Synthesis and insecticidal evaluation of novel sulfide-containing amide derivatives as potential ryanodine receptor modulators
Yan Zhang,Yuxin Li,Huan Li,Junfeng Shang,Zhengming Li,Baolei Wang
State Key Laboratory of Elemento-Organic Chemistry,College of Chemistry,Nankai University,Tianjin 300071,China
ABSTRACT With the aim of discovering new bioactive pesticides for crop protection,a series of novel sulfidecontaining amide derivatives A were efficiently synthesized via a strategy of modifying the “amide” structure of anthranilic diamide insecticides.The single-crystal structures of A2-3 and A4-5 were firstly reported.The bioassay results showed that most of the synthesized compounds display moderate to high insecticidal activities.Particularly,some sulfone-containing compounds, e.g., A2-3,A3-3 and A6-3,not only possessed favorable lethality rate(50%–100%)against P.xylostella at a concentration of 0.1 mg/L,but also held good activities towards a variety of agricultural pests such as M.separata, C.pipiens pallen, H.armigera and O.nubilalis;the larvicidal activities of A4-1 and A6-1 towards P.xylostella were close to that of chlorantraniliprole at 0.01 mg/L.The calcium imaging experiments revealed that the representative compounds A2-3 and A6-3 are potential ryanodine receptor(RyR)modulators.The structure–activity relationships were discussed in detail.These results provide useful information for further design and development of novel insecticides.
Keywords:Sulfide-containing amide derivatives Sulfoxide Sulfone Insecticidal activities Calcium channel
The discovery and development of new pesticides with novel structures and excellent biological activities are perpetual objectives for agrochemical researchers[1–5].The phthalic diamides and anthranilic diamides which are two kinds of amide derivatives have been given great attention in agrochemistry area during the recent 15 years[6–9].This is mainly because compounds with such structural features usually possess exceptional insecticidal activities with broad spectrum towards various pests,such as Lepidoptera,Coleoptera,Isoptera and Diptera pests;most importantly,some of which—flubendiamide,chlorantraniliprole and cyantraniliprole(Fig.1,I–III)have been successfully developed as commercial insecticides by Nihon Nohyaku Co.,Ltd.and DuPont,respectively[10–12].These insecticides are known as highly potent and selective modulators of insect ryanodine receptor(RyR,also known as the calcium ion channel receptor)[11,13].Recently,another anthranilic diamide insecticide cyclaniliprole(Fig.1,IV)has also been successfully developed by Ishihara Sangyo Kaisha[14].However,because of the overuse of these insecticides,resistance issue has been developed in some pests,such asTuta absoluta,Plutellidae PlutellaandSpodoptera exigua[15–17].For example,from 2010 to 2011,there was a high-level resistance inPlutellidae Plutellapopulations reported in Philippines and China[16].Moreover,food safety concerns about the application of chlorantraniliprole and flubendiamide have also been reported recently[18].Accordingly,developing new insecticides to overcome resistance and safety issues associated with the diamide insecticides is urgently needed.The structural modification based on these diamide insecticides has been considered as one of the significant and feasible ways to do the insecticide innovations.
The common structural characteristic of these insecticides is that there are two amide groups in each of the molecules.For the structural modification on the anthranilic diamide insecticides,e.g.,chlorantraniliprole,there are a few reports about the investigations on the amide bond modifications compared to many explorations of phenyl group or pyridylpyrazole motif transformations[19–22].The acyl thiourea[9,23],heterocycle such as aziridine[24],β-lactam[24]and benzotriazinone[25],and aminomethylphosphonate[26]motifs as new amide bond substitutes of such diamide structure have been reported,resulting in promising insecticidal or fungicidal activities of the corresponding new compounds.Therefore,more new forms of amide bond modifications based on anthranilic diamide structures are expected to produce potential breakthroughs for discovering novel agrochemicals.

Fig.1.The structures of some diamide- and sulfide-containing insecticides,and the designed target compounds A in this paper.
Sulfides,an important class of agrochemicals,have attracted much attention because of their versatile bioactivities and easy derivatizable structures.Many sulfur-containing compounds have been applied in almost all kinds of agrochemicals,such as herbicides[27],insecticides[28,29]and fungicides[30].It is mentioned by Ando[31]that the sulfur atom of organosulfur-containing compounds can offer reactive site for environmental degradation and for further derivatization,and ultimately lead to versatile bioactivities.It is also reported that more than 30% of all the agrochemicals contained at least one sulfur atom[32].Therefore,developing new sulfur-containing compounds may be an important aspect in future pesticide research.
Thioethers,sulfoxides and sulfones are three kinds of sulfides.The sulfur-containing groups of which are important pharmacophores of many insecticides.For examples,vaniliprole(Fig.1,V)[28]has a trifluoromethylthio moiety(thioether motif),fipronil(Fig.1,VI)[29]and flubendiamide(Fig.1,I)have sulfinyl and sulfonyl groups,respectively.With this in mind,based on a strategy of replacing one of the amide moieties of anthranilic diamide insecticides with sulfide/sulfinyl/sulfonyl group,a series of novel thioether-containing amide derivatives and their oxidation products-sulfoxide/sulfone-containing amide derivatives were synthesized in this paper.Their insecticidal activities and mode of action for the calcium ion modulations were investigated.
