Selective C-C bonds formation, N-alkylation and benzo[d]imidazoles synthesis by a recyclable zinc composite
Gunxin Zhu,Zheng-Cho Dun,Hiyn Zhu,Dongdong Ye,Dwei Wng,∗
aThe Key Laboratory of Synthetic and Biological Colloids,Ministry of Education,School of Chemical and Material Engineering,Jiangnan University,Wuxi 214122,China
bState Key Laboratory of Pulp and Paper Engineering,South China University of Technology,Guangzhou 510640,China
cSchool of Chemical and Environmental Engineering,Hubei Minzu University,Enshi 445000,China
ABSTRACT Earth abundant metals are much less expensive,promising,valuable metals and could be served as catalysts for the borrowing hydrogen reaction,dehydrogenation and heterocycles synthesis,instead of noble metals.The uniformly dispersed zinc composites were designed,synthesized and carefully characterized by means of XPS,EDS,TEM and XRD.The resulting zinc composite showed good catalytic activity for the N-alkylation of amines with amines,ketones with alcohols in water under base-free conditions,while unsaturated carbonyl compounds could also be synthesized by tuning the reaction conditions.Importantly,it was the first time to realize the synthesis of 2-aryl-1H-benzo[d]imidazole derivatives by using this zinc composite under green conditions.Meanwhile,this zinc catalyst could be easily recovered and reused for at least five times.
Keywords:Unsymmetrical Zinc Borrowing hydrogen Recyclability Selective
Functionalized imines and amines are important intermediates for organic synthesis,biological,and pharmaceutical applications because they are widely used as pharmacophores in many biologically active compounds and agrochemicals[1,2].Although the traditional methods of alkylation of primary amines with alkyl halides to functionalized amines are studied,great quantities of unexpected wastes create an undesirable ecological footprint.In addition,the reaction of amines with alkyl halides in the presence of large amounts of bases,which often suffers from overalkylation,and exhibits low selectivity for the desired products[3].Hence,the development of efficient and sustainable methods is of infinite interest.To address these problems,borrowing hydrogen strategy is stand out from other methods[4].N-Alkylation of amines and alcohols or two amines was documented for the preparation of imines or amines through the borrowing hydrogen strategy.
Earth abundant metals,like Mn,Fe,Co,Zn,Ni,are much economic,promising,valuable catalysts for borrowing hydrogen reaction,dehydrogenation and heterocycles synthesis.Kirchneret al.recently described an excellent example of cobalt-catalyzed borrowing hydrogen reaction and predicted that earth abundant metals are the most economic and promising catalysts for modern industry[5].Therefore,the research on earth abundant metals for borrowing hydrogen reaction and dehydrogenation is highly interesting and desirable[6-10].
Recently,our group developed several triazole-skeleton ligand bridged transition-metal complexes,which showed good catalytic activities in dehydrogenation and borrowing hydrogen reactions[11-16].However,most of the central metals are noble metals,low catalyst stability or catalysts are difficult to be recovered and reused[17-19].Herein,we have described the synthesis of an unsymmetrical thienyl-pyrazoly-triazole ligand(TPT)and the corresponding heterogeneous zinc composite on molecular sieve(MS),which was carefully characterized by means of XPS,EDS,TEM and XRD(Scheme 1).The resulting zinc composite revealed good catalytic activity for borrowing hydrogen reaction of amines with amines in water under base-free conditions.Moreover,2-aryl-1H-benzo[d]imidazole derivatives were synthesized with this zinc composite in water under base-free conditions.
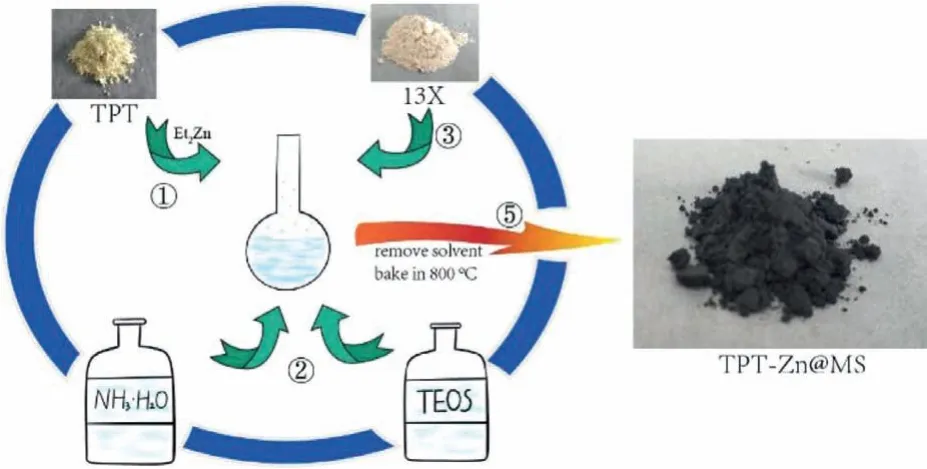
Scheme 1.The designed PPT-Zn@SBA-15.
