Dualistic role of platelets in living donor liver transplantation: Are they harmful?
Chen Liang, Kazuhiro Takahashi,Kinji Furuya,Nobuhiro Ohkohchi,Tatsuya Oda
Abstract Platelets are anucleate fragments mainly involved in hemostasis and thrombosis,and there is emerging evidence that platelets have other nonhemostatic potentials in inflammation, angiogenesis, regeneration and ischemia/reperfusion injury (I/R injury), which are involved in the physiological and pathological processes during living donor liver transplantation (LDLT). LDLT is sometimes associated with impaired regeneration and severe I/R injury, leading to postoperative complications and decreased patient survival. Recent studies have suggested that perioperative thrombocytopenia is associated with poor graft regeneration and postoperative morbidity in the short and long term after LDLT. Although it is not fully understood whether thrombocytopenia is the cause or result, increasing platelet counts are frequently suggested to improve posttransplant outcomes in clinical studies. Based on rodent experiments, previous studies have identified that platelets stimulate liver regeneration after partial hepatectomy. However, the role of platelets in LDLT is controversial, as platelets are supposed to aggravate I/R injury in the liver. Recently, a rat model of partial liver transplantation (LT)was used to demonstrate that thrombopoietin-induced thrombocytosis prior to surgery accelerated graft regeneration and improved the survival rate after transplantation. It was clarified that platelet-derived liver regeneration outweighed the associated risk of I/R injury after partial LT. Clinical strategies to increase perioperative platelet counts, such as thrombopoietin, thrombopoietin receptor agonist and platelet transfusion, may improve graft regeneration and survival after LDLT.
Key Words: Platelet; Liver transplantation; Regeneration; Ischemia/reperfusion injury;Kupffer cell; Oxidative stress
INTRODUCTION
Living donor liver transplantation (LDLT) has been developed as an important option for patients with end-stage liver disease, particularly in the virtual absence of deceased donors. During LDLT, changes in the platelet count and platelet function may occur, and these alterations may lead to deterioration of hemostatic function[1]. Transient thrombocytopenia has been considered as a common phenomenon after LDLT[2]. It is characterized by an average reduction of 60% in platelet counts on postoperative day(POD) 3 and recovers to normal levels on POD 10 after LDLT[3]. The reduction in platelet number can be caused by hemodilution, immunologic reactions, decreased platelet production, or sequestration of platelets in the liver graft upon reperfusion[1]. Moreover, platelet function declines during LDLT, as it was demonstrated that a large number of degranulated platelets were detected in the sinusoids of the liver graft after reperfusion[4].
Recent studies have suggested that postoperative thrombocytopenia is not simply an academic observation but is associated with catastrophic events, such as postoperative bleeding, cerebral hemorrhage and infection, which eventually lead to poor graft regeneration, increased postoperative morbidity and decreased patient survival in the short and long term after LDLT[5]. However, the precise mechanism is unknown, and it is unclear whether increasing perioperative platelet counts could improve posttransplant outcomes. The aim of this article is to summarize and discuss the clinical and experimental evidence of the role of platelets in LDLT. We also referred to the potential beneficial and detrimental effects of “platelet therapy” in the form of thrombopoietin (TPO) receptor agonists that augment graft regeneration.
PLATELETS
Platelets are anucleate fragments of cytoplasm derived from megakaryocytes in the bone marrow[6].The average life span of circulating platelets is approximately 9 d, and they are destroyed by phagocytosis in the spleen and liver[7]. The main function of platelets is to react to hemorrhage by clumping and initiating blood clots[8], which are regulated and kept in balance in hemostasis. However,multiple changes occur in patients with chronic liver disease and post transplantation (LT) conditions,including changes in prohemostatic and antihemostatic pathways, which may consequently lead to either bleeding diatheses or thrombotic disorders[9]. Clinical approaches to increase platelet levels are necessary to compensate for the increased blood loss and requirements for platelets. However, due to the fear of thrombosis and transfusion-related injury[5], the safety and strategies of increasing perioperative platelet counts are still under debate.
Apart from the well-known role of platelets in hemostasis, there is emerging evidence that platelets have other functions in inflammation, angiogenesis, immune response, wound healing, regeneration,and ischemia/reperfusion (I/R) injury[10-12]. Platelets contain three types of secretory granules: alpha granules, dense granules, and lysosomal granules. Each granule contains physiological substances such as platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), insulin-like growth factor-1(IGF-1), vascular endothelial growth factor (VEGF), serotonin, epidermal growth factor (EGF), and transforming growth factor-β[13-16]. When platelets are activated in specific situations, these biologically active substances are released and may induce nonhemostatic processes. All these physiological or pathological processes are involved in the alterations that occur in patients undergoing LDLT.
