Near UV luminescent Cs2NaBi0.75Sb0.25Cl6 perovskite colloidal nanocrystals with high stability
Huixin Yng,Ynmei Guo,Guoning Liu,∗,Ruowei Song,Jinxi Chen,Yongbing Lou,∗,Yixin Zho
aSchool of Chemistry and Chemical Engineering,Jiangsu Key Laboratory for Science and Application of Molecular Ferroelectrics,Jiangsu Engineering Laboratory of Smart Carbon-Rich Materials and Devices,Southeast University,Nanjing 211189,China
bSchool of Environmental Science and Engineering,Shanghai Jiao Tong University,Shanghai 200240,China
ABSTRACT Near UV highly luminescent colloidal Cs2NaBiCl6 nanocrystals(NCs)were synthesized by a simple lowcost ligand-assisted reprecipitation method.In our strategy,metal chloride precursors were added to the mixture of anti-solvent and ligand at room-temperature.The obtained Cs2NaBiCl6 NCs exhibited a bright blue emission with significantly improved photoluminescence quantum yield(PLQY)of 39.05%.The optical properties and stability were greatly enhanced by doping Sb where Cs2NaBi0.75Sb0.25Cl6 showed a high PLQY of 46.57%,and both the powder and the colloidal solution exhibited superior stability.
Keywords:Perovskite nanocrystals Ligand-assisted reprecipitation Stability Cs2NaBiCl6 Cs2NaBi0.75Sb0.25Cl6
In the past decade,colloidal lead halide perovskite nanocrystals(NCs)have aroused widespread interests due to their excellent photoelectric properties and potential application in light-emitting devices(LEDs)[1-3].However,the instability in air and aqueous medium as well as lead toxicity issues hindered their employments[4-6].Therefore,finding a lead-free alternative with optical properties comparable to the compounds has become the focus of current research.One of the most effective strategies was to replace the lead with divalent cations such as Sn2+or Ge2+.Unfortunately,the related materials exhibited poor crystal structural stability which was attributed to the facile oxidation to Sn4+or Ge4+in air,bringing about defects to cause photoluminescence(PL)quenching[7].Another popular alternative approach was to substitute the two Pb2+ions with a pair of singly-charged and triply-charged metal ions to form two kinds of octahedron,which led to a A2M’M’’X6(A:monovalent cation,X:halogen anion,M′:+ 1 cation,M’’:+ 3 cation)three dimensional(3D)double perovskite structure[8,9].The lead-free double perovskite NCs were expected to be excellent candidate in photoelectric field because of their 3D structure,ecofriendly and stability[10].
Currently,different colloidal double perovskite NCs such as Cs2AgBiX6,Cs2AgInX6,Cs2AgSbX6,Cs2NaBiCl6and Cs2NaInCl6have been synthesizedviavarious methods.One of the mostly used approaches was hot-injection in which one precursor was injected into another solution under the condition of N2environment and high temperature.Another strategy was the ligand-assisted reprecipitation(LARP)route,where ions in the polar solvent was immediately destabilized by adding into an anti-solvent,thereby causing a nucleation explosion[11].However,low photoluminescence quantum yield(PLQY)and ambient instability still remained to be the obstacles of wide application for double perovskite NCs,which were attributed to the indirect band gaps[12,13].For the purpose of improving the PLQYs of these NCs,different doping strategies have been explored such as incorporating Cu2+,Mn2+,and lanthanide ions[14,15].Alternative processes,mainly aimed at engineering the band gap of double perovskites,involved the synthesis of quinary compounds by employing a combination of B+or B3+ions.Previous reports showed that impurity ions(e.g.,Mn2+,Yb3+/Er3+and Bi3+ions)could be successfully introduced into Cs2AgInCl6NCs and remarkable PL improvements were obtained[16,17].PLQY of 36.6% for Bi-alloyed Cs2AgInCl6NCs was observed which was comparable to traditional lead perovskite NCs[18,19].The PLQY of Na and Bi alloyed Cs2AgInCl6NCs was as high as 64%,and the emission color varies with its composition and size[20,21].In addition to Ag-based perovskite NCs,Cs2NaBiCl6NCs were mainly prepared by hot-injection with very low PLQY.Besides,Cs2NaBiCl6NCs showed high flexibility for different ion doping to enhance its photoelectric properties.Recently,Mn-doped Cs2NaBiCl6NCs with an improved PLQY of 15% were reported[22-24].As investigated by Yaoet al.,Ag-doped Cs2NaBiCl6NCs exhibited a high PLQY of 20%,which has attracted great attention on the doped lead-free Cs2NaBiCl6NCs[25].
