Stereotactic radiotherapy and the potential role of magnetic resonance-guided adaptive techniques for pancreatic cancer
Tai Ermongkonchai,Richard Khor,Vijayaragavan Muralidharan,Niall Tebbutt, Kelvin Lim, Numan Kutaiba,Sweet Ping Ng
Abstract
Key Words: Magnetic resonance imaging; Pancreatic cancer; Radiotherapy; Stereotactic; Adaptive techniques
INTRODUCTION
Pancreatic cancer is one of the leading causes of cancer deaths, with a 5 -year overall survival (OS) rate of less than 10 %[1 ]. Surgical resection is the only curative option, but is seldom feasible due to a lack of early detection markers, late presentation with locally advanced disease or the lesion being medically inoperable[2 -6 ]. In the cohort of patients who received surgery in the PREOPANC-1 randomised trial, a subgroup analysis in patients with borderline resectable disease demonstrated a survival advantage in those receiving preoperative chemoradiotherapy compared to those receiving immediate surgery[7 ].However, the data for definitive radiotherapy (RT) in unresectable pancreatic cancers is conflicting.Traditionally, locally advanced pancreatic cancers (LAPC) unsuitable for curative surgery are treated with chemotherapy regimens or conventionally fractionated radiotherapy (CFRT), or both[8 ]. However,the role of RT is controversial as radiation-induced toxicities remain a concern. Conventional radiotherapy is often associated with significant grade ≥ 3 toxicities while achieving a median OS of only 5 to 15 mo[2 ]. The LAP-07 trial demonstrated that the survival outcomes of those who received conventionally fractionated chemoradiotherapy is not superior to chemotherapy alone. However,despite its known caveats, the trial indicated that there is a benefit from RT in multimodal regimens in achieving improved local control (LC), which approached 70 % at 12 mo[9 ].
Stereotactic body radiotherapy (SBRT) is an emerging RT technique due to its ability to deliver highly ablative radiation doses in several fractions[10 ]. A study by Park et al[4 ] found that the use of a fivefraction SBRT regimen achieved improved quality-of-life scores and tolerable acute toxicities, with comparable late grade ≥ 3 toxicities to intensity-modulated radiotherapy (IMRT) (15 .9 % SBRT vs 13 .7 %IMRT)[4 ]. But while SBRT strives for more accuracy and precision, there are some obstacles that prevent further dose escalation without compromising safety. First is the susceptibility of the pancreas to intrafractional movement during respiratory cycles and digestion. Secondly, the adjacent surrounding organs-at-risk (OAR) which comprises of the stomach, duodenum and small intestine are highly radiosensitive, therefore care needs to be taken to limit doses to these structures to avoid significant treatment-related toxicity. And finally, the current imaging modalities and fiducial markers provide poor visualisation of targets during treatment planning[11 ].
The recent development of magnetic resonance-guided RT (MRgRT) provides potential to circumvent these challenges, as magnetic-resonance imaging (MRI) offers excellent soft-tissue contrast that can guide dosimetric adjustments to the target volume and limit OAR exposure. This review will evaluate the role of SBRT in the treatment of LAPC, its shortcomings, and present the potential use of MR-guided adaptive techniques to mitigate those caveats.
MATERIALS AND METHODS
Searches were conducted in the online databases PubMed and Ovid (Medline) from August to September 2020 , using Medical Subject Headings (MeSH) terms/keywords of pancreatic cancer,stereotactic, radiotherapy and magnetic-resonance. Records were included if it studied the treatment outcomes of SBRT and/or MRgRT in unresectable pancreatic cancers. The excluded literature were review articles or studies done on metastatic disease. Studies that involved resectable tumours or used chemoradiotherapy as adjuvant treatment post-surgery were also omitted. Only results in the English language were included. Additional literature was also sought from references of included studies. A final shortlist of studies was selected based on relevance. A study was considered as relevant if it investigated the effect of SBRT and/or MRgRT on any survival metric in patients with inoperable LAPC.
