Long-term outcomes of endoscopic submucosal dissection and surgery for undifferentiated intramucosal gastric cancer regardless of size
Gil Ho Lee, Eunyoung Lee, Bumhee Park, Jin Roh, Sun Gyo Lim, Sung Jae Shin, Kee Myung Lee, Choong-Kyun Noh
Abstract
Key Words: Early gastric cancer; Undifferentiated cancer; Expanded indication; Endoscopic submucosal dissection; Surgery
lNTRODUCTlON
Endoscopic submucosal dissection (ESD) is recommended as a treatment modality for early gastric cancer (EGC) because it allows curativeen blocresection and complete histopathological evaluation[1 ,2 ].With the development of endoscopic instruments and techniques, the indication for ESD has expanded,and short- and long-term outcomes of ESD have been favorably reported in various studies[3 -8 ].Accordingly, ESD can be performed for patients with undifferentiated (UD) intramucosal EGC without lymphovascular invasion when the lesion size is ≤ 2 cm and there is no ulceration. Compared with surgery, ESD may be an alternative treatment option for UD intramucosal EGC within expanded indications[9 -12 ]; however, concerns regarding lymph node (LN) metastasis in patients with UD intramucosal EGC remain[12 ].
Even if UD intramucosal EGC meets the criteria of expanded indications, additional surgical treatment is recommended if the lesion size alone is a non-curative factor (lesion diameter > 2 cm)[2 ]. In this case, physicians are concerned about determining the appropriate treatment modality and whether additional treatment should be performed. Various risk factors for LN metastasis have been reported in several surgical reports based on a lesion size of 2 cm[13 -16 ]. Therefore, the role of ESD is limited in patients with UD intramucosal EGC because of the lesion size. However, patients may choose ESD for several reasons such as refusal of surgical treatment or older age. A recent multicenter study reported that mortality was not significantly higher in patients who underwent endoscopic resection for UD intramucosal EGC with tumor size > 2 cm as the only non-curative factor than in those who underwent additional surgery[17 ].
Compared with surgery, ESD can reduce the period of hospital stay after treatment and improve quality of life. To date, no study has compared long-term outcomes between ESD and surgical treatment based on propensity-score matching in patients with UD intramucosal EGC who are within or beyond expanded indications but satisfy the criteria of curative resection except for lesion size. Thus, this study aimed to evaluate the clinical outcomes and adverse events of ESD compared with those of surgery in patients with UD intramucosal EGC using propensity-score matching analysis. Furthermore, we compared long-term clinical outcomes of ESD and surgery after matching for patients with UD intramucosal EGC who are beyond the expanded indication but meet the criteria of curative resection,except for lesion sizes > 2 cm.
MATERlALS AND METHODS
Patients
We retrospectively analyzed patients who underwent ESD (n= 212 ) or surgery (n = 1373 ) for UD intramucosal EGC at the Ajou University Medical Center (Suwon, Republic of Korea) between January 1 , 2005 and August 31 , 2020 . Among those patients, patients with included expanded indications and curative resection[2 ] were enrolled. Patients who satisfied the condition of curative resection but had a lesion size > 2 cm (beyond expanded indications, tumor size > 2 cm as the only non-curative factor)were also included. The expanded indications with curative resection for UD intramucosal EGC were described as follows: intramucosal tumor; UD type; without ulceration;en blocresection; tumor-free lateral and deep resection margin; without lymphovascular (or LN) invasion; and lesion size ≤ 2 cm[2 ].Exclusion criteria were as follows: previous history of gastric cancer; previous history of other malignancy; or initial multiple gastric cancers. Additionally, we excluded patients who underwent additional surgery after ESD. The study protocol was approved by Ajou University Hospital Institutional Review Board and Ethics Committee (Approval No. AJIRB-MED-MDB-21 -101 ). The requirement for informed patient consent was waived owing to the retrospective nature of the study. All co-authors had access to study data and reviewed and approved the final manuscript.