LDLT
LT is one of the most definitive choices for patients with end-stage liver disease and acute liver failure,and LDLT has been recognized as an important option for patients, particularly small pediatric patients and adults who are disadvantaged by the current deceased donor allocation system[17,18]. The feasibility of LDLT is based on the regenerative capacity of the liver, the evolution of surgical techniques in splitting the liver, and the widespread shortage of deceased liver grafts. In the LDLT procedure, a part of the healthy liver is surgically resected from a living person and transplanted to a recipient immediately after the recipient’s diseased liver is removed[18]. After LDLT, the liver graft undergoes two different processes, namely, liver regeneration and I/R injury[18]. In liver regeneration, the remnant partial liver graft has to rapidly grow to meet the demands of the recipient’s reduced metabolic and synthetic capacities[19]. At the same time, reactive oxygen species (ROS) and inflammatory factors are generated, leading to various responses related to I/R injury[20].
LDLT is sometimes associated with impaired regeneration and severe I/R injury in the liver graft,resulting in small-for-size syndrome (SFSS). SFSS is usually induced by size mismatching between donors and recipients and is characterized by synthetic dysfunction, elevated aminotransferases, and prolonged cholestasis[21]. The increased transaminitis and cholestasis may be attenuated with supportive care and time after LDLT, but sometimes irreversible damage, such as hypoglycemia,cholestasis, encephalopathy, renal failure and acidosis, may occur, which could be critical for recipients[21]. Thus, strategies to improve graft conditions are essential in clinical practice.
LIVER REGENERATION
Liver regeneration is mainly mediated by the proliferation of hepatocytes. In addition, nonparenchymal cells such as Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs), and hepatic stellate cells contribute to liver growthviatheir own proliferation and proliferation-stimulatory effects on hepatocytes[22]. Proliferation is generated when normally quiescent parenchymal cells and nonparenchymal cells undergo one or two rounds of replication to restore liver mass by a process of compensatory hyperplasia[23]. Liver regeneration is usually induced under two conditions: trauma or surgical resection-induced tissue loss and toxins or virus-induced hepatocellular death[24]. Hepatic progenitor cells are liver stem cells with differentiation capacities that can be activated during hepatic stress or injury. According to the participation of hepatic progenitor cells, the origin of the cells compensating for liver mass could be different. The regenerative process after tissue loss is usually driven by some of the existing cells in the liver without activating the progenitor cell compartments. In contrast, when acute liver failure is induced by some toxins, such as galactosamine, intrahepatic progenitor cells can replicate and differentiate into different cell types, such as cholangiocytes, hepatocytes and epithelial cells, to compensate for impaired liver functions[25].
Due to the central role of the liver in body homeostasis, intensive research was conducted to identify factors that might contribute to hepatic growth and regeneration. The essential circuitry required for liver regeneration encompasses three types of pathways, namely, cytokine, growth factor, and metabolic pathways that link liver function with cell growth and proliferation. Tumor necrosis factor-alpha (TNFα) and interleukin-6 (IL-6) are important cytokines involved in liver regeneration, as it was reported that both liver mRNA and serum levels of TNF-α and IL-6 stimulated liver regeneration after hepatectomy[26]. The elevations in TNF-α and IL-6 lead to the activation of the transcription factors nuclear factorkappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3), which consequently increase the expression levels of cyclin D1 and trigger cellular proliferation[27]. In addition, growth factors, such as HGF, EGF, IGF-1 and PDGF, play essential roles in driving cell cycle progression during liver regeneration[28]. With the release of growth factors, numerous intracellular signaling pathways are activated to regulate liver regeneration.
LIVER I/R INJURY
I/R injury is tissue damage induced when the blood supply returns to tissue after a period of ischemia or hypoxia. It is an important cause of liver damage during hepatectomy and LT, which consequently induces graft dysfunction after surgery[29]. During the ischemic period, the absence of oxygen creates a condition in which inflammation and oxidative damage accumulate in the tissue under oxidative stress,which results in deregulation of the phenotype of all liver cellular components[29].