RESULTS
Figure 1 illustrates the search and screening processes done to assess the eligibility of studies. A total of 1630 records were found from the databases using the search strategy, with an additional 10 retrieved from references of included studies. There was a total of 411 duplicates, and after removal of these we resulted with 1229 records. Screening was conducted by the primary author. The first screening phase was done by screening the titles and abstracts of the 1229 records, which resulted in 93 potential studies.The second screening phase assessed full texts, and 46 further studies were excluded for reasons such as use of novel therapies, investigating metrics not relevant to survival outcomes in pancreatic cancer, or usingin-vivoanimal models. This resulted in 47 eligible texts and out of those, 36 were used to synthesise the discussion. The final 36 texts chosen represented the latest seminal work pertaining to SBRT and MRgRT in treatment of pancreatic cancer.
DISCUSSION
SBRT in patients with LAPC
For patients with unresectable LAPC, chemotherapy has been the mainstay of treatment. Early radiation techniques such as CFRT and IMRT have called into question the value of irradiation in LAPC management due to their considerable toxicity profiles[4 ,9 ], with minimal to no impact on survival outcomes[9 ]. However, SBRT is an advanced radiation technique which can be delivered on the same linear accelerator at most centres. It has gained attraction due to three main reasons: Firstly, it allows delivery of high biologically effective doses (BED) split into several fractions (typically 3 -5 ). Secondly,the technique allows a sharp radiation dose falloff at the edge of target volumes, thereby reducing doses to OARs[12 ]. Thirdly, it offers an overall shorter treatment time, as SBRT is normally delivered in 1 -3 wk, compared to CFRT which takes 5 -6 wk[13 ,14 ]. Hence, SBRT ensures there is minimal interruption to chemotherapy, which is important given that the main pattern of failure in this disease is distant metastasis (DM)[3 ]. In addition to this, patients with limited prognoses will be able to complete RT courses in 3 wk instead of 6 wk (which may account for a quarter of their remaining lifespan). This greatly improves quality-of-life, as it requires less commuting and reduces associated costs on patients and families[10 ,13 ].
Table 1 summarises the studies of SBRT in unresectable pancreatic cancer. The majority of studies demonstrated an OS of 10 -16 mo, freedom from local disease progression (FFLP) rates of approximately 80 % and progression-free survival (PFS) of 8 -10 mo with SBRT[2 -4 ,13 ,15 -17 ]. A systematic review by Petrelliet al[18 ] assessed prospective trials and retrospective studies of SBRT use in LAPC, with the pooled results showing a median OS of 17 mo[18 ]. Other studies compared SBRT’s efficacy compared to other RT techniques. A retrospective review by Zhonget al[13 ] showed that patients who received SBRT had improved median OS times and 2 -year OS rates relative to CFRT[13 ]. Similar results were found by Dohopolskiet al[3 ] who also demonstrated a higher median OS for the SBRT group (12 .6 mo) compared to its counterpart (11 .2 mo)[3 ]. Other studies also showed that patients who had SBRT achieved at least similar outcomes as those who had IMRT[4 ,19 ]. Park et al[4 ] demonstrated no significant difference in median OS between those who had SBRTvsIMRT[4 ]. However, Shaib et al[19 ] found that SBRT achieves at least a month longer median OS (8 .6 mo vs 6 .7 mo; P < 0 .001 ) and more than double compared to supportive care alone (8 .6 mo vs 3 .4 mo; P < 0 .001 )[19 ]. However, large prospective trialsare needed to definitively conclude SBRT’s efficacy compared to conventional techniques, but the evidence so far suggests that SBRT is associated with better survival outcomes.