The intermediates 2 were synthesized referring to a reported similar procedure(Scheme 1)[33–35].The synthesis for compound 3a is according to a similar procedure in literature(Scheme 1)[36]:6-chlorobenzo[d]thiazol-2-amine 2a(20 mmol),KOH(5 times the weight of thiazole amine)and water(100 mL)were placed in a round-bottom flask and heated under reflux for 8 h.Upon completion of the reaction and cooling down to room temperature,the system was poured into ice water and adjusted pH value to 7 with 1 mol/L hydrochloric acid.After that,the mixture was extracted with ethyl acetate(3 × 30 mL)and the combined organic phase was dried over anhydrous Na2SO4.The solvent was removed to afford the corresponding aryl thiol.Without further purification,the crude aryl thiol was mixed with KOH(40 mmol)and water(50 mL),and the mixture was warmed up to reflux for 1 h,then cooled down to room temperature.After being added the methyl iodide(20 mmol),the system was further turned to reflux for about 3 h until completion of the reaction,then cooled down.The reaction system was poured into ice water and conducted extraction with ethyl acetate(3 × 20 mL).The combined organic phase was washed with brine and dried over Na2SO4.After removal of solvent,the residue was further purified using column chromatography with ethyl acetate and petroleum ether(1:2,v/v)as solvents to afford the intermediate 4-chloro-2-(methylthio)aniline(3a).

Scheme 1.Synthetic route of the intermediates 2 and 3.
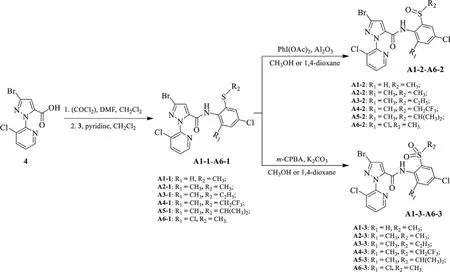
Scheme 2.Synthetic route of the title compounds A.
The synthetic procedure for compounds 3b,3c,3e and 3f was similar to that of 3a in general.The difference is the reaction condition for the first step-thiazole amine 2(2a or 2c,20 mmol),KOH(5 times the weight of thiazole amine)and ethylene glycol(100 mL)were mixed and heated under reflux for about 12 h.The synthesis for compound 3d:compound 2b(20 mmol),KOH(5 times the weight of thiazole amine),and ethylene glycol(100 mL)were mixed and the mixture were refluxed with stirring for about 12 h.Upon completion of the reaction and cooling down to room temperature,the system was poured into ice water and adjusted pH value to 7 with 1 mol/L hydrochloric acid.After that,the mixture was extracted with ethyl acetate(3 × 30 mL)and the combined organic phase was dried over anhydrous Na2SO4.The solvent was removed to afford the corresponding aryl thiol.Without further purification,the crude aryl thiol was mixed with K2CO3(22 mmol)andN,N-dimethylformamide(DMF)(20 mL),and the mixture was warmed up to reflux for 3 h,then cooled down to room temperature.After being added trifluoroiodoethane(20 mmol),the system was further turned to reflux for about 7 h until completion of the reaction,then cooled down.The reaction system was poured into ice water and conducted extraction with ethyl acetate(3 × 20 mL).The combined organic phase was washed with brine and dried over Na2SO4.After removal of solvent,the residue was further purified using column chromatography with ethyl acetate and petroleum ether(1:3,v/v)as solvents to afford the intermediate 4-chloro-2-methyl-6-((2,2,2-trifluoroethyl)thio)aniline(3d).
The synthesized alkylthioarylamine(3,10 mmol),pyridine(10.1 mmol)and dichloromethane(20 mL)were placed in a 100 mL round-bottom flask and the system was cooled to 0 °C using an ice bath.A dichloromethane(10 mL)solution of pyrazole acyl chloride,which was freshly prepared by carboxylic acid 4(10 mmol)according to a reported procedure[23,26],was added dropwise to the above cold system.After that,the reaction mixture was warmed to room temperature and stirred for about 5 h(monitored by TLC),then was concentrated under reduced pressure.The residue was redissolved with ethyl acetate(30 mL),and the solution was washed with brine.The organic phase was dried over anhydrous Na2SO4.After removal of the solvent,the residue was conducted for column chromatography with ethyl acetate and petroleum ether(1:3,v/v)as solvents to afford the thioethercontaining amide compounds A1-1–A6-1(Scheme 2).
Methylthio-containing amide compound A1-1(1 mmol),PhI(OAc)2(3 mmol)and Al2O3(1 mmol)were mixed in methanol(20 mL),the reaction system was stirred at room temperature for 3 h[monitored by thin layer chromatography(TLC)]and concentrated under reduced pressure.To the residue CH2Cl2(20 mL)was added,the mixture was washed with brine and the organic phase was dried over anhydrous Na2SO4.After removal of the solvent,the residue was further purified by column chromatography with ethyl acetate and petroleum ether(1:2,v/v)as solvents to afford the sulfoxide-containing compound A1-2.Using the same procedure,the sulfoxide-containing compounds A2-2–A6-2(1,4-dioxane solvent used in the case of A4-2,Scheme 2)and A4-4(Scheme 3)were synthesized successfully.