ESD procedure and surgery
All ESD procedures were performed by expert endoscopists using single-channel (GIF-Q260 J; Olympus,Tokyo, Japan) or two-channel (GIF-2 TQ260 M; Olympus) endoscopy. After identifying the lesion,circumferential marking was done 5 mm outside the tumor margin using a needle knife (Dual knife;Olympus) or argon plasma coagulation (Erbe Elektromedizin, Tübingen, Germany). Epinephrine mixed fluid (0 .01 mg/mL) was injected into the submucosal layer to lift the lesion from the muscle layer, and dissection was performed using an insulated-tip knife (IT knife; Olympus). The resected specimen was retrieved using a Swirl Net (Olympus), and all samples were fixed in 10 % buffered formalin solution and embedded in paraffin.
Patients underwent total or subtotal gastrectomy with LN dissection according to the treatment guidelines of the Japanese Gastric Cancer Association (2 ). Therefore, patients who were enrolled in the surgery group underwent laparoscopy-assisted or open gastrectomy with D1 or D1 +β LN dissection.The surgeons decided on the extent of gastric resection according to the tumor location.
Gross and histopathologic evaluations
Tumor locations were categorized into upper, middle, or lower third of the stomach based on the longitudinal axis of the stomach. Endoscopic findings were classified into elevated, flat, and depressed according to the predominant type based on the Japanese Research Society for Gastric Cancer classification system[18 ]. A standard histopathological examination, including hematoxylin and eosin staining, was conducted. Tumor size, presence of ulceration, histologic type, depth of invasion,lymphatic and vascular invasions, and presence of tumor cells in the resection margin were assessed.Pathological diagnoses were made according to the Japanese Classification of Gastric Cancer[1 ].
Follow-up schedules after gastric cancer resection
Follow-up endoscopy was performed 3 mo after ESD. A subsequent endoscopy with abdominal computed tomography (CT) was performed every 6 -12 mo for 2 years and annually thereafter for 5 years after the treatment. In surgically resected patients, follow-up endoscopy and abdominal CT scans were performed every 6 mo for the first 2 -3 years and then annually until 5 years after the initial treatment.
Clinical outcomes
The primary outcome of this study was overall survival (OS). The secondary outcomes were recurrencefree survival (RFS) and adverse events of short-term clinical outcomes. In this study, we defined OS as the duration between treatment and death owing to any cause; RFS was defined as the duration between treatment and first recurrence or death with evidence of recurrence. We collected data regarding survival status from the National Cancer Center (Goyang, South Korea); however, cause of death was not obtained for privacy after follow-up loss.
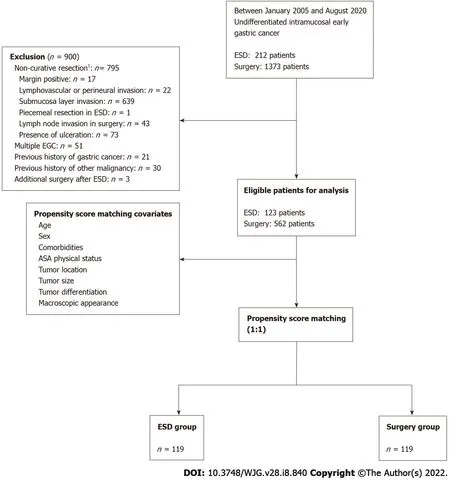
Figure 1 Flow diagram of study population. 1 If the patient met within expanded indications except for lesion size factor > 2 cm (the only non-curative factor),we enrolled that patient for analysis. ASA: American Society of Anesthesiologists; EGC: Early gastric cancer; ESD: Endoscopic submucosal dissection.
We defined local recurrence as a recurrence at the resection site after ESD or a recurrence at the anastomosis site after surgery. A synchronous lesion was defined as the occurrence of a new lesion detected at a different site from the previous treatment site within 1 year after gastric cancer resection. A metachronous lesion was defined as the occurrence of a new lesion detected at a different site from the previous treatment site more than 1 year after initial treatment. Distant metastasis was defined as a tumor metastasis in another organ.