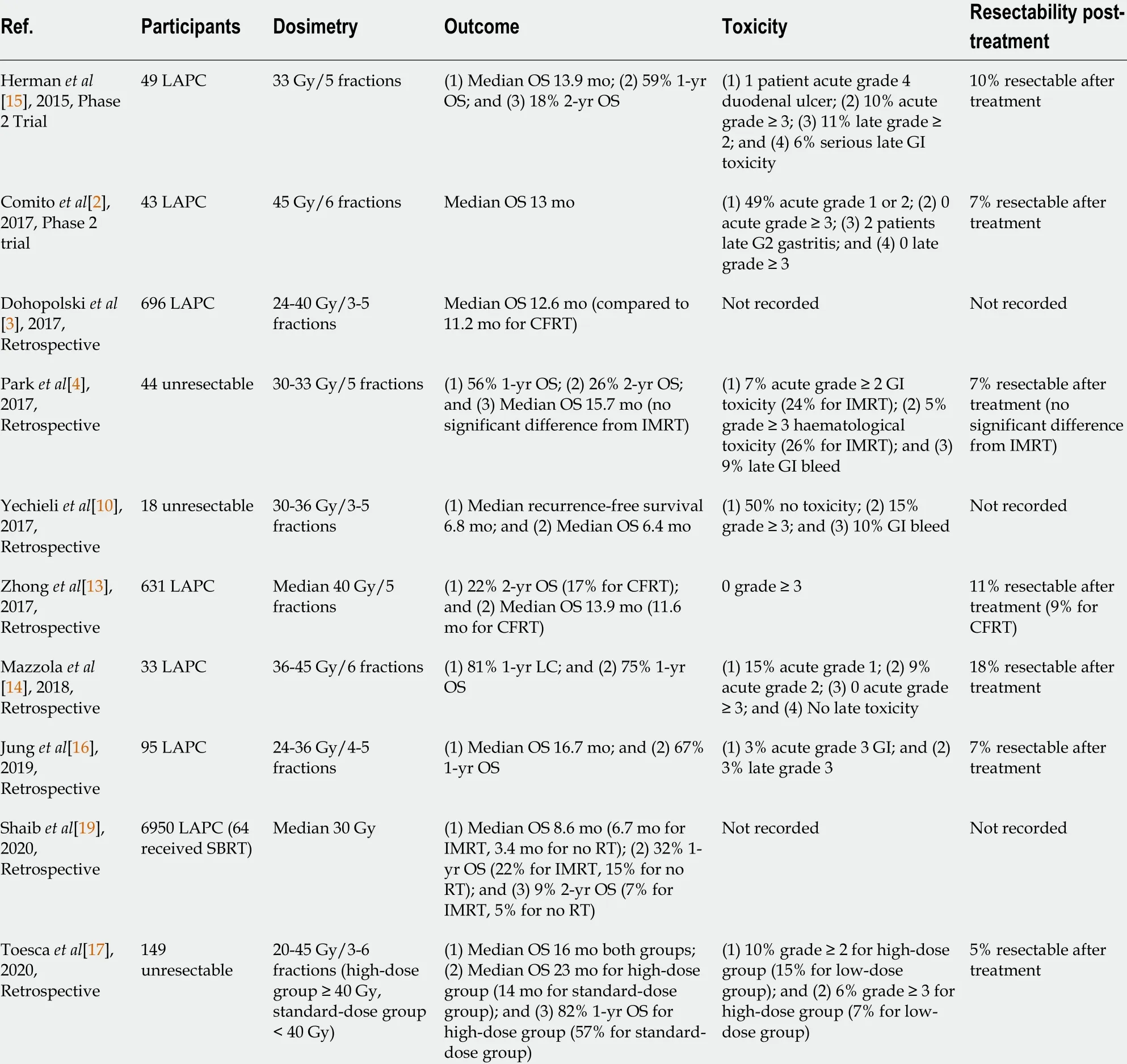
Table 1 Studies of stereotactic body radiotherapy in locally advanced pancreatic cancer
Another advantage of SBRT is its favourable toxicity profiles. Studies in Table 1 report no more than 15 % and 10 % of patients receiving SBRT suffering from acute grade ≥ 3 toxicities and late side effects(such as duodenal bleeding and gastric ulcer perforation), respectively. Compared to IMRT, SBRT had significantly lower acute grade ≥ 2 gastrointestinal toxicity rates (7 % vs 24 %)[4 ]. Petrelli et al[18 ]’s systematic review found late grade 3 to 4 toxicity rates of up to 11 % in their studies, with only 3 of their included studies reporting > 10 % risk of severe gastrointestinal ulceration[18 ]. The patients of those studies all received higher dosesperfraction due to previously failed RT[18 ], suggesting that a relationship exists between delivered doses and toxicity severity in SBRT treatment. The lower toxicity rates may be attributed to SBRT’s rapid dose falloffs and the utilisation of motion mitigation methods.The pancreas is a retroperitoneal organ embedded around gastrointestinal structures, hence it undergoes significant motion during respiratory cycles and physiological processes such as digestion.The two commonly used motion mitigation methods during SBRT are respiratory gating and abdominal compression. Respiratory gating uses an external surrogate marker that represents the internal tumour position, where the radiation beam is only delivered when this marker correlates to a certain phase of the respiratory cycle. Abdominal compression requires applying pressure onto the abdomen to suppress diaphragmatic movements, but is less preferred due to patient discomfort and the occasional displacement of OARs closer to the radiation volume[11 ]. A prospective study by Campbell et al[11 ]confirmed that both methods reduce motion and OAR exposure compared to no mitigation, however respiratory gating achieves greater motion reduction than abdominal compression by more than 20 %[11 ].