Sb3+,with an electronic structure of ns2,is an important metal ion for double perovskites owing to its close energy levels to other B3+ions and unique luminescence characteristics[26].Currently,Sb-doping strategy was successfully applied in Cs2SnCl6,Cs2AgInCl6and Cs2NaInCl6.For instance,Sb3+alloyed Cs2NaInCl6microcrystals showed PLQYs of 78.9% and 79% respectively.Also,by the incorporation of Sb3+,an excellent PLQY(~37%)of the vacancy-ordered double perovskite Cs2SnCl6was obtained[27].Besides,through a gradual substitution of Sb with In in a solid solution experiment of Cs2AgSb1−xInxCl6,the bandgap transformed from direct to indirect[28].In this paper,Cs2NaBiCl6NCs prepared by a simple LARP approach showed bright blue photoluminescence emission with a record high PLQY of 39.05%.After incorporating Sb,the PLQY increased to 46.57%,that is,for the Cs2NaBi0.75Sb0.25Cl6stoichiometry.Moreover,the powder and the solution of Cs2NaBi0.75Sb0.25Cl6exhibited superior stability under air which would make it promising for commercial application.
The crystal structures of Cs2NaBi1-xSbxCl6(x=0,0.25,0.5,0.75,1)NCs were shown in Scheme 1.The Cs2NaBiCl6crystal adopted a typical 3D framework of double perovskite with Cs atoms in the center and octahedrons consisting of corner-sharing[NaCl6]5−and[BiCl6]3−while Sb3+ions were incorporated into Cs2NaBiCl6NCs and occupied Bi3+sites to form Cs2NaBi1-xSbxCl6crystals[29].The synthesis of Cs2NaBi1-xSbxCl6(x=0,0.25,0.5,0.75,1)NCs were carried out by a simple low-cost LARP route as well as by tuning the molar ratios of BiCl3and SbCl3in the precursor.Details were discussed in Supporting information.Also,the influence of different amounts of oleic acid(OA)and precursors were explored for better optical properties,where 100 μL precursor solution and 3%OA were found to be the optimal condition for the reaction(Figs.S1a-d in Supporting information).As shown in Fig.1a,the X-ray diffraction(XRD)patterns of Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6NCs were consistent with Cs2NaBiCl6(PDF#77–1831),indicating that the NCs were pure and highly crystalline in theFm 3 mspace group.The ordered arrangements of Na+and Bi3+were evidenced by the strong intensity of the(1 1 1)peak.The(4 0 0)peak shifted toward higher angles as the amount of Sb3+increased,which was attributed to the lattice contraction(Fig.S2 in Supporting information)[26].Notably,the intensity of(1 1 1)and(3 1 1)peaks gradually decreased with higher Sb3+ratios(x >0.25),while an impure phase Cs3Sb2Cl9appeared in Sb-rich condition(Fig.1a)[30].The low alloying level of Sb was probably due to the larger radius difference between Na+(0.102 nm)and Sb3+(0.076 nm)comparing to that of Na+(0.102 nm)and Bi3+(0.103 nm).In order to reveal the accurate stoichiometric ratios of the metal ions(Bi3+and Sb3+)in Cs2NaBi0.75Sb0.25Cl6,the inductively coupled plasmaoptical emission spectrometry(ICP-OES)measurement was conducted.The feeding molar amount of(Bi3+and Sb3+)was equal to that of Na+so that the calculated molar ratios of Bi/Na and Sb/Na could represent the actual values of 1 −xandx(Table S1 in Supporting information).The results indicated that the actual amount of Sb3+was much lower than the chemical formula composition,which was common in other doped double perovskites[26,12].As shown in transmission electron microscopy(TEM)images,the Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6NCs were sphericalshaped(Figs.1c and d)with average sizes of 10.03 ± 0.34 nm and 7.01 ± 0.16 nm,respectively(Figs.1e and f).From the highresolution TEM(HRTEM),it could be observed that the lattice distances of the NCs were measured to be 0.33 nm,corresponding to the(3 1 1)plane(insets in Figs.1c and d).The analysis above revealed that the alloy of Sb3+would not produce additional vacancies and interstitial defects.Therefore,no additional surface trapping states would occur after doping and the intrinsic optical properties were significantly enhanced[31].