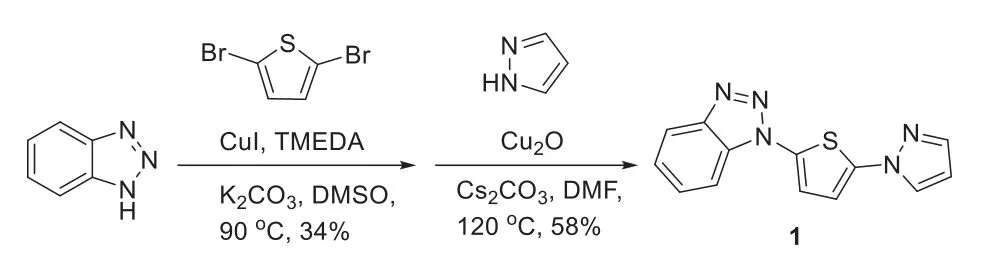
Scheme 2.The synthesis of thienyl-pyrazoly-triazole.

Fig.1.SEM image(a),EDS pattern(b)and TEM images(c,d)of TPT-Zn@MS.
The thienyl-pyrazoly-triazole ligand(TPT,1)was synthesized in two steps with moderate yield(Scheme 2)and the detailed steps of TPT synthesis and purification were provided in Supporting information.TPT-Zn@MS was obtained through a one-pot method,as follows:A flame-dried flask was charged with TPT(1.0 equiv.)and freshly distilled THF under nitrogen at room temperature,then diethylzinc(1.0 mol/L in hexanes;2.0 equiv.)was added dropwise under vigorous stirring condition.The flask was removed after 30 min,then silica was introduced into the above flask byin situhydrolysis of the added TEOS with ammonium hydroxide.After that the flask was heated to reflux at 65 °C for 5 h,the power of 13X molecular sieve(MS)was added to the solution and refluxed with vigorous stirring for another 5 h.The resulting mixture was grounded to a fine powder after removed the reaction solvent,and pyrolyzed at 800 °C with a constant argon flow for 2 h.Then the black power was cooled down to room temperature,and washed with HCl solution,water respectively.Finally,the resulting material(TPT-Zn@MS)was obtained for characterizations.
The prepared TPT-Zn@MS material was characterized by X-ray photoelectron spectrometry(XPS),scanning electron microscope(SEM),energy dispersive X-ray spectroscopy(EDS)and transmission electron microscopy(TEM).
The TEM images(Fig.1)demonstrate the existence of crystal phases of zinc composite and the interplanar spacing of crystal phases is be afforded(Fig.1d),which shows that the interplanar spacing of zinc composite is 0.205 nm.Furthermore,there are many crystal phases belong to 13X molecular sieve,suggesting the material remained active salts after firing and Zn was uniformly dispersed.Meanwhile,scanning electron microscope(SEM)and energy dispersive X-ray spectroscopy(EDS)were conducted to better characterize TPT-Zn@MS.As shown in Fig.1a,this composite is amorphous,and we random sampled on five different areas to perform EDS test,the maximal element content of Zn is at 4.75 wt%and minimal element content is at 2.21 wt%,suggesting the distribution of Zn element was uniformly dispersed.Other element contents are as follows:Na(2.89 wt%),Al(2.331 wt%),Si(6.59 wt%),O(31.087 wt%),S(0.49 wt%).
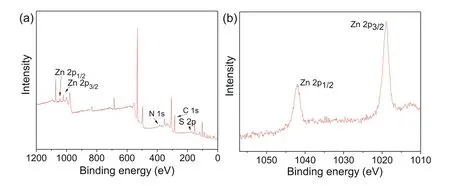
Fig.2.(a)XPS spectra of TPT-Zn@MS,(b)narrow spectra of Zn.
X-ray photoelectron spectroscopy(XPS)was subsequently performed to study the surface chemistry of TPT-Zn@MS.The Zn element content on the surface is 2.681 wt%,the result is achieved agreeable to the EDS results.In addition,low intensity of the N 1s and S 2p shows N and S elements maybe reacted with ultralow air,so that the content of N and S was under low level(Fig.2).