Figure 1 PRISMA diagram of literature search.
Interestingly, while the studies only included unresectable patients, a small proportion were able to receive surgical resection after their SBRT course. As shown in Table 1 , the rate of conversion to surgical resectability by SBRT was 5 %-18 %. In these studies, surgical resectability was decided upon multidisciplinary review including operating surgeons. This is important because if SBRT can induce local tumour regression and subsequently convert the tumour from unresectable to resectable, then it can possibly improve the chances of cure. The study by Mazzolaet al[14 ] yielded the highest rates of resectability at 18 %, all of which were participants that received higher doses of SBRT at 42 -45 Gy in 6 fractions[14 ].Meanwhile, Petrelliet al[18 ] found that higher total doses and number of fractions are significantly associated with 1 -year locoregional control[18 ]. These results suggest that dose escalation may be the key determinant in achieving LC and thus conversion to surgical resectability. Currently, for fivefraction regimens, dose escalations of up to 60 Gy is feasible without compromising adequate target coverage and OAR constraints[20 ,21 ].
Alternative fractionation schemes
Recent evidence indicates that patients may benefit from alternative fractionation regimens, especially for those with gross tumour abutment into surrounding structures or invasion into peripancreatic nodes. The rationale is to prolong the treatment regime (≥ 10 fractions) such that higher overall BEDs can be delivered while still accounting for OAR toxicity. Reyngoldet al[22 ] studied ablative schemes of 75 Gy in 25 fractions (BED = 97 .5 Gy) and 67 .5 Gy in 15 fractions (BED = 97 .88 Gy) for patients with significant tumour abutment to the stomach/intestines, demonstrating a median OS of 18 .2 mo and a 2 -year OS of 38 %[22 ]. This is an improvement from standard 1 -5 fraction regimens, as the reported 2 -year OS from those studies ranged from 9 %–26 %[4 ,13 ,15 ,19 ].
Caveats of current SBRT
Despite the advances of SBRT, its overall management of LAPC is limited by its imaging modalities.SBRT utilises computed tomography (CT)-based techniques such as 4 -Dimensional CT (4 DCT) and Cone Beam CT to assess tumour movement and carry-out the motion mitigation techniques[23 ]. This is a limitation because CT has poor soft-tissue contrast and is unable to accurately determine the appropriate therapy volumes. Furthermore, CT often involves larger planning target volumes (PTV) or use of an internal target volume (ITV) to account for tumour motion, thus putting the surrounding OARs at increased toxicity risk and ultimately preventing any possibility of dose escalation[24 ].Furthermore, 4 DCT only provides the average of motion amplitude over several respiratory cycles.Since the fourth dimension represents “phase” of respiration rather than being real-time, tumour motion might even be underestimated[25 ]. This explains why despite SBRT’s evidence in reducing acute toxicity, there are still significant concerns with late toxicity as previous published studies report rates of up to 47 % of late grade ≥ 2 toxicity[2 ]. Therefore, SBRT is constrained by dose-limitations placed on the surrounding OARs. Another concern is its steep dose gradient and the marginal misses that may result[26 ]. This is made more challenging given that conventional CT tends to underestimate the true pathologic size of the pancreatic tumour[27 ]. To optimise SBRT’s therapy volumes and dose distribution, a better imaging modality needs to be incorporated.