Scheme 1.Schematic presentation of the crystal structures of the Cs2NaBiCl6 material and their Cs2NaBi1-xSbxCl6 mixed Bi/Sb cationic analogues.
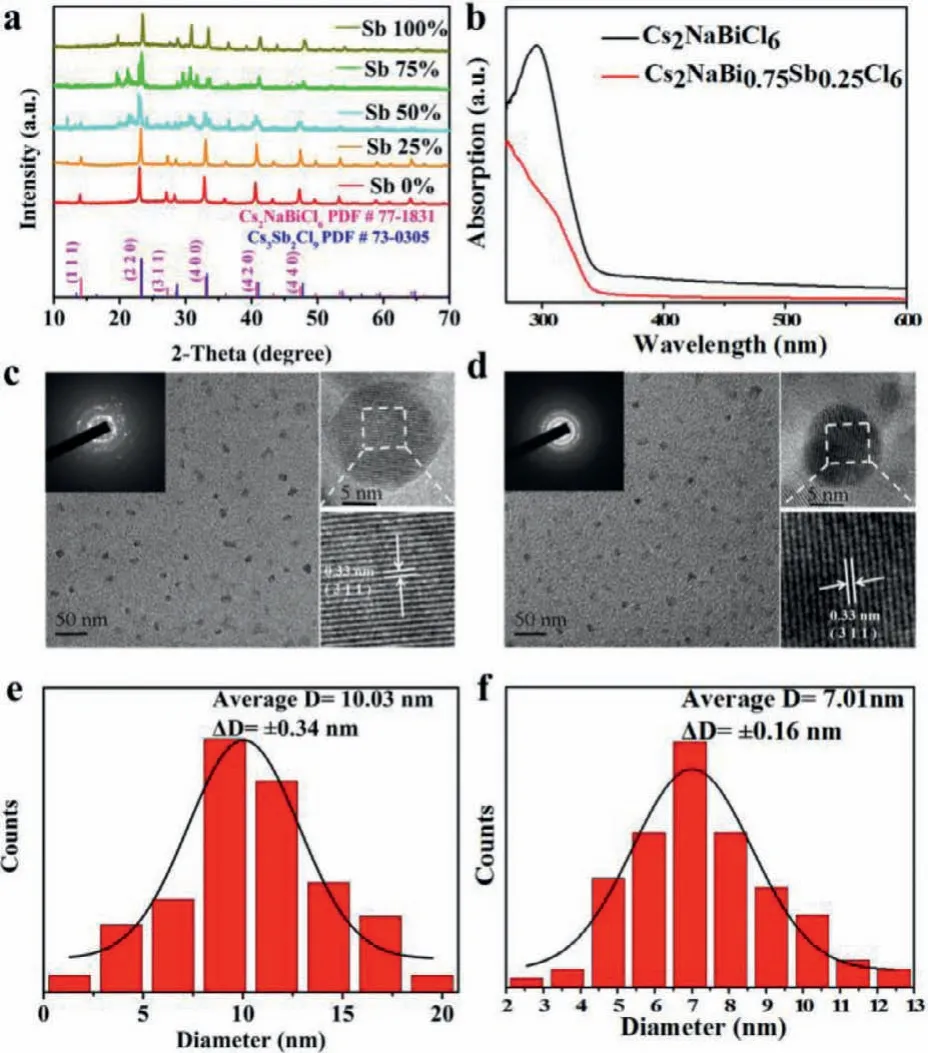
Fig.1.(a)XRD patterns of Cs2NaBi1-xSbxCl6(x=0,0.25,0.5,0.75,1).(b)Steadystate absorption spectra of Cs2NaBiCl6 and Cs2NaBi0.75Sb0.25Cl6.(c,d)Low-resolution TEM images of Cs2NaBiCl6 and Cs2NaBi0.75Sb0.25Cl6 NCs(insets:electron diffraction patterns and associated HRTEM images).(e,f)The size distributions of Cs2NaBiCl6 and Cs2NaBi0.75Sb0.25Cl6 NCs.