LSECs, which are essential in controlling vascular homeostasis and toxicant clearance, are especially vulnerable to I/R injury[30]. It was described that I/R injury could induce membrane discontinuation,vacuolization, and cell shape rounding in LSECs[31]. Concomitant with the deregulation of LSECs, the lack of oxygen and energy during the ischemic period produces edema in KCs, and biomolecules, such as damage-associated molecular patterns or pathogen-associated molecular patterns, can be released by neighboring hepatic cells to activate KCs[32]. Activated KCs can initiate the inflammatory response by releasing ROS and proinflammatory cytokines, including TNF-α, interleukin-1, interferon-c and interleukin-12[33].
I/R injury is associated with two forms of cell death, namely, apoptosis and necroptosis. Apoptosis is a form of programmed cell death that is characterized by a series of cellular alterations, such as DNA breaks, plasma membrane blebbing, cell shrinkage and chromatin condensation[34]. Most of the biochemical and morphological changes in cells are mediated by a subset of the caspase family.Necroptosis, which is a programmed form of necrosis, occurs from extracellular signals or intracellular cues and involves the process of cellular swelling, plasma membrane rupture, and the release of proinflammatory molecules[35]. In the process of apoptosis, TNF-α leads to the activation of initiator caspases such as caspase-8 and caspase-10. These caspases cleave and activate downstream effector caspases, including caspase-3 and caspase-7, which promote the release of pro-apoptotic molecules to execute apoptosis[34]. Necroptosis is also typically driven in response to the engagement of TNF-α.Activation of the TNF receptor facilitates receptor-interacting protein kinase (RIPK) 1 to assemble with RIPK3 and concomitantly phosphorylates mixed lineage kinase domain-like (MLKL), which is a crucial downstream effector protein of necroptosis. The phosphorylation of MLKL induces plasma membrane permeabilization and the release of cell damage-associated molecular patterns, which results in cell destruction[36]. Overall, TNF-α serves as a central regulator in the process of apoptosis and necroptosis during hepatic I/R injury.
EVIDENCE FROM CLINICAL STUDIES
Platelets and partial hepatectomy
Hepatectomy is the surgical resection of the liver mainly performed for the treatment of primary or metastatic hepatic malignancies. This technique is conducted based on the regeneration capacity of the liver. Although surgical techniques and perioperative management have been substantially improved in recent years, partial hepatectomy is still associated with a high postoperative mortality rate of 1% to 5%[37]. Perioperative thrombocytopenia has been recognized as a common phenomenon during liver resection. It was reported that platelet counts drop immediately after surgery with a nadir on POD 2-3 and return to normal levels by POD 14[38]. The potential reasons concerning preoperative thrombocytopenia may be decreased platelet production, hemodilution, splenic sequestration, medications, or infections[2], but the precise mechanism remains unclear.
Recently, the association of the perioperative platelet count with posthepatectomy liver failure and mortality has been investigated. By conducting retrospective studies, several researchers stated that a low postoperative platelet count was associated with poor recovery and worse outcomes after liver surgery[37,39]. Takahashiet al[40] reported that a greater than 40% decrease in the platelet count was an independent risk factor for delayed liver function recovery after partial hepatectomy. They observed that the platelet count in the delayed recovery group returned to preoperative levels significantly later than that in the adequate recovery group, which indicated that the extra platelets were consumed to compensate for the delayed recovery, resulting in delayed restoration of the platelet counts in the delayed recovery group[40]. In addition, several other parameters regarding perioperative platelet counts, such as the platelet-to-lymphocyte ratio, alkaline phosphatase-to-platelet ratio index, aspartate aminotransferase to platelet count ratio index, and fibrosis-4 index, were reported to be effective criteria for predicting poor surgical outcomes after partial hepatectomy[5]. Although the underlying mechanisms are not fully understood, these reports indicated that increasing the perioperative platelet count may improve the outcomes after partial hepatectomy.
Platelets and deceased donor liver transplantation
The total number of deceased donor liver transplantation (DDLT) has dramatically increased with innovations in both immune suppression and surgical techniques. Posttransplant thrombocytopenia has been recognized as a common phenomenon since the prevalence of DDLT began to increase[1]. In 1968,it was first reported that an acute drop in platelet count to less than 10 × 103/μL was observed on POD 3 in some patients undergoing DDLT[1]. By using111In-labeled platelets, researchers found that transplant recipients had a delayed recovery of platelet counts after DDLT[41]. Subsequent studies have demonstrated that retransplantation, low preoperative platelet counts, massive intraoperative platelet transfusions, and poor general preoperative conditions were factors associated with posttransplant thrombocytopenia[42]. However, they did not pay attention to the meaning of posttransplant thrombocytopenia in DDLT.