Emerging role of MRgRT
MRgRT has been proposed as the solution to the inconsistencies of onboard imaging with RT. MRI provides superior soft-tissue visualisation compared to CT and thus allows better delineation of the target tumour from surrounding OARs. Its real-time feedback also tracks inter-fractional and intrafractional organ changes[8 ,28 ,29 ]. Another benefit of MRI is its exploration of multiple breathing cycles over different days to quantify daily changes[25 ]. Therefore, MRgRT can be used to guide treatment plan adaptations, such that therapy volumes account for intra-treatment tissue changes[30 ]. This led to the advent of Stereotactic MR-guided Adaptive Radiotherapy (SMART), which is the application of the principles of MRgRT combined with SBRT. A non-randomised trial by Heerkenset al[25 ] assessing the feasibility of MRgRT with SBRT showed that it is safe with dosimetric plans of at least 24 Gy, with no cases of acute or late grade ≥ 3 toxicity. They were also able to deliver higher doses under free-breathing conditions while ensuring adequate target coverage and OAR sparing[25 ]. SMART has become a promising technique in LAPC by possibly enabling SBRT dose escalation without exposing OARs to higher toxicity risk[8 ,25 ].
Table 2 summarises recent studies of MRgRT use in LAPC. Rudra et al[31 ] investigated the use of MRgRT with standard-dose and high-dose SBRT plans, and were able to demonstrate that dose escalation is possible. Patients in the high-dose group (receiving 40 -52 Gy) achieved significantly higher survival rates compared to those in the standard-dose group (receiving 30 -35 Gy), despite the former cohort having worse prognostic factors such as older age and higher Carbohydrate Antigen 19 -9 biomarker levels[31 ]. There was no incidence of severe toxicity amongst the higher dose group, with all cases of grade ≥ 3 gastrointestinal toxicities reported from the standard-dose cohort[31 ]. A study by Lutersteinet al[8 ] on a patient case yielded similar results. The patient with clinical stage III (T4 N1 M0 )LAPC was given a high BED of 72 Gy via SMART after chemotherapy and achieved LC at 16 mo postradiation (21 mo since diagnosis) with no significant side effects or toxicities[8 ]. Furthermore, a multiinstitutional study at the American Society for Radiation Oncology suggested that adaptive plans that allow safe delivery of BED > 70 Gy can achieve higher OS rates than BED < 70 Gy without impacting surrounding OARs[8 ]. These results indicate that MRgRT’s precision can potentially address prior issues with RT. And since previous studies recommend that five-fraction regimens should use a dose prescription of 40 Gy to cover the gross tumour[32 ], the advances of MRgRT provides potential to maximise this limit in the future without compromising safety.
With the implementation of MRgRT in its early stages, some caveats have emerged such as workflow disruptions. Utilising MRI to guide therapy also poses new challenges unique to the MRI magnet,including but not limited to patient selection and MRI safety. This needs particular consideration as the MRI magnet is now being used outside of a radiology department where MRI safety protocols are firmly embedded into work practices. As with any novel modality or technological advancement, there will be a learning curve and an initial period to bolster awareness of safety requirements.
Concomitantly, adaptive techniques also require increased time investment as plans need to be reoptimised between fractions. Hence, MRgRT is costly and resource-intensive because it involves multidisciplinary teams to re-contour images, review and re-approve the adapted plans daily[28 ,30 ].There is now an emerging interest to use artificial intelligence tools such as auto-contouring methods and radiomics to increase the workflow efficiency of treatment planning.
Strengths and limitations of the review
Our review methodology covers a wide range of literature, but it comes with limitations. The review mostly sought evidence from large retrospective studies without individual data for each patient.Hence, it was difficult to identify confounding factors that may exist due to the variability of patient characteristics. The review also excluded studies on patients with DM as it aimed to investigate SBRT’s effect locally. This may artificially elevate survival rates as those without DM will naturally have better outcomes.