Statistical analysis
We performed propensity-score matching analysis using the radius method to balance covariates across groups and reduce selection bias in the observational study. The propensity score was estimated using a logistic regression model with seven matching variables such as age, sex, comorbidities, lesion size,tumor location, gross morphology, histology appearance, and American Society of Anesthesiologists(ASA) physical status classification system score. Based on these propensity scores, the ESD and surgery groups were matched in a 1 :1 ratio on an allowable absolute difference between exact propensity scores.The standardized mean differences were computed to measure the balance of covariates between groups before and after propensity-score matching.
We compared demographics and clinical characteristics, clinical outcomes, and adverse events between the ESD and surgery groups using the independentt-test or Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables, as appropriate. Survival curves were plotted, and 5 -year survival rates with 95 % confidence intervals (CIs)were estimated using the Kaplan-Meier method. Differences in OS and RFS were examined using the log-rank test between the ESD and surgery groups and within and beyond the expanded indication and between the ESD and surgery groups among patients beyond the expanded indication separately. The multivariable Cox proportional hazard model with treatment modality and ESD indication was used to estimate the hazard ratio (HR) with 95 %CIs to assess the recurrence risk.
All statistical analyses were performed using SAS software, version 9 .4 (SAS Institute Inc., Cary, NC,United States), and R software, version 3 .6 .2 (R Project for Statistical Computing), and all P values < 0 .05 were two sided and considered statistically significant.
RESULTS
Study population
In our center, 212 and 1373 patients with UD intramucosal EGC underwent cancer resectionviaESD and surgery, respectively. Patients who failed to meet the expanded ESD indication and curative resection criteria, except lesion size, were excluded. For long-term outcome analysis, we finally enrolled 123 and 562 patients in the ESD and surgery groups, respectively. Before matching, the mean age ± SD of the ESD group was older than that of the surgery group (55 .3 ± 12 .4 vs 53 .0 ± 11 .8 , P < 0 .001 ). The proportions of male patients were 55 .3 % and 50 .2 % in the ESD and surgery groups respectively (P=0 .305 ). These two groups showed differences with respect to hypertension history, ASA physical status,tumor location, and histology type. In particular, the proportion of patients with signet ring cell carcinoma was higher in the surgery group than in the ESD group (73 .1 % vs 54 .5 %, P < 0 .001 ); however,there was no significant difference between the two groups with respect to lesion size and ESD indication. There was also no significant difference between the ESD and surgery groups [43 (35 .0 %)vs233 (41 .5 %), P = 0 .183 ] with respect to the proportion of beyond expanded indications with lesion size >2 cm (the only non-curative factor). We performed propensity-score matching to compare long-term outcomes of the ESD and surgery groups on a one-to-one basis, and all differences in baseline characteristics after matching were eliminated (Table 1 ). The flow diagram of enrolled patients is shown in Figure 1 , and the distribution of propensity scores is shown in Supplementary Figure 1 .
Clinical outcomes and adverse events
The median hospital stay [interquartile range (IQR)] was shorter in the ESD group than in the surgery group [4 .0 (4 .0 -5 .0 ) vs 9 .0 (8 .0 -10 .0 ) d, P < 0 .001 ]. Regarding intensive care unit admission related to treatment complications, treatment complications caused by severe bleeding was noted one patient in the ESD group, who eventually died. Although the incidence of all adverse events was not different between the two groups, five cases (4 .2 %) of perforations and eight cases (6 .7 %) of bleeding occurred in the ESD group, all of which were early complications within 30 days. Surgical complications, including anastomotic leakage (n= 1 , 0 .8 %), bowel obstruction (n = 3 , 2 .5 %), and hernia (n = 3 , 2 .5 %), were mostly successfully treated with conservative treatment; however, three hernia cases required additional surgery. Late complications were not observed in the ESD group; however, four cases (3 .4 %) were observed in the surgery group (Table 2 ).