In order to further clarify the presence of Sb3+,the X-ray photoelectron spectroscopy(XPS)technique was conducted.The full spectra revealed that Cs,Na,Bi,Cl existed in both Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6NCs(Fig.S3 in Supporting information).As shown in Fig.S3d,two peaks of Sb 3d appearing at 539 eV and 531 eV in Cs2NaBi0.75Sb0.25Cl6sample confirmed the successful incorporation of Sb3+ions in Cs2NaBiCl6NCs.Furthermore,compared the high-resolution XPS spectra of Bi 4f and Na 1s of Cs2NaBi0.75Sb0.25Cl6and Cs2NaBiCl6NCs(Fig.S4 in Supporting information),the binding energy of Bi 4f shifted slightly to lower energy while Na 1s has little variations.The slight shift could be attributed to the lattice contraction caused by the introduction of Sb3+ions,probing the above speculation that Sb3+ions occupied Bi3+sites instead of Na+sites[32].
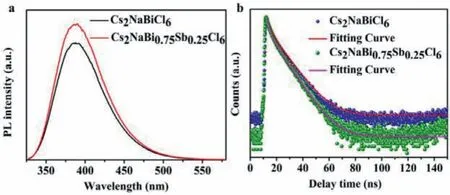
Fig.2.(a)PL spectra of Cs2NaBiCl6 and Cs2NaBi0.75Sb0.25Cl6 NCs.(b)Time-resolved PL traces of Cs2NaBiCl6 and Cs2NaBi0.75Sb0.25Cl6 NCs measured with TCSPC.
Next,the steady-state optical properties of Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6NCs were investigated.Bright blue-violet emission could be seen under UV lamp(Fig.S5 in Supporting information).From the absorption spectra,it was observed that a prominent absorption peak appeared at 295 nm for Cs2NaBiCl6while a broad peak at 312 nm was shown for Cs2NaBi0.75Sb0.25Cl6NCs(Fig.1b).The 295 nm absorption peak could be assigned to1S0→3P2forbidden transition whereas the 312 nm peak was associated with1S0→3P1partially allowed transition of the localized[BiCl6]3−octahedron in colloidal solution[33].The band gap,calculated from the corresponding Tauc plot,fell at 3.04 eV and 2.92 eV which illustrated a red-shift trend(Fig.S6 in Supporting information).According to the previous research,the valence band was shifted up by the incorporation of Sb3+5s orbitals while the 5p orbitals of Sb3+caused a lower conduction band,resulting in a narrower band gap[31].