Methylthio-containing amide compound A1-1(1 mmol),m-CPBA(3 mmol)and K2CO3(1 mmol)were mixed in methanol(20 mL),the reaction system was heated to reflux for 3 h(monitored by TLC)and then concentrated under reduced pressure.To the residue CH2Cl2(20 mL)was added,the mixture was washed with brine and the organic phase was dried over anhydrous Na2SO4.After removal of the solvent,the residue was further purified by column chromatography with ethyl acetate and petroleum ether(1:1,v/v)as solvents to afford the sulfone-containing compound A1-3.Using the same procedure,the sulfone-containing compounds A2-3–A6-3(1,4-dioxane solvent used in the case of A4-3,Scheme 2)and A4-5(Scheme 3)were synthesized successfully.
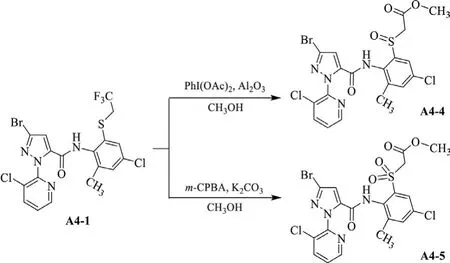
Scheme 3.Synthetic route of the ester by-products A4-4 and A4-5.
The insecticidal evaluation and calcium imaging experiments of the synthesized compounds were conducted according to the reported methods[19,21,23,37–39],the detailed procedures were presented in the Supporting information.
The key intermediates 2 can be efficiently prepared from the corresponding 2-substituted-4-chloroaniline(1)as the starting materialviaa synthetic route shown in Scheme 1.The intermediates alkylthio-containing arylamine 3 were prepared from a KOHdecomposition of benzothiazole amine 2,followed by an alkylation of the generatedo-aminophenthiol under base-catalyzing in moderate to high yield(45%–83%).For the substrates 2,when R1is H,the first step reaction of the corresponding compound is easier than that of CH3or Cl,indicating an influence of the steric hindrance effect from substituent in 4-position of benzothiazole(R1)on its reactivity.The following comparison of reaction temperature and time can well reflect this influence:boiling in water(b.p.100 °C),8 h(R1=H);boiling in ethylene glycol(b.p.197°C),12 h(R1=CH3or Cl).Interestingly,for the alkylation reagents R2I,when R2is alkyl group[CH3,C2H5and CH(CH3)2],the second step reaction for preparing 3 is much easier than that of trifluoroethyl group(CH2CF3),which may be due to the easy elimination side-reaction of the reagent CF3CH2I under refluxing conditions in strong base solution(KOH-H2O)(while in K2CO3-DMF system,the reaction underwent smoothly).
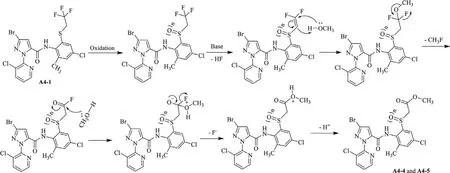
Scheme 4.Possible synthetic mechanism for compounds A4-4 and A4-5.
The thioether-containing amide compounds A1-1–A6-1(Scheme 2)can be preparedviathe reaction of alkylthio-containing arylamine(3)and pyridylpyrazole acyl chloride generated from pyridylpyrazole acid(4)using pyridine as acid binding agent with good yields.When using triethylamine as acid binding agent,it was found that such reaction will produce certain amount of disubstituted by-product,e.g.,compounds A7 and A8(Scheme S1 in Supporting information)from their respective reactants 3b and 3d.This indicates the weaker base(pyridine)is better for synthesizing the monosubstituted target structures.Treated with different oxidants,the thioether-containing product(A1-1–A6-1)can be directly oxidized to the corresponding sulfoxide-containing product(A1-2–A6-2 and A4-4)in PhI(OAc)2-Al2O3system or sulfone-containing product(A1-3–A6-3 and A4-5)inm-CPBAK2CO3system.It is especially worth mentioning that the reflux condition for the sulfone-containing product was necessary in view of the situation that only sulfoxide-containing products can be obtained at room temperature.According to the literature[40],methanol was more beneficial to the oxidation reaction when a solvent was used.When using compound A4-1(R2=CH2CF3)as the reactant to do the oxidation in the methanol solvent,neither PhI(OAc)2-Al2O3norm-CPBA-K2CO3could efficiently give the desired target products(A4-2 and A4-3).However,an abnormal side reaction was found to form the ester by-products(A4-4 and A4-5,Scheme 3),which was further confirmed by a single crystal structure of A4-5(Fig.2).By taking into account the reaction conditions,the mechanism of this abnormal reaction was suggested as shown in Scheme 4.We speculated that this is due to the extremely strong electron-withdrawing effect of the generated sulfinyl/sulfonyl group in the reaction led to the easy occurence of following elimination and alcoholysis of trifluoroethyl group in methanol.As a result,aprotic solvent—1,4-dioxane as an alternative of methanol was chosen to do such oxidation reactions,smoothly affording the target products(A4-2 and A4-3).