Long-term outcomes
The median follow-up period was 45 mo (IQR, 21 -65 mo) and 59 mo (IQR, 36 -81 mo) in the ESD and surgery groups, respectively. During the follow-up period, three (2 .5 %) and five (4 .2 %) patients died in the ESD and surgery groups, respectively. Among those patients, gastric cancer-related death was identified in one patient (0 .8 %) in each group. The incidence of total recurrence was higher in the ESD group (n= 7 , 5 .3 %) than in the surgery group (n = 2 , 1 .7 %) (Table 3 ). Local recurrence was identified in four patients (3 .4 %) who underwent ESD, and all whom had EGC. A synchronous lesion was identified in one patient (0 .8 %), who had EGC and was treated with surgery. A metachronous lesion was identified in three patients (2 .5 %) of the ESD group. Distant metastasis was identified in one patient(0 .8 %) who underwent surgery with peritoneal metastasis. The number of patients whose tumor size was ≤ 2 cm before ESD or surgery and therefore, satisfied the criteria for expanded-indication lesions but had a size of > 2 cm in the final pathology analysis was 22 (18 .5 %) and 20 (16 .8 %) in the ESD and surgery groups, respectively. Of these patients, one patient in the ESD group had a recurrence but no mortality in both groups. Clinical and tumor data for all recurrent patients are given in Table 4 .
We analyzed RFS and OS using Kaplan-Meier survival plots (Figure 2 ). Regarding RFS, according to the treatment modality, the ESD group had inferior results compared with the surgery group (P= 0 .031 )(Figure 2 A). The 5 -year RFS rates were 93 .3 % (95 %CI: 85 .1 -97 .0 ) and 99 .2 % (95 %CI: 94 .2 -99 .9 ) in the ESD and surgery groups, respectively. However, there was no significant difference between the two groups with respect to OS (ESDvssurgery, 5 -year OS, 97 .2 %; 95 %CI: 91 .6 -99 .1 vs 99 .0 %; 95 %CI: 93 .0 -99 .9 ,P=0 .948 ) (Figure 2 B). Among non-curative factors, we analyzed RFS (within vs expanded, 5 -year RFS,97 .7 %; 95 %CI: 93 .0 -99 .3 vs 94 .6 %; 95 %CI: 85 .6 -98 .0 , P = 0 .777 ) and OS (within vs expanded, 5 -year OS,98 .5 %; 95 %CI: 94 .1 -99 .6 vs 97 .4 %; 95 %CI: 90 .1 -99 .4 , P = 0 .698 ) for patients with lesion size > 2 cm(beyond the expanded indication but meeting the criteria of curative resection except for lesion size > 2 cm) and within expanded indication, in which no difference according to the indication was observed(Figure 2 C and D). While there was no difference in OS (ESD vs surgery, 5 -year OS, 97 .5 %; 95 %CI: 83 .5 -99 .6 vs 97 .7 %; 95 %CI: 84 .9 -99 .7 , P = 0 .610 ) according to the treatment modality in patients with beyond expanded indication with lesion size > 2 cm only, the ESD group had a significantly lower RFS than the surgery group (5 -year RFS, 86 .2 %; 95 %CI: 64 .9 -95 .0 vs 100 .0 %; 95 %CI: 100 .0 -100 .0 , P = 0 .013 ) (Figure 2 Eand F). In the multivariable analysis, ESD was a significant risk factor for recurrence after cancer resection in patients with UD intramucosal EGC (HR, 5 .2 ; 95 %CI: 1 .0 -25 .8 , P = 0 .045 ). The lesion included in the beyond expanded indication was not associated with recurrence risk. Moreover, ESD as a treatment modality increased the HR for recurrence in the beyond expanded indication with lesion size > 2 cm compared with the within expanded indication; however, this result was not statistically significant (Table 5 ).