The first report clarifying the relationship between thrombocytopenia and DDLT was presented in 1992, when McCaughanet al[43] conducted an analysis of a large cohort of 541 DDLT patients and identified that the decreased counts after DDLT were an independent risk factor for graft survival. Since then, several consecutive studies have been reported to demonstrate perioperative thrombocytopenia as a negative factor for grafts and patient survival in the short and long term after DDLT[42,44,45]. In 2014,Lesurtelet al[9] suggested the 60-5 criteria in which a platelet count of < 60 × 103/μL on POD 5 was an independent risk factor associated with severe postoperative complications, early graft failure, and patient mortality in the short term after DDLT.
Although clinical studies have identified that postoperative thrombocytopenia deteriorates graft and patient survival after DDLT[9], thrombocytosis has not been proven to be a positive factor for DDLT.Some studies stated that a higher preoperative platelet count was associated with I/R injury and arterial thrombosis in DDLT[46,47]. As a result, it is difficult to perform prospective trials by increasing perioperative platelet counts.
Platelets and LDLT
LDLT is different from DDLT in that the partial liver graft needs to regenerate under the condition of I/R injury[18]. Transient thrombocytopenia has been regarded as an independent risk factor for LDLT.Several separate authors stated that a low postoperative count had a higher chance of developing early allograft dysfunction and was a strong predictor of postoperative complications in recipients undergoing LDLT[3,48]. It was demonstrated that an immediate posttransplant platelet count of < 68 ×103/μL or a platelet count of < 30 × 103/μL on POD 3 was an independent risk factor for major postoperative complications and was associated with early graft dysfunctions[3,48]. Takahashiet al[19]reported that a platelet count of < 60 × 103/μL on POD 5 was independently associated with the incidence of postoperative morbidity in the mid-term after LDLT and was especially related to smallfor-size syndrome such as ascites and infection.
Increasing perioperative platelet counts has been considered to be positively associated with LDLT.Kimet al[49] performed a retrospective study in a population of 87 recipients with LDLT and reported that the number of platelets transfused was significantly associated with graft regeneration. Moreover,some consecutive studies were conducted to provide further evidence regarding the benefits and risks of platelet transfusion. They described that platelet transfusion enhanced graft regeneration in recipients after LDLT without increasing morbidity and mortality rates[50,51].
Living donor hepatectomy is sometimes associated with postoperative complications, leading to posthepatecomy liver failure. Previous studies reported that the morbidity rates in liver donors ranged from 8.3% to 78.3%[52,53]. The remnant liver volume ratio, which was recommended to exceed the minimum of 30% to 35% for donor safety[54], is closely related to postoperative morbidity such as liver failure, and platelets have been highlighted as playing an important role in this condition. Yoshinoet al[55] retrospectively collected data from 254 donors undergoing LDLT and showed that a lower preoperative platelet count was an independent risk factor for postoperative complications, such as bile leakage, subphrenic effusion, infectious ascites, postoperative anemia, and liver failure, after living donor hepatectomy. Emondet al[56] demonstrated that even in healthy donors, the fluctuation of platelet count within the normal range was negatively associated with potential portal hypertension and subclinical liver dysfunction, indicating that platelet count might serve as a surrogate marker to predict potential liver failure in healthy donors.
Although postoperative thrombocytopenia after LDLT was associated with low graft regeneration, it is unclear whether postoperative thrombocytopenia is the “cause” of low graft regeneration or just a“result” that appears as an unfavored postoperative condition of the patients. As posttransplant thrombocytopenia was reported to be associated with LDLT, clinical studies concerning this field are necessary. However, due to the fear of thrombosis and other complications, strategies to increase platelet counts are difficult to implement in clinical practice. Thus, basic studies explaining the precise mechanism of platelets in liver regeneration, I/R injury and LT are warranted.
EVIDENCE FROM BASIC STUDIES
The role of platelets in liver regeneration
Platelets are considered to stimulate liver regeneration, as they can secrete physiological substances such as IGF-1 and HGF[57], which play important roles during liver regeneration[22]. In addition,platelet-derived serotonin was demonstrated to be an inducer of liver regeneration, as it was reported that the liver failed to regenerate after partial hepatectomy in mice lacking intraplatelet serotonin[11].Previous studies revealed that platelets accumulated in the liver after hepatectomy with a 2-fold increase compared with prehepatectomy levels[58], and electron microscopy showed that platelets translocated from the sinusoidal space into the space of Disse and directly contacted hepatocytes[59]. It was shown that marked changes in proliferation-related signaling pathways and mitosis occurred after changing the platelet levels in mice after hepatectomy[59]. These results suggest that platelets accumulate in the liver after hepatectomy and may provide signals for rapid hepatocyte proliferation.