The title compounds A were identified by melting points,1H nuclear magnetic resonance(NMR),13C NMR and19F NMR spectra.The measured elemental analysis or high resolution mass spectrometry(HRMS)data were also consistent with the corresponding calculated values.When R1and R2are the same,the characteristic proton peak of N-H in1H NMR spectra exhibited a chemical shift sequence of sulfoxide-containing amides(A1-2–A6-2 and A4-4,δ9.25–11.65 ppm)>sulfone-containing amides(A1-3–A6-3 and A4-5,δ8.71–10.36 ppm)>thioethercontaining amides(A1-1–A6-1,δ7.59–8.84 ppm).The pyrazole-H showed a similar sequence—sulfoxide-containing amides(δ7.05–7.31 ppm)>sulfone-containing amides(δ6.98–7.08 ppm)>thioether-containing amides(δ6.89–6.98 ppm).While the trend of chemical shift for the protons on alkyl carbon attached to sulfur atom generally was sulfone-containing amides(δ3.05–4.18 ppm)>sulfoxide-containing amides(δ2.71–3.88 ppm)>thioethercontaining amides(δ2.32–3.34 ppm).These results are mainly due to the different electronic effect of–S–,–S(=O)–and–S(=O)2–groups.Moreover,when R2=CH2CF3,the1H NMR and13C NMR spectra for compounds A4-1,A4-2 and A4-3 showed up chemical shifts ofmoiety atδ3.34–3.97 ppm andδ36.7–57.5 ppm,respectively,which were both split into9.6 Hz)andowing to the couplingsplitting of F to neighboring H or C.Meanwhile,the typical carbon chemical shift for the CF3group appeared atδ119.6–125.1 ppm as quartets(J=277.8–279.8 Hz).In the19F NMR spectra of the compounds,the fluorine signals were observed atδ−93.9–60.5 ppm(A4-1,A4-2,A4-3 and A8),among which the F signal of sulfoxidecontaining compound A4-2(δ−93.9 ppm)exhibited a much more upfield shift than that of the others(δ−66.0–−60.5 ppm).
The structures of compounds A2-3(CCDC No.1961832)and A4-5(CCDC No.1961841)were further confirmed by single crystal Xray diffraction analysis,which are shown in Fig.2.The corresponding crystal data were provided in the Supporting information.From the molecular structures,it can be seen that the dihedral angle between pyrazole ring and pyridine ring are 69.274(210)o(A2-3)and 51.603(75)o(A4-5),respectively,which revealed that the two heterocyclic rings are not coplanar.Furthermore,the torsion angles of N(3)-N(2)-C(5)-N(1)in A2-3,and N(1)-N(2)-C(5)-N(3)in A4-5 are 69.926(758)oand −123.348(220)o,respectively,indicates that the pyridine ring flipped about 180obetween the two molecular structures,which can be clearly seen from the Fig.2 that the relative positions of the pyridine ring towards pyrazole ring in two molecules are reverse.This may be derived from maintaining the lowest energy optimal configuration of the molecular structure to reduce the steric hindrance of groups.Therefore,the electronic effect and large steric hindrance of substituent in benzene ring(A4-5:R2=CH2CO2CH3;A2-3:R2=CH3)were the main possible fac-tors leading to this result,which also may be the same reason for the great difference of the bioactivity between the two compounds.
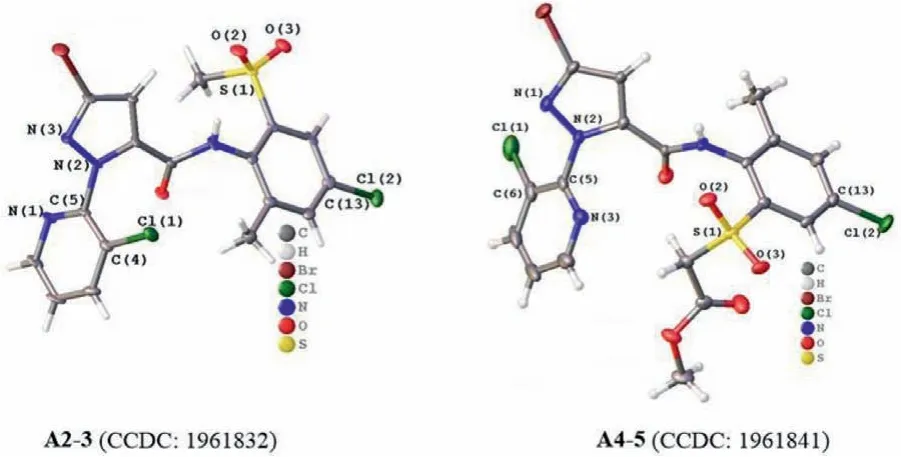
Fig.2.The single-crystal structures of A2-3 and A4-5.