The included evidence came with strengths and limitations. Firstly, SBRT has mature follow up data from several large retrospective analyses, with evidence dating back over a decade. This provided ample evidence to suggest that SBRT is a safe and beneficial technique for multimodal management of LAPC. However, the heterogeneity in study designs contributes to a large variability in the data. Since LAPC management differs on a case-by-case basis according to tumour staging and the physician’s clinical judgement, many of these studies include patient cohorts that received different chemotherapy regimens from each other. As a result, it is unsure how much survival benefit can be attributed to SBRT.It is also noteworthy that many of these studies could involve selection bias, since the most unwell patients often received no treatment and went into palliative care. This led to “healthier” subjects chosen for SBRT and thus better OS rates. Many of the included studies are retrospective analyses of database records, presenting another source of selection bias. Meanwhile, there is limited research on MRgRT so far, thus definitive conclusions about this technique cannot be made. There is a need for largeprospective trials on SBRT and MRgRT, with comparisons to other treatment modalities to validate the results of previous retrospective studies. However, given LAPC’s generally poor outcomes, long-term prospective studies will be challenging.

Table 2 Studies of magnetic resonance-guided radiotherapy in locally advanced pancreatic cancer
CONCLUSION
SBRT is an advanced radiation technique that allows delivery of ablative doses in several fractions. It is highly precise, time-efficient and can limit OAR exposure when combined with motion mitigation techniques. SBRT is associated with improved treatment outcomes and safer toxicity profiles compared to other conventional RT techniques. And by implementing MR-guided imaging techniques with SBRT,the excellent soft-tissue contrast of MRI enables the physician to make daily plan adaptations such that target volumes are optimised according to intra- and inter-fractional tissue changes. This enables the possibility of dose escalation, which may be the key in achieving long-term LC and converting unresectable LAPC into operable cases. The current evidence on MR-guided SBRT is still limited, but early protocols have suggested its promise. Further research should focus on validating the feasibility,safety and efficacy of MRgRT with comparison to other treatment modalities.
ARTICLE HIGHLIGHTS
Research results
SBRT is associated with improved survival outcomes and toxicity profiles compared to conventional RT techniques. A small proportion of unresectable patients were able to undergo surgical resection after their SBRT course. Conversion to resectability was associated with higher doses. However, dose escalation in SBRT is limited by the onboard computed tomography (CT) imaging due to its poor softtissue contrast. MRgRT may address these issues as magnetic resonance imaging (MRI) provides excellent tissue visualisation and is appropriate for real-time scanning. Early data indicates MRgRT as a safe and efficacious technique.
Research conclusions
SBRT may lead to improved survival outcomes and safer toxicity profiles compared to conventional RT,but is ultimately limited by onboard CT imaging. The addition of MRI-guided techniques allows the potential for dose escalation, which may be the key to achieving surgical resectability and possibly increasing the chances of cure.
Research perspectives
There is a need for large prospective trials to definitively conclude if SBRT is superior to other RT techniques. Large studies are also required to validate the safety, feasibility and efficacy of MRgRT with comparison to other RT techniques.
FOOTNOTES
Author contributions:Ermongkonchai T performed the literature search and wrote the manuscript; Khor R,Muralidharan V, Tebbutt N, Lim K, Kutaiba N and Ng SP performed editing and contributed to the quality of the manuscript.
Conflict-of-interest statement:No conflict-of-interest to be declared by authors of this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:Australia
ORCID number:Tai Ermongkonchai 0000 -0002 -2210 -5773 ; Richard Khor 0000 -0002 -7057 -2747 ; Vijayaragavan Muralidharan 0000 -0001 -8247 -8937 ; Niall Tebbutt 0000 -0003 -2613 -5168 ; Kelvin Lim 0000 -0002 -5216 -8994 ; Numan Kutaiba 0000 -0003 -4627 -9847 ; Sweet Ping Ng 0000 -0003 -1721 -0680 .
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年7期
World Journal of Gastroenterology2022年7期
- World Journal of Gastroenterology的其它文章
- Gallbladder biliary lithotripsy: A new rationale applied to old treatment
- Will the collaboration of surgery and external radiotherapy open new avenues for hepatocellular carcinoma with portal vein thrombosis?
- Early gastric cancer: A challenge in Western countries
- Crohn’s disease-related ‘gastrocnemius myalgia syndrome’ successfully treated with infliximab: A case report
- Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome
- Clinical online nomogram for predicting prognosis in recurrent hepatolithiasis after biliary surgery: A multicenter, retrospective study