It was suggested that direct contact between platelets and hepatocytes contributed to liver regeneration. When recruited in the liver, platelets translocate from the liver sinusoids to the space of Disse and trigger the release of soluble mediators from platelets such as HGF, IGF-1, serotonin and VEGF, which leads to hepatocyte proliferation[60]. LSECs and KCs were also reported to interact with platelets to stimulate liver regeneration. It was identified that platelets induced the release of IL-6 from LSECs through direct contact with LSECs[61]. On the other hand, platelets could attach to KCs, and the hepatic expression of TNF-α and IL-6, which are predominantly produced by KCs, increased in response to the interaction between platelets and KCs[62]. Due to the secretion and stimulation capacities of platelets, researchers found that the TNF-α/NF-κB, IL-6/STAT3, and phosphatidylinositol 3-kinase(PI3K)/Akt pathways are the three major cascades in which platelets exert their effects during the process of liver regeneration[62]. The pathways are associated with the transition of quiescent hepatocytes to the cell cycle and progression beyond the restriction point in G1 phase of the cycle[22],which finally stimulates hepatocyte proliferation.
In addition, platelet-derived messenger RNA was considered to have an impact on liver regeneration.By coculturing platelets with hepatocytes, it was found that platelets accumulated in the perinuclear region of hepatocytes, and messenger RNA from platelets was transferred throughout the hepatocyte cytoskeleton[63]. This result suggested that platelets were internalized into hepatocytes and transferred proliferation-related messenger RNA and stimulated hepatocyte proliferation[63].
Overall, basic studies have identified that platelet-derived liver regeneration occurs through four different mechanisms: (1) direct effects on hepatocytes; (2) cooperative effects with LSECs; (3) collaborative effects with KCs; and (4) the transfer of messenger RNA to hepatocytes (Figure 1).
The role of platelets in I/R injury
There is emerging evidence that platelets have pathological functions in hepatic I/R injury. Cyweset al[12] used a reperfusion model to study the contribution of platelets to I/R injury. They isolated the rat liver and perfused the liverex vivowith Krebs-Henseleit solution containing platelets. They speculated that the degree of platelet adherence to LSECs was related to hepatic injury in perfused rat livers[12],and the number of apoptotic LSECs increased by 6-fold in isolated liver perfused with platelets. These reports indicated that platelets are directly responsible for hepatic injury and contribute to the development of apoptosis in LSECs after reperfusion. Adhesion molecules such as selectins and integrins, which are expressed on platelets and LSECs, are thought to mediate the interaction between platelets and LSECs and result in liver damage[10].
KCs are considered to act in synergy with platelets in the mechanism of I/R injury, as activated KCs release a large amount of both proinflammatory and anti-inflammatory mediators, such as TNF-α, IL-6,interleukin-10 and interleukin-13, which aggravate liver injury[64]. Electron microscopy showed platelets attached to KCs in the early period after hepatic ischemia[65]. It was demonstrated that platelet-related I/R injury after hepatic reperfusion was mainly characterized by the activation of KCs,which potentially release proinflammatory cytokines and generate ROS[66].
ROS, which contribute to inflammatory responses in I/R injury[67], are pivotally related to platelets.First, oxidases or proinflammatory molecules located in platelets are able to produce ROS[68]. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is considered to be the most relevant source of ROS in platelets. Patients with a hereditary deficiency of NADPH oxidase had an almost complete loss of platelet-related ROS products[69]. Xanthine oxidase is another potential source of ROS[70], but its precise relationship with platelet physiology is still unclear. In addition, platelet proinflammatory molecules, such as P-selectin and CD40 ligand, are demonstrated to be associated with intraplatelet ROS generation[71]. Second, ROS formation is functionally associated with platelet activation. It has been reported that catalase, which can reduce the cytosolic concentration of hydrogen peroxide, inhibits platelet aggregation[72]. Moreover, the inhibition of NADPH oxidase by chemical inhibitors, such as diphenyleneiodonium and apocynin, was observed to be related to the suppression of platelet activation[73].
In contrast, platelets have been demonstrated to indirectly inhibit I/R injury. Oberkofleret al[74]reported that the platelet-serotonin-VEGF-interleukin 10/matrix metalloproteinase 8 axis mediated the protective effects of preconditioning on I/R injury in mice. Additionally, it was reported that inducible nitric oxide synthase, an aggravating enzyme for I/R injury[75], was inhibited in macrophages after coculture with platelets under lipopolysaccharide-induced inflammatory conditions[76].