To look deep into the luminescence features of Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6NCs,PL spectra and PLQYs of these NCs were studied.The highest emission intensity was observed for 25% Sbdoped Cs2NaBiCl6NCs.Meanwhile,the slight decrease in luminescence intensity was clearly visible in PL spectrum for the higher Sb3+contents,which was due to the concentration quenching and the presence of by-product Cs3Sb2Cl9.(Fig.S7 in Supporting information)[33].In contrary to the weak emission with a low PLQY of Cs2NaBiCl6NCs in published literature,the pristine Cs2NaBiCl6prepared by LARP route exhibited a PLQY up to 39.05%(Fig.S8 in Supporting information).By using hot-injection method,inhomogeneous morphologies and impurity CsCl were found under different temperature which probably led to the low PLQY.Thus,the LARP approach used in this work effectively avoided such shortcomings and the ligand(OA)passivated the surface defects of the NCs,resulting in an improved PLQY[25].Interestingly,the PL peak position of Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6showed no variation(Fig.2a),which indicated that the PL transition was derived from the radiative recombination of the internal energy states instead of the band-edge electrons/holes recombination[31].It was proposed that the self-trapped excitons(STEs)played an important role in the emission process.The observable PL in Cs2NaBiCl6was attributed to the dark state STEs[31].After trace Sb3+doping,the emission intensity was greatly improved while maintaining the same peak position at 388 nm,which illustrated that the dark STEs in Cs2NaBiCl6transformed to bright STEs in Cs2NaBi0.75Sb0.25Cl6[12].The transition from dark state to bright state was probably related to two aspects.Firstly,the parity-forbidden transition could be broken by the interaction of[SbCl6]3−and[BiCl6]3−,resulting in effective PL emission.Secondly,reduced lattice vibrations could increase radiative recombination pathway[12].To prove the smaller lattice vibrations caused by Sb3+doping,Raman spectra was investigated(Fig.S9 in Supporting information).It was obvious that the peak position of Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6remained the same,but the vibration intensity decreased after alloying Sb[12].More importantly,the PLQY value of Cs2NaBi0.75Sb0.25Cl6NCs was measured to be 46.57%(Fig.S8),suggesting a significant improvement compared to other ion alloyed Cs2NaBiCl6NCs(Table S2 in Supporting information).For the purpose of better understanding the mechanism,the wavelength-dependent PL emission spectrum and PL excitation(PLE)spectrum of Cs2NaBi0.75Sb0.25Cl6were carried out.The PL curves were almost the same at different excited wavelength which proved that the emission was not aroused from permanent defects(Fig.S10a in Supporting information)[12].The almost same PLE profile verified the same excited state origin of these emission bands(Fig.S10b in Supporting information)[31].As shown in Scheme S1(Supporting information),the emission energy of STE could be calculated by the formula:ESTE=Eg-Eb- Est- EdwhereEgrepresents the band gap,Ebrepresents the binding energy of free exciton,Estrepresents the self-trapping energy,andEdrepresents the lattice deformation energy[34].Based on the strong exciton-phonon coupling existed in pure Cs2NaBiCl6and Cs2NaSbCl6[26],Cs2NaBi0.75Sb0.25Cl6promoted the formation of STEs and produced smallEstandEd.Therefore,the values ofESTEandEgwas very close which could lead to the strong blue emission[31].
In order to shed more light on the recombination dynamics of Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6NCs and clarify the implication of Sb,time-resolved PL(TR-PL)measurements using timecorrelated single photon counting(TCSPC)was carried out.The two decay curves could be fitted well by a biexponential function(Fig.2b).For Cs2NaBiCl6,a fast decay component(short lifetimeτ1)assigned to charge carrier trapping processes was about 2.5 ns and a slow decay component(long lifetimeτ2)associated with the band-to-band radiative recombination was 9.8 ns.For Cs2NaBi0.75Sb0.25Cl6,a long-lived lifetime of 8.7 ns and a shortlived lifetime of 1.9 ns were obtained(Table S3 in Supporting information).It was well-known that the ground state of Sb3+was represented by the1S0atomic term,and four energy levels of1P1,3P0,3P1and3P2constituted the excited state(sp).The1S0−1P1transition was allowed,and the1S0−3P1transition was partially-allowed because of the spin-orbit coupling,while the1S0−3P2and1S0−3P0transitions were completely prohibited at the electric dipole transition level[35].Upon the theory and research of Reisfeldet al.,it was reasonable to speculate that there could exist two recombination routes.One was the excited state of3P0→1S0recombination in Sb3+with a faster decay rate and the other was the excited state of3P1→1S0with a slower decay rate[35].It meant that the incorporation of Sb3+increased the possibility of radiation recombination by breaking the local symmetry so that the initial parity-forbidden transition could occur,which led to the improvement of PLQY[21].Besides,both the percentages ofτ1andτ2of Cs2NaBiCl6were 50% while the percentages ofτ1andτ2of Cs2NaBi0.75Sb0.25Cl6were 49% and 51%.The similar percentages of two NCs represented the same emission mechanism which is consistent with the inference that Sb alloying caused STEs to change from dark state to bright state.