Table 1 Baseline characteristics of the study population
DlSCUSSlON
We comparatively analyzed the long-term outcomes of patients who underwent ESD and surgery for UD intramucosal EGC with complete resection regardless of lesion size using propensity score-matched analysis. ESD was similar to surgery in terms of OS; however, RFS in the ESD group was lower than that in the surgery group. In both the groups, LN metastasis was absent during the follow-up period, andonly one case of distant metastasis was observed in the surgery group. ESD was advantageous because of shorter hospital stays and fewer late complications compared with surgery. Although complete resection was performed for patients with UD intramucosal EGC, ESD was identified as a recurrence risk factor in terms of treatment modality compared with surgery. In patients with lesion size > 2 cm as the only non-curative factor, there was a difference in RFS depending on the treatment modality, with ESD having an inferior outcome.

Table 4 Clinical and tumor information for recurrent patients

Table 5 Cox proportional hazard model for risk of recurrence after initial treatment
In our study, there was a clear bias in the patient population depending on treatment modality.Before matching, patients in the ESD group were older and had a higher ASA physical status score,fewer lesions located in the upper third of the stomach, and lower signet ring cell carcinoma rate than those in the surgery group. While the expanded indication proposed by Gotodaet al[19 ] is based on the pathology of specimens, clinicians should select the treatment modality for EGC based on gross and pathological findings. However, this method sometimes makes it difficult to determine a treatment modality in clinical practice. Besides, there are cases in which patients beyond the expanded indication of ESD refuse surgery and thus undergo ESD in hopes of endoscopic resection instead of surgery. In contrast, there are a significant number of patients who meet the expanded indications and undergo surgery. As a result, patients and lesion characteristics are not the same between patients who undergo ESD and those who undergo surgery. Our results suggested that patients were hesitant to undergo surgery if they were older or if their ASA physical status score was high. For this reason, selection bias was inevitable in this study group. Therefore, we performed propensity-score matching analysis to reduce selection bias owing to treatment modality.

Figure 2 Kaplan-Meier survival plots for recurrence-free survival and overall survival according to treatment modality for gastric cancer.A: Recurrence-free survival (RFS), endoscopic submucosal dissection (ESD) vs surgery; B: Overall survival (OS), ESD vs surgery; C: RFS, within the expanded indication vs beyond expanded indication; D: OS, within the expanded indication vs beyond expanded indication; E: RFS, ESD vs surgery in patients with beyond expanded indication; F: OS, ESD vs surgery in patients with beyond expanded indication. ESD: Endoscopic submucosal dissection.
With the development of ESD techniques and instruments and the accumulation of various clinical outcomes, the scope of ESD has been gradually expanding. However, issues regarding the role of ESD in patients with UD intramucosal EGC are continuously raised. Currently, the ESD criteria for UD intramucosal EGC are strict. ESD is performed only when the lesion has no ulceration and is ≤ 2 cm and when it satisfies the complete resection conditions of negative resection margins and the absence of lymphovascular and perineural invasion in the final pathological examination[2 ]. Lesion size > 2 cm is a non-curative factor, and various studies have reported a difference in LN metastasis based on lesion size of 2 cm[13 ,14 ,15 ,20 ]. However, in cases of UD-EGC with a lesion size > 2 cm, LN metastasis was reported to be 0 % (0 /54 ) when neither ulceration nor lymphovascular invasion was present[16 ], all of which were from surgical studies. To our knowledge, only one study analyzed LN metastasis in patients who underwent endoscopic resection for UD intramucosal EGC with lesion size > 2 cm. Yang et al[17 ]reported an incidence of 1 .1 % (2 /176 ), which showed no increase in mortality during observation after ESD based on Cox regression analysis. Similar results were also obtained in our study. LN metastasis was not observed among 119 patients who underwent ESD. However, 71 .4 % (5 /7 ) of the total recurrence cases had a lesion size > 2 cm, which revealed a higher recurrence rate than that in patients with a lesion size ≤ 2 cm (11 .9 % vs 2 .6 %). Furthermore, patients with lesion size > 2 cm in the ESD group had a lower RFS than those in the surgery group.