With above encouraging zinc composite in hand,the catalytic activity of TPT-Zn@MS was next investigated.Initially,the challenging reaction involvingN-alkylation of amines and amines was selected to test the catalyst activity of TPT-Zn@MS,especially in water.Compared to classicalN-alkylation conditions,such as,strong base,organic solvents,sealed flask,this reaction was attempted in water under base-free conditions.After a series of conditions screening,it was disclosed that the reaction could occur in water under base-free conditions.For a higher yield,the additive experiments were carried out and the results showed that AgNTf2/KF could produce a much higher yield.The introduction of phase transfer catalyst could further enhance the yield of desired product(Table 1,entry 11).It was observed that the reaction of amine and amine could not take place in the absence of catalyst(Table 1,entry 16).It should be noted that the reaction could take place under solvent-free conditions,however,only moderate yield was achieved(entry 21).
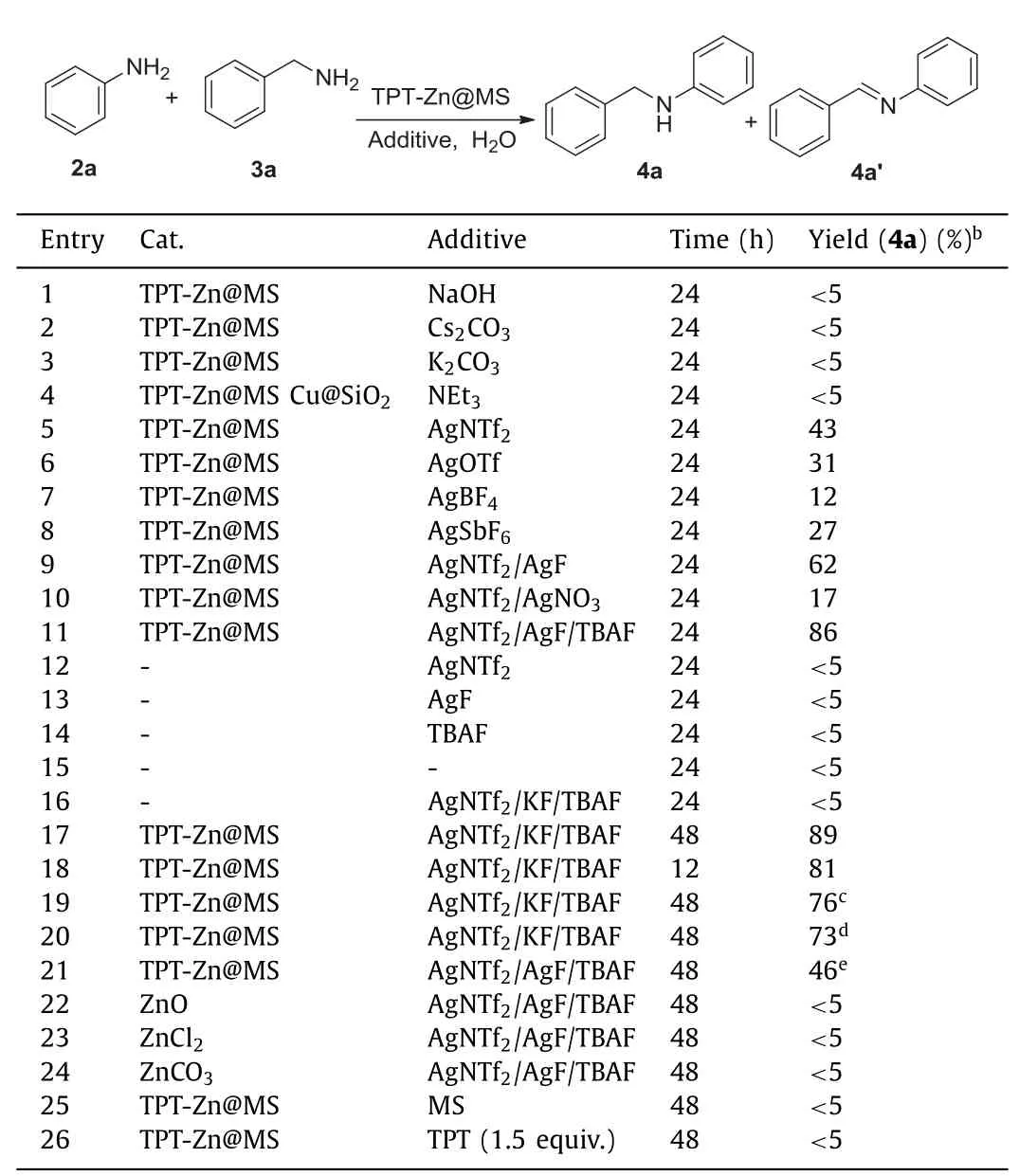
Table 1 Optimization of reaction conditions.a
After establishing the optimal conditions,the substrate scope was then extended by utilizing a variety of substituted aromatic amines and various benzylamine derivatives.As showed in Scheme 3,all the amines were smoothly converted into the correspondingN-phenylbenzylamines and moderate to high isolated yields were obtained regardless of the electronic properties of starting materials.
