112(5): 4171‒4181.
Zhao Z X, Sun R X, Su Y, Hu J Y, Liu X L. 2021. Fate, residues and dietary risk assessment of the fungicides epoxiconazole and pyraclostrobin in wheat in twelve different regions, China., 207: 111236.
7 May 2021;
2 August 2021
Tang Xiangru (tangxr@scau.edu.cn)
Copyright © 2022, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2022.01.007
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- Functions of Nitrogen, Phosphorus and Potassium in Energy Status and Their Influences on Rice Growth and Development
- Comparisons of Metabolic Profiles for Carbohydrates, Amino Acids, Lipids, Fragrance and Flavones During Grain Development in india Rice Cultivars
- Cloning and Functional Analysis of Calcineurin Subunits A and B in Development and Fecundity of Nilaparvata lugens (Stål)
- Improving Rice Blast Resistance by Mining Broad-Spectrum Resistance Genes at Pik Locus
- Genetic Improvement of Rice for Bacterial Blight Resistance: Present Status and Future Prospects
- Pectin Methylesterases Enhance Root Cell-Wall Phosphorus Remobilization in Rice