Although the role of platelets in hepatic I/R injury is controversial, it is supposed that platelets could directly aggravate hepatic I/R injury in three ways: (1) adhesion to LSECs; (2) cooperative effects with KCs; and (3) platelet-derived ROS formation (Figure 2).
The role of platelets in partial LT
Platelets are suggested to be positively associated with LDLT in that partial liver grafts require postoperative liver regeneration under I/R injury[77]. This is compatible with previous studies that proved that platelets stimulate liver regeneration after hepatectomy in animal models[59]. Although the positive role of higher perioperative platelet counts has been suggested, the precise mechanisms clarifying how platelets interact with other cells under I/R conditions were reported recently. Lianget al[61] reported that TPO-induced preoperative thrombocytosis contributed to a better outcome in a rat model of partial LT. In this study, platelets stimulated liver regeneration after partial LTviaseveral proliferation-related cytokines and pathways. I/R injury was not aggravated, as shown by unchanged levels of aggravating parameters such as ROS, apoptosis or necrosis. They further used a critical model of 20% partial LT and identified that thrombocytosis could prolong the survival rate in rats. This research explained that thrombocytopenia is not a “result” but a “cause” of postoperative complications.

Figure 1 Platelets and liver regeneration. Platelets translocate into the space of Disse and release insulin-like growth factor-1, hepatocyte growth factor, and vascular endothelial growth factor. The direct contact of platelets with liver sinusoidal endothelial cells (LSECs) results in the excretion of interleukin-6 (IL-6) from LSECs. In addition, the attachment of platelets activates Kupffer cells (KCs) and enhances the release of tumor necrosis factor-alpha and IL-6 from KCs to promote liver regeneration. Moreover, platelets are internalized into hepatocytes and trigger the functional transfer of messenger RNA stored in platelets, which stimulates hepatocyte proliferation. KCs: Kupffer cells; LSECs: Liver sinusoidal endothelial cells; IGF-1: Insulin-like growth factor-1; HGF: Hepatocyte growth factor; VEGF:Vascular endothelial growth factor; LSECs: Liver sinusoidal endothelial cells; IL-6: Interleukin-6; KCs: Kupffer cells; TNF-α: Tumor necrosis factor-alpha.

Figure 2 Platelets and ischemia/reperfusion injury. Liver sinusoidal endothelial cells (LSECs) express selectins and integrins to stimulate the interaction between platelets and LSECs, and platelets result in the excretion of interleukin-6 (IL-6) from LSECs. The generation of tumor necrosis factor-alpha, IL-6 and reactive oxygen species (ROS) from KCs is elevated after the cooperative effect between platelets and KCs. Furthermore, platelets can produce ROS independently and consequently aggravate ischemia/reperfusion injury. KCs: Kupffer cells; LSECs: Liver sinusoidal endothelial cells; IL-6: Interleukin-6; TNF-α: Tumor necrosis factoralpha; ROS: Reactive oxygen species; I/R injury: Ischemia/reperfusion injury.
The most ambiguous factor concerning platelets and partial LT is TNF-α, which is a pleiotropic cytokine possessing two opposite effects on hepatocytes, namely, promoting proliferation and inducing apoptosis. TNF-α binds to its receptor and activates signaling pathways such as the NF-κB pathway and cyclin protein families to stimulate cellular proliferation[78]. On the other hand, TNF-α can induce apoptosis through caspase cascades[78]. The Akt signaling pathway, which could be activated by IGF-1[59], was reported to suppress TNF-α-mediated apoptosis through NF-κB activation[78,79]. It was supposed that the elevated secretion of IGF-1 under thrombocytosis enhanced the phosphorylation of Akt and NF-κB and consequently prevented liver grafts from undergoing apoptosis[61]. However,direct evidence proving the interaction between the Akt pathway and IGF-1 or TNF-α was not provided in previous studies. Partial transplantation models using Akt agonists or inhibitors are necessary to clarify the precise mechanisms (Figure 3).