The results of our analysis after matching for UD intramucosal EGC patients with complete resection,irrespective of lesion size, showed that RFS was inferior in the ESD group compared with the surgery group. However, no LN metastasis or distant metastasis was identified during the follow-up period. In other words, all recurrence cases occurred in the stomach, all were treated successfully after recurrence,and no further recurrences occurred during the follow-up period. However, taking ESD into account over surgery as the preferred treatment for UD intramucosal EGC irrespective of lesion size should be carefully considered. LN metastasis was not observed; however, local recurrence was found in four patients (4 /119 , 3 .4 %), all of whom had an initial tumor size > 2 cm that occurred less than a year after initial treatment, and surgery as a rescue treatment was performed because endoscopic treatment was impossible. In the study by Yanget al[17 ], the local recurrence rate was 2 .3 % (4 /176 ), and one case had LN metastasis. Therefore, ESD must overcome the problem of local recurrence, which does not need to be considered in surgery. Furthermore, we should consider the why local recurrence occurs even after complete resection. The initial pathologic evaluation could have been incorrect or a new cancer may have occurred. We repeated the pathologic evaluation for these cases; however, the initial diagnosis did not change. Additional studies and data accumulation are required to examine why local recurrence occurs within a short period, although complete or curative resection is performed after the initial pathologic evaluation. In this study, in the 212 patients considered, the margin negative resection rate in the ESD group before matching was 92 .0 % (195 /212 ). This was similar to previous studies where endoscopic resection was performed in UD EGC[21 -26 ].
Therapeutic endoscopists always consider that a sufficient lateral margin can reduce the possibility of local recurrence. While it would be best to secure as much safety margin as possible, the operator should consider the duration of intervention, acute complications (bleeding or perforation), or delayed complications (bleeding, stricture). These considerations may be more prominent in UD EGC. A recently published study mentioned that local recurrence may be related to sequential molecular changes in various cancer-related proteins in histological margin-free endoscopically resected EGCs[27 ]. In this study, a tumor-free distance of 5 .5 mm was considered insufficient as a safety margin. Besides, a subepithelial spread beneath the normal mucosa may exist in UD EGC, especially in signet ring cell cancer, and this subepithelial spread could reach up to 6 mm[28 ]. These studies suggest that securing sufficient margin in the endoscopic resection of UD EGC using the ESD method might reduce the rate of local recurrence. In the endoscopic resection of UD EGC using the ESD method, the endoscopically predicted and the actual size of the lesion is often different. In the Japanese algorithm, an additional biopsy was recommended from the surrounding mucosa of UD EGC to accurately evaluate the margin of the lesion[29 ]. In addition, other studies have reported that narrow-band imaging with magnifying endoscopy may help in accurately predicting the tumor extent in UD EGC[30 ]. Prospective randomized studies are required to evaluate whether the various attempts to accurately determine the tumor margin and resection with sufficient margin can reduce the rate of local recurrence.
In our study, all recurrence cases in the ESD group occurred in the lower or middle third of the stomach, except for one case (6 /7 , 85 .7 %). Although it is difficult to judge because of only a small number of recurrence cases, there is a report that incidence increased in the middle and lower third of the stomach in metachronous cancer after ESD[31 ]. This may be why fewer synchronous or metachronous recurrences were observed in the surgery group. In our study, 16 .8 % of patients underwent total gastrectomy after matching (20 /119 ). Since the portion of the remnant stomach is small,even in patients who have undergone subtotal gastrectomy, surgery can be advantageous in terms of recurrence. In our study, only one recurrence occurred in the upper third of the stomach in the ESD and surgery groups. It is challenging to select ESD as the initial treatment option if surgical treatment is performed as a rescue treatment in a short period, given that ESD has a probability of local recurrence.Therefore, our results suggest that ESD should not be actively recommended to patients with UD intramucosal EGC with lesion size > 2 cm even without ulceration on preoperative workup.