At an initial test concentration of 200 mg/L,all the title compounds A exhibited obvious insecticidal activities againstM.separata(20%–100%).As shown in Table 1,we can get the following structure–activity relationship(SAR).When R2was fixed as CH3,compounds bearing CH3or Cl group for R1(e.g.,A2-3 and A6-3,100%)exhibited much higher larvicidal activity than those of bearing non-substituent(R1=H)[e.g.,A1-3,(20 ± 5)%].At lower concentrations,some of the compounds still showed good activity againstM.separata.For examples,A2-3,A3-3,A4-1 and A6-3 held lethality rate of 50%–100% at 25 mg/L;particularly,A2-3 and A6-3 at 10 mg/L possessed lethality rate of(60 ± 2)% and(45 ± 1)%,respectively.Through SAR analysis,it was found that when fixing R1and R2as electron-donating groups,smaller substituents in R2produce better bioactivity[sequence:CH3>C2H5>CH(CH3)2].While when fixing R2as CH3,the insecticidal activity trend corresponding to R1substituent was CH3~Cl.Furthermore,when R1and R2were fixed as CH3or Cl,and CH3or C2H5,respectively,it showed an activity sequence of sulfone-containing compounds>sulfoxide-containing compounds ≥thioether-containing compounds in the same series of compounds,that is,A2-3>A2-2>A2-1;A3-3>A3-2 ≈A2-1;A6-3>A6-2>A6-1.This indicated that the stronger electron-withdrawing ability of the sulfursubstituents is favorable for the improvement of the bioactivity,in other words,the more O atoms the S atom connects,the higher insecticidal activity the corresponding compound has.However,when R1=CH3and R2=CH2CF3,the larvicidal activity sequence was thioether-containing compound[A4-1,(50 ± 2)%(25 mg/L)]>sulfoxide-containing compound[A4-2,(60 ± 3)%(100 mg/L)]>sulfone-containing compound[A4-3,(20 ± 0)%(100 mg/L)].Compounds A4-4 and A4-5,unexpectedly obtained from the oxidation reaction of thioether compound A4-1 in methanol solvent,showed common larvicidal activity againstM.separataat 100 mg/L[(35± 1)% and(50 ± 2)%].So we speculated that bearing electronwithdrawing and bulky groups together for R2are unfavorable to the increase of bioactivity againstM.separata,meanwhile the CF3moiety in the case of A4-1 may also contribute to the bioactiv-ity improvement,possibly due to the effect of fluorine atom(e.g.,lipophilicity and metabolic stability).In addition,from Table 1 the title compounds containing sulfone moiety were found to possess much better bioactivity than that of the others.
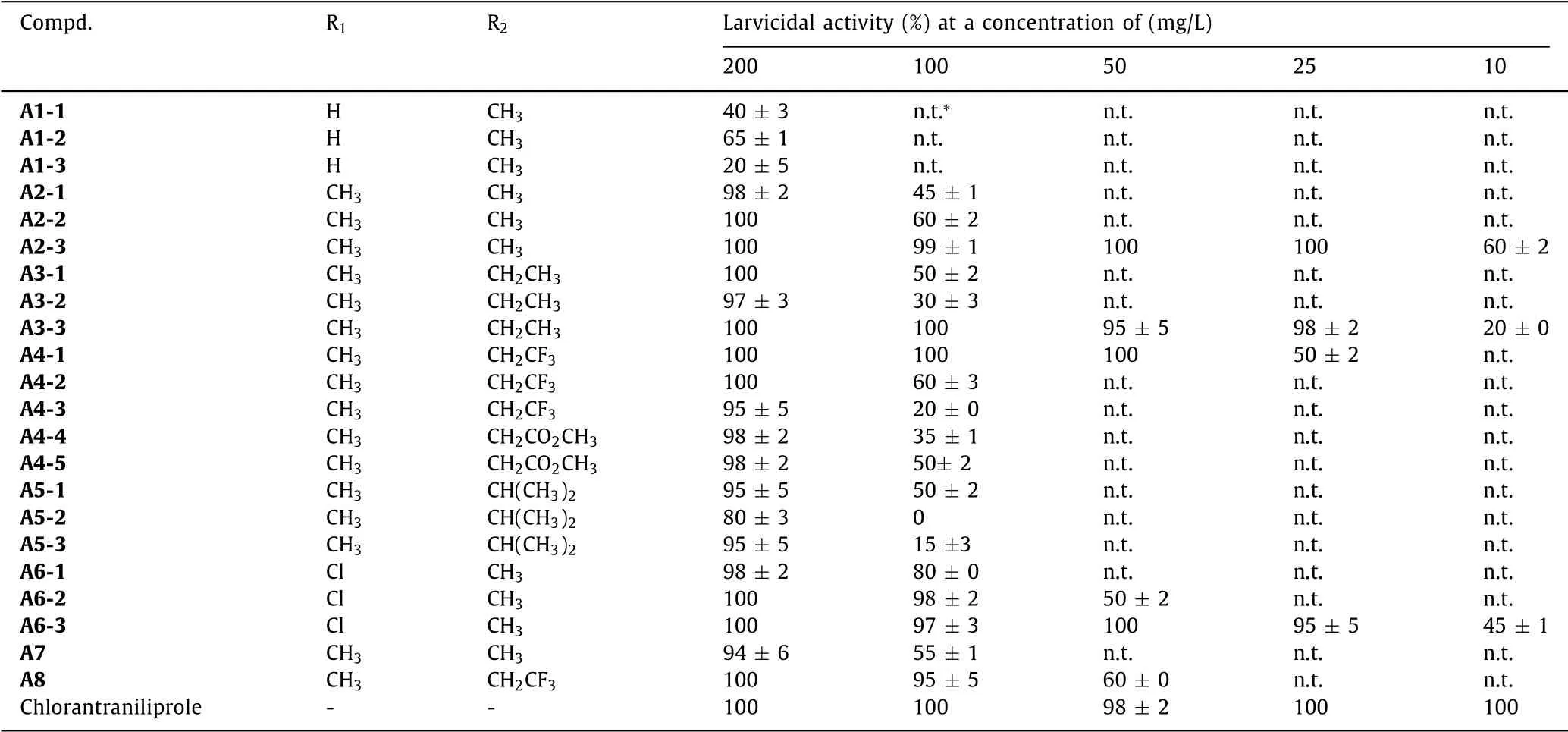
Table 1 Insecticidal activity of compounds A against M.separata.