PERSPECTIVES FOR PLATELET THERAPY
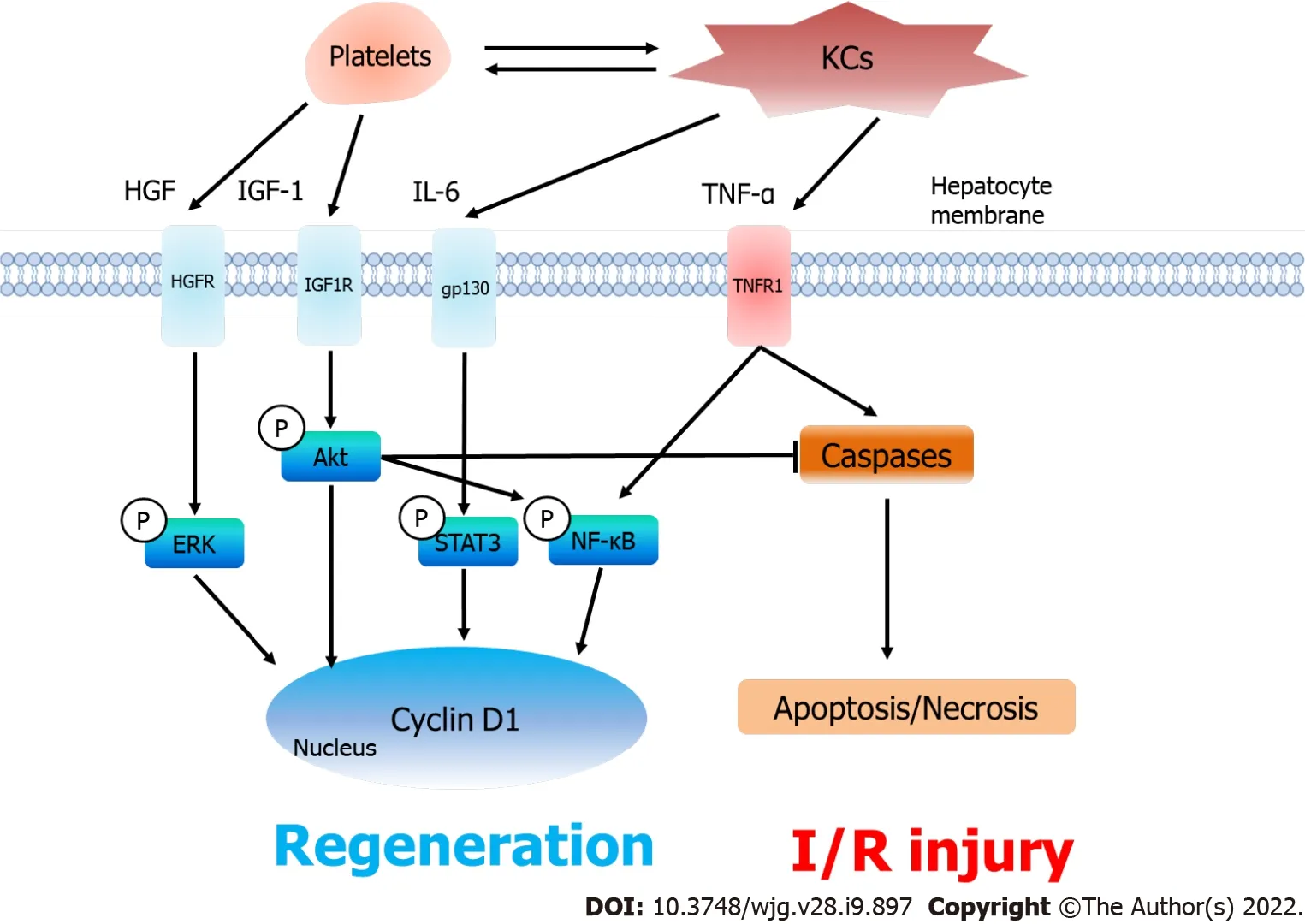
Figure 3 Platelets, liver regeneration and ischemia/reperfusion injury after partial liver transplantation. After accumulating in the liver graft,platelets excrete hepatocyte growth factor (HGF) and insulin-like growth factor-1 (IGF-1) and collaborate with KCs to increase the release of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). As a result, the serum levels of HGF, IGF-1, IL-6 and TNF-α increase under thrombocytosis, which consequently induces the phosphorylation of the ERK, Akt, STAT3 and nuclear factor-kappa B signaling pathways to promote liver regeneration (Cyclin D1). On the other hand, platelets do not aggravate in ischemia/reperfusion injury. The phosphorylated Akt pathway inhibits TNF-α-induced apoptosis and necrosis in the liver graft. KCs: Kupffer cells; HGF:Hepatocyte growth factor; IL-6: Interleukin-6; IGF-1: Insulin-like growth factor-1; TNF-α: Tumor necrosis factor-alpha; HGFR: HGF receptor; IGF1R: IGF-1 receptor;gp130: Glycoprotein 130; TNFR1: Tumor necrosis factor receptor; ERK: Extracellular signal-regulated kinase; STAT3: Signal transducer and activator of transcription 3; NF-κB: Nuclear factor-kappa B; Caspase: Cysteinyl aspartate specific proteinase; I/R injury: Ischemia/reperfusion injury.