Fig.3.(a)The percentages of PL intensity of Cs2NaBiCl6(3.4 mg/mL)and Cs2NaBi0.75Sb0.25Cl6(2.3 mg/mL)solutions at different times when exposed to air.The XRD patterns of(b)Cs2NaBi0.75Sb0.25Cl6 powder and(c)Cs2NaBiCl6 powder after 7 days and 5 months being placed in the air.(d)The percentages of PL intensity at different times when 3% water were added to 5 mL supernatant of two NCs.
The stability of perovskite NCs was also directly relevant to the industrial applications.To evaluate the air stability of Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6NCs solutions,the PL evolution was measured with different storage time in ambient atmosphere.As illustrated in Fig.3a,the PL intensity in first few days of two solutions increased slightly which was assigned to the elimination of dangling bonds[36].With the increasing time placed in air,the PL intensity gradually declined owing to the formation of surface defects after air exposure[36].It was worth noting that the Cs2NaBi0.75Sb0.25Cl6solution retained 91% of original photoluminescence after 40 days while Cs2NaBiCl6solution retained only 83% of original photoluminescence after 26 days(Fig.3a).Furthermore,the powder of Cs2NaBi0.75Sb0.25Cl6NCs exhibited better stability in air,maintaining the XRD pattern even after 5 months(Fig.3b),where impurity appeared in the XRD pattern of undoped Cs2NaBiCl6sample(Fig.3c).The higher air stability of Cs2NaBi0.75Sb0.25Cl6NCs compared to undoped NCs was attributed to the reduction of volume defects which resulted in the improvement of short-range order of the Cs2NaBi0.75Sb0.25Cl6NCs[12].Moreover,the water-stability of the Cs2NaBiCl6and Cs2NaBi0.75Sb0.25Cl6colloidal solution were investigated by adding 3% deionized water.It was observed that Cs2NaBi0.75Sb0.25Cl6remained 68% of initial intensity after 8 days while the intensity of Cs2NaBiCl6was dropped to merely 20% after 5 days(Fig.3d).The better water-stability was probably due to the formation of SbOCl on the surface.The XPS peaks located at 540.0 eV and 532.9 eV were associated with Sb3+3d3/2and 3d5/2.An additional peak of Sb-O at 531.7 eV appeared(Fig.S11a in Supporting information),which was attributed to the presence of Sb-O on the crystal surface due to the hydrolysis of antimony chloride.Besides,the XRD patterns of Cs2NaBi0.75Sb0.25Cl6before and after adding water were investigated(Fig.S11b in Supporting information),where SbOCl phase was identified in Cs2NaBi0.75Sb0.25Cl6after adding 3% water[27].All the results demonstrated the superior stability of Cs2NaBi0.75Sb0.25Cl6comparing to various ion doping strategies used for Cs2NaBiCl6NCs in the literatures(Table S4 in Supporting information).The excellent air-stability of Cs2NaBi0.75Sb0.25Cl6NCs laid a solid foundation of its wide application and the research results provided a new example for the future study of Sb-based double perovskite.
In summary,Cs2NaBiCl6NCs were fabricatedviaa roomtemperature LARP approach with a high PLQY of 39.05%.The strategy was extended to the synthesis of Cs2NaBi0.75Sb0.25Cl6NCs,which showed a strong blue photoluminescence with an improved PLQY of 46.57%.Additionally,the powder and the solution of Cs2NaBi0.75Sb0.25Cl6NCs exhibited superior stability in ambient environment.The excellent optical performance and outstanding stability of Cs2NaBi0.75Sb0.25Cl6perovskite NCs prepared by this simple route would expand its potential application for optoelectronic devices.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was sponsored by the National Natural Science Foundation of China(Nos.21475021,21427807 and 21777096),the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.071.
 Chinese Chemical Letters2022年1期
Chinese Chemical Letters2022年1期
- Chinese Chemical Letters的其它文章
- Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation:A review
- Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers
- Porphyrin-based heterogeneous photocatalysts for solar energy conversion
- Systematic evaluation of advance in application and discharge mechanism of solution electrode glow discharge
- Insoluble carbonaceous materials as electron shuttles enhance the anaerobic/anoxic bioremediation of redox pollutants:Recent advances
- Selective N-terminal modification of peptides and proteins:Recent progresses and applications