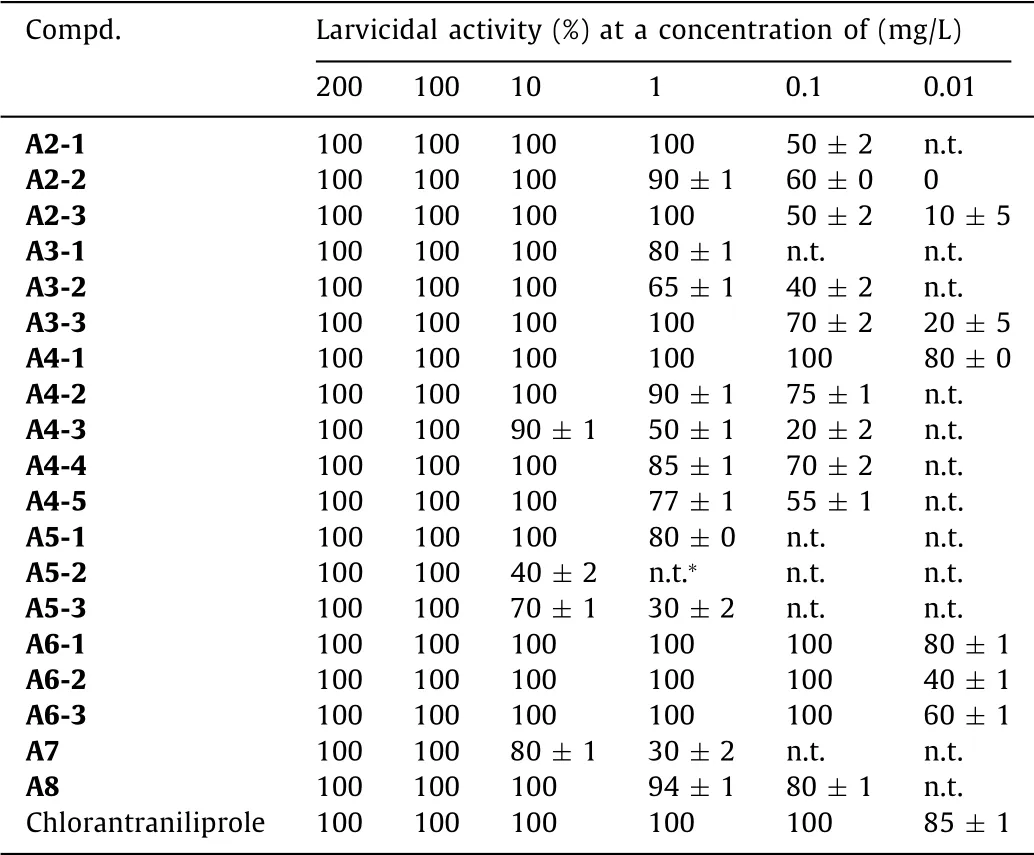
Table 2 Insecticidal activity of partial compounds A against P.xylostella.