Platelet transfusion and TPO receptor agonists are some alternatives to increase perioperative platelet levels in the clinical setting. Platelet transfusion in LT has been controversial, as prophylactic platelet transfusion was reported to have a prothrombotic effect in patients with liver disease[80]. On the other hand, platelet transfusion has been considered to have positive effects on LDLT due to the regeneration capacity of the liver graft, according to previous studies[49-51]. However, several potential problems,such as anaphylaxis reaction, platelet transfusion refractoriness, and transfusion-related lung injury,could be critically harmful to patients[81,82]. Clinical studies have indicated that TPO receptor agonists are more effective than platelet transfusion for chronic liver disease with thrombocytopenia, as shown by the success ratio, effect duration, and nonincidence rate of adverse events[83].
TPO receptor agonists are currently the main focus of pharmaceutical treatment options for thrombocytopenia[84]. There are currently four types of TPO receptor agonists on the market: eltrombopag,avatrombopag, lusutrombopag, and romiplostim. Eltrombopag, avatrombopag, and lusutrombopag are oral TPO receptor agonists approved for patients with thrombocytopenia, and romiplostim is a subcutaneous TPO receptor agonist[85]. Eltrombopag was revealed to increase platelet counts significantly in patients with chronic liver disease, along with an antitumor effect on hepatocellular carcinoma[86]. However, eltrombopag was reported to induce hepatic decompression and thromboembolic events[5]. In addition, romiplostim was shown to have serious side effects leading to bone marrow reticulin fibrosis[87]. Avatrombopag was proven to be generally well tolerated without the occurrence of hepatotoxicity[88]. Although a few thrombotic events were reported[85], there were no serious adverse effects or critical events reported in previous studies. Lusutrombopag was recently released for patients with thrombocytopenia in Japan and the USA[83], with additional effects being reported such as an increase in hematocytes in a patient with compensated liver cirrhosis[89]. There is still no report regarding the side effects of lusutrombopag in clinical practice. For these reasons,avatrombopag and lusutrombopag are promising and may serve as a suitable “platelet therapy” to increase perioperative platelet counts.
CONCLUSION
This review presented accumulated evidence for the role of platelets in LT, especially LDLT, based on clinical and basic studies. Platelets have both beneficial and detrimental effects on liver grafts, with generally positive roles in liver regeneration and potentially negative roles in I/R injury. Clinical and basic studies have broadened our horizons about altering platelet counts in patients undergoing LDLT,and “platelet therapy” may provide prophylactic or therapeutic strategies to enhance the beneficial effects on LDLT.
FOOTNOTES
Author contributions:Liang C, Takahashi K, Furuya K, Ohkohchi N, and Oda T contributed equally to this work;Liang C and Takahashi K wrote the paper; all authors provided final approval of the version that was submitted.
Conflict-of-interest statement:There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Japan
ORCID number:Chen Liang 0000-0002-4528-7303; Kazuhiro Takahashi 0000-0003-1089-0644; Kinji Furuya 0000-0002-2630-3072; Nobuhiro Ohkohchi 0000-0003-2779-1247; Tatsuya Oda 0000-0001-6115-0158.
S-Editor:Chang KL
L-Editor:A
P-Editor:Chang KL
 World Journal of Gastroenterology2022年9期
World Journal of Gastroenterology2022年9期
- World Journal of Gastroenterology的其它文章
- Radiomics-clinical nomogram for response to chemotherapy in synchronous liver metastasis of colorectal cancer: Good, but not good enough
- Relationship between clinical remission of perianal fistulas in Crohn’s disease and serum adalimumab concentrations: A multi-center cross-sectional study
- Applications of endoscopic ultrasound elastography in pancreatic diseases: From literature to real life
- Postoperative morbidity adversely impacts oncological prognosis after curative resection for hilar cholangiocarcinoma
- Cystic fibrosis transmembrane conductance regulator prevents ischemia/reperfusion induced intestinal apoptosis via inhibiting PI3K/AKT/NF-κB pathway