This study had some limitations. First, this was a retrospective single-center study. A randomized study is required to compare long-term outcomes of ESD and surgery, but this is difficult to perform for UD intramucosal EGC. Second, baseline characteristics and tumor information were different between the groups; however, we analyzed the data after propensity-score matching to minimize the difference between baseline characteristics and reduce selection bias of treatment modality. Third, the number of patients with UD intramucosal EGC with lesion size > 2 cm was significantly lower in the pre-matching ESD group than in the surgery group (43 vs 233 patients). Although the data were corrected as much as possible with propensity-score matching, most ESD patients were assigned after matching; thus,selection bias could exist. Finally, although we confirmed survival based on data from the National Cancer Center registry, we did not check the cause of the death in all patients who died. Therefore, we did not evaluate gastric cancer-related deaths in both groups after follow-up loss.
CONCLUSlON
In conclusion, ESD is a treatment modality for stomach preservation with fewer late complications and shorter hospital stays than surgery. For patients with UD intramucosal EGC, if the lesion size is the only non-curative factor, ESD may be an alternative treatment option when surgery is not possible. However,all cases of local recurrence were identified within 1 year in our study, all of which were cancer,although LN metastasis was not observed after ESD. Therefore, even for complete resection, endoscopic surveillance is essential. Especially in cases with lesion sizes > 2 cm, endoscopic surveillance should be more thoroughly performed.
ARTlCLE HlGHLlGHTS

ACKNOWLEDGEMENTS
We would like to thank Cho W, Medical Information & Media Center, Ajou University School of Medicine for providing editing services for images and illustrations.
FOOTNOTES
Author contributions:Lee GH and Lee E contributed equally to this work; Lee GH planned the study design,reviewed the data, analyzed the data and drafted the manuscript; Lee E and Park B analyzed and reviewed the statistical data; Roh J reviewed the pathologic data; Lim SG planned the study design and collected the data; Shin SJ and Lee KM interpreted the data and supervised the report; Noh CK conceptualized, drafted the manuscript and critically revised the manuscript; all the authors approved the final version of the article and agree to be accountable for all aspects of the work.
lnstitutional review board statement:The study protocol was approved by Ajou University Hospital Institution’s Review Board and Ethics Committee (Approval No. AJIRB-MED-MDB-21 -101 ).
lnformed consent statement:Patients were not required to give informed consent because the analysis used anonymous clinical data obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:No potential conflicts of interest were disclosed.
Data sharing statement:The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: http://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:South Korea
ORClD number:Gil Ho Lee 0000 -0001 -7695 -0828 ; Eunyoung Lee 0000 -0002 -9440 -9119 ; Bumhee Park 0000 -0002 -5271 -1571 ; Jin Roh 0000 -0002 -3995 -355 X; Sun Gyo Lim 0000 -0003 -2045 -5099 ; Sung Jae Shin 0000 -0003 -1849 -4435 ; Kee Myung Lee 0000 -0003 -3785 -693 X; Choong-Kyun Noh 0000 -0002 -3607 -8120 .
Corresponding Author's Membership in Professional Societies:The Korean Society of Gastroenterology, No. 1193012 .
S-Editor:Yan JP
L-Editor:A
P-Editor:Yan JP
 World Journal of Gastroenterology2022年8期
World Journal of Gastroenterology2022年8期
- World Journal of Gastroenterology的其它文章
- Treatment strategy for pancreatic head cancer with celiac axis stenosis in pancreaticoduodenectomy: A case report and review of literature
- Mucosal bacterial dysbiosis in patients with nodular lymphoid hyperplasia in the terminal ileum
- Mixed neuroendocrine-nonneuroendocrine neoplasms of the gastrointestinal system: An update
- Differential DNA methylation analysis of SUMF2 , ADAMTS5 , and PXDN provides novel insights into colorectal cancer prognosis prediction in Taiwan
- Inverse correlation between gastroesophageal reflux disease and atrophic gastritis assessed by endoscopy and serology