From the insecticidal activity data of compounds A againstP.xylostellalisted in Table 2,we can see that all the tested compounds exhibited high larvicidal activity(40%–100%)towardsP.xylostellaat 10 mg/L,and most of compounds also showed obvious insecticidal activity(20%–100%)at lower concentration of 0.1 mg/L.Compounds A4-1,A6-1,A6-2 and A6-3 can still possess lethality rate of 40%–80% even at 0.01 mg/L concentration,especially the larvicidal activities of trifluoethylthio-containing compound A4-1[(80 ±0)%]and methylthio-containing compound A6-1[(80 ± 1)%]were found very close to that of positive control chlorantraniliprole[(85± 1)%]towardsP.xylostella.Through SAR analysis,it was found that when fixing R1as CH3,the activity trend of R2to the insecticidal activity of the compounds againstP.xylostellawas similar to that againstM.separata,that is,smaller substituents in R2produce better bioactivity:-CH3>C2H5>CH(CH3)2.However,when R1was fixed as CH3,the tested compounds with CH2CF3or CH2CO2CH3of R2almost possessed better bioactivity than those with CH3,C2H5and CH(CH3)2,which indicates a R2group with weaker electron-donating ability may be favorable for the bioactivity improvement of such kind of structures.Noticeably,all the compounds had favorable larvicidal activity when R1=Cl and R2=CH3,showing a lethality rate of 40%–80% at 0.01 mg/L.When R2was fixed as CH3,the bioactivity sequence corresponding to substituent R1was Cl>CH3.Moreover,compounds A2-2,A2-3,A3-3,A4-1,A6-1,A6-2 and A6-3 were further tested lethal concentration 50%(LC50)values towardsP.xylostellaand the results are shown in Table 3.These compounds possessed LC50value of 0.0046–0.1306 mg/L,higher than that of control chlorantraniliprole(0.0031 mg/L);among which,thioether-containing compound A6-1 with LC50value of 0.0046 mg/L exhibited as the best insecticide,and had a great potential for further investigation.
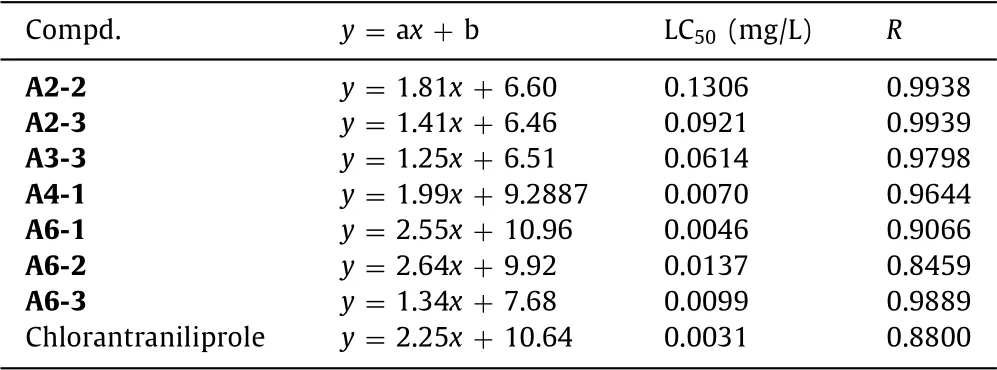
Table 3 LC50 values of partial compounds A against P.xylostella.
Partial compounds with favorable activities in preliminary screening were selected to further test their insecticidal activities againstC.pipiens pallens,H.armigeraandO.nubilalis,and the results are shown in Table 4.We can see that towardsC.pipiens pallens,the tested compounds showed high insecticidal activity(95%–100%)at 25 mg/L;however,when decreasing the test concentration to 10 mg/L,the compounds showed only moderate insecticidal activity(15%–55%).When fixing R1as CH3,the insecticidal activity of sulfone-containing compounds displayed a trend of C2H5[A3-3,(55 ± 1)%]>CH3[A2-3,(40 ± 0)%]for R2group.Meanwhile,when R2was fixed as CH3,the insecticidal activity trend of R1was CH3[A2-3,(40 ± 0)%]>Cl[A6-3,(15 ± 3)%]for sulfonecontaining compounds.Moreover,it also showed an activity trend of sulfoxide-containing compound[A2-2,(50 ± 2)%]>sulfonecontaining compound[A2-3,(40 ± 0)%],when both bearing CH3for R1and R2.All the four tested compounds displayed favorable larvicidal activities(95%–100%)towardsH.armigeraandO.nubilalisat 200 mg/L.In particular,sulfone-containing compounds A2-3 and A6-3 held lethality rate of 97%–100% at 100 mg/L,showing as the best insecticides among the four compounds.At lower concentration of 50 mg/L,both of the compounds merely exhibited moderate larvicidal activities against such two kinds of pests.The overall relationships between these compound structures and the insecticidal activities towardsH.armigeraandO.nubilalisillustrated the similar activity trends of sulfone-containing compound>sulfoxide-containing compound,CH3>Cl for R1,and CH3>C2H5for R2.
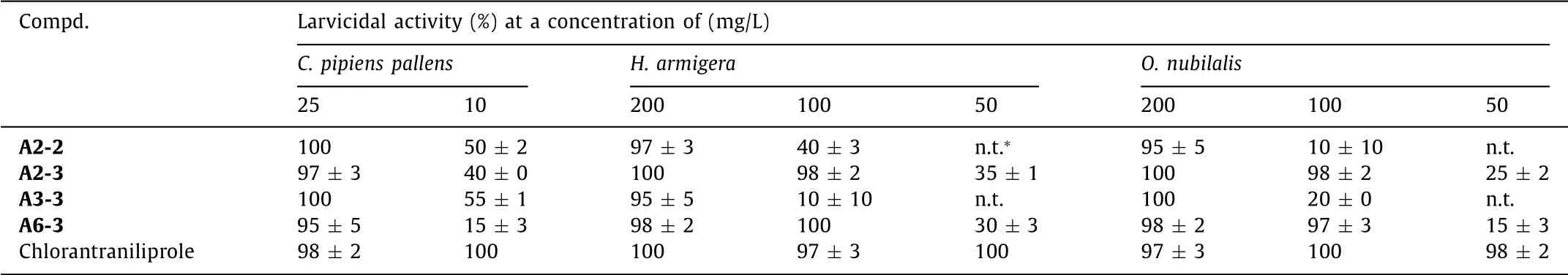
Table 4 Insecticidal activities of partial compounds A against C.pipiens pallens,H.armigera and O.nubilalis.
To further study the mechanism of the novel sulfide-containing insecticidal compounds,the effects on the calcium channels of high bioactive compounds A2-3 and A6-3 were tested by using central neurons isolated from third-instar larvae ofM.separatawith the calcium imaging technique after neuron loading with fluo-3 AM.Fig.3 showed the change in[Ca2+]iversusrecording time when the central neurons were treated with A2-3,A6-3 and chlorantraniliprole in the absence of extracellular calcium.There are two intracellular calcium release channels in neurons named IP3 receptor(IP3R)and RyR.Free calcium could be released from calcium storesviaRyR or IP3R into the endoplasmic reticulum.2-Aminoethoxydiphenyl borate(2-APB)as IP3R antagonist has been used as blocker to probe aspects of calcium signaling.According to the experiment results,it was found that the peaks of[Ca2+]iwere all increased by treating the tested compounds with 5 mg/L A2-3 and A6-3(Figs.3A and C).These results demonstrated that the novel compounds could activate the calcium channels in the central neurons of the third-instar larvae ofM.separata.For further study which is the main channel to release the calcium,RyR or IP3R blocker was used to preincubate the neurons about 5 min.The experiments showed that within the error range,the increasing of calcium had little effect after the blocking agents used(Figs.3B and D).Therefore,ryanodine receptor in the central neurons ofM.separatais the possible target—that is,these novel compounds may have the same target as that of chlorantraniliprole.
In summary,a series of novel sulfide-containing amide derivatives A were efficiently synthesizedviaa strategy of modifying the “amide” structure of anthranilic diamide insecticides,and their structures were identified by melting points,1H NMR,13C NMR,19F NMR and elemental analysis or HRMS.The molecular structures of A2-3 and A4-5 were further confirmed by the X-ray singlecrystal diffraction analysis and firstly reported.The bioassay results showed that most of the compounds A possess moderate to high insecticidal activities.Especially,some sulfone-containing compounds exhibited favorable bioactivities,e.g.,compounds A2-3,A3-3 and A6-3 not only possessed high lethality rate againstP.xylostella(50%–100%)at 0.1 mg/L,but also held good insecticidal activities towards a variety of agricultural pests such asM.separata,C.pipiens pallen,H.armigeraandO.nubilalis.It is worth noting that the larvicidal activity towardsP.xylostellafor partial thioethercontaining compounds[A4-1:(80 ± 0)%;A6-1:(80 ± 1)%]was close to that of chlorantraniliprole[(85 ± 1)%]at 0.01 mg/L.The calcium imaging experiments revealed that the highly insecticidalA2-3 and A6-3 as the representative compounds are potential RyR modulators.In addition,the structure–activity relationships were discussed in detail.The research results in this paper provide useful information for further design and development of novel compounds with promising insecticidal activities.
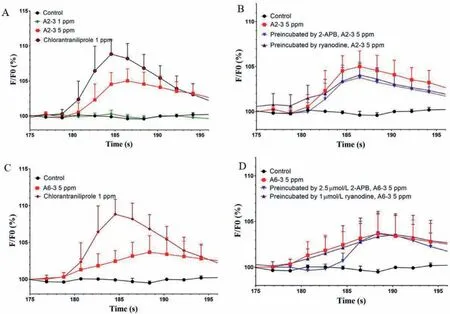
Fig.3.Effects of A2-3 and A6-3 and chlorantaniliprole on[Ca2+]i on the central neurons of M.separata when extracellular calcium was absent(EGTA replaced calcium).
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
Project supported by the National Key Research and Development Program of China(No.2017YFD0200505),the National Natural Science Foundation of China(Nos.21772103 and 31972287),and Tianjin Natural Science Foundation(No.17JCYBJC19900).We thank Lixia Xiong and Na Yang of the Biological Assay Center,Nankai University,for kind bioassay assistance of compounds.This article is dedicated to the 100thAnniversary of Chemistry at Nankai University.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.05.027.
 Chinese Chemical Letters2022年1期
Chinese Chemical Letters2022年1期
- Chinese Chemical Letters的其它文章
- Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation:A review
- Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers
- Porphyrin-based heterogeneous photocatalysts for solar energy conversion
- Systematic evaluation of advance in application and discharge mechanism of solution electrode glow discharge
- Insoluble carbonaceous materials as electron shuttles enhance the anaerobic/anoxic bioremediation of redox pollutants:Recent advances
- Selective N-terminal modification of peptides and proteins:Recent progresses and applications