It was confirmed that the substituent groups of aromatic amines and benzylamines,such as methoxyl,methyl and chloro,were smoothly reacted and it was noticed that the aromatic amines containing methoxyl or chloro groups produced much higher yields(4c,4e,4f).It was observed that benzylamines with electron-withdrawing group producedN-phenylbenzylamines in admirable yields(4g,4i,4j).In addition,2-thiophenemethylamine could also react with aromatic amine and generated the desired product in moderate yield(4l).
Encouraged by such wonderful results,we further employed TPT-Zn@MS to ketones and various benzyl alcohols.After optimizing the condition of the reaction of ketones and various benzyl alcohols,it was demonstrated that the C-C bond formation could be smoothly transformed by this Zn composite,and then the substrate scope was explored and the results were summarized in Scheme 4.The experiments showed the products were obtained with good to excellent yields.It was noticed all combinations of ketones and benzyl alcohols provided high selectivity of the process on the formation of C=C bond.Under suitable conditions,both electron-rich and electron-deficient substrates could be reacted efficiently and the desired products were achieved with good yields(7b,7e,7h,7k,7l,7n).The electron-rich benzyl alcohols bearing methyl,methoxyl groups afforded the products in high yields(7c,7d,7f,7g,7i).The electron-efficient ketones bearing chloro,bromo groups were also suitable to this transformation in good yields(7j,7m,7o).
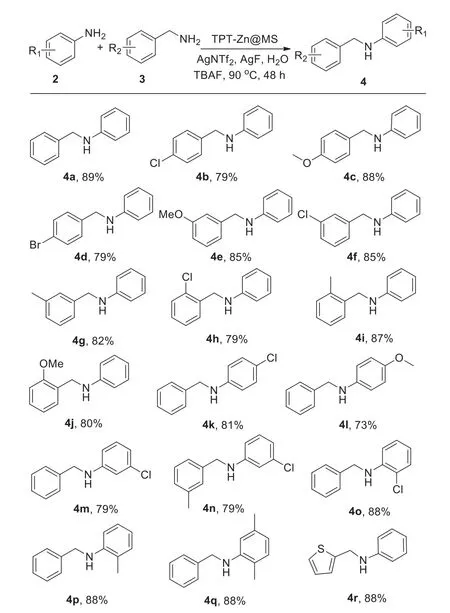
Scheme 3.Reaction of aromatic aimines and benzylamines.Reagents and conditions:2(1.0 mmol),3(1.2 mmol),TPT-Zn@MS(10 mg),AgNTf2(0.3 mmol),KF(0.3 mmol),TBAF(0.3 mmol),water(3.5 mL),90 °C,48 h,N2.Isolated yields.
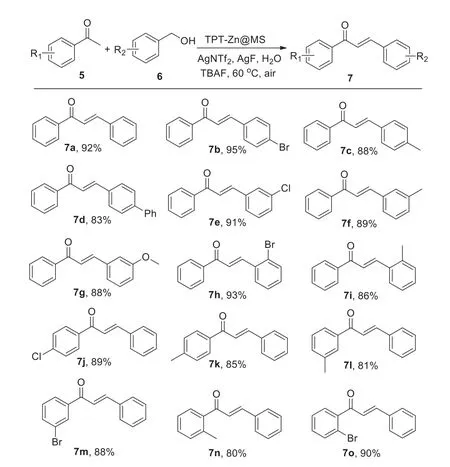
Scheme 4.Selective synthesis of unsaturated carbonyl compounds.Conditions:5(1.0 mmol),6(1.2 mmol),TPT-Zn@MS(10 mg),AgNTf2(0.3 mmol),KF(0.3 mmol),TBAF(0.3 mmol),water(3.5 mL),60 °C,air,12 h.Isolated yields.
Interestingly,when we changed the conditions of the reaction of ketones and benzyl alcohols,an unexpected result was observed(Scheme 5).The 3-phenylpropiophenone as the main product was provided with the same TPT-Zn@MS catalytic system.We further demonstrated the C-C bond formation scope of ketones and benzyl alcohols with this reaction system,and the 3-phenylpropiophenone derivatives were provided in good to high yields.This system was able to tolerate the -Cl,-Me,-Ph and -OMe groups,even the strong electron-rich substrate like 3,5-dimethoxybenzyl alcohol was also reacted smoothly(8f).In addition,the 2-acetonaphthone and 2-thiophenemethanol were suitable to this transformation,leading to the corresponding products in 90% and 76% yields,respectively(8k,8l).
2-Aryl-1H-benzo[d]imidazole derivatives are an important and valuable natural products or intermediates and are found in natural alkaloids,pharmaceuticals and bioactive molecules[20].Recently,we developed copper-catalyzed the synthesis of 2-aryl-1Hbenzo[d]imidazole derivatives,however,strong base,organic solvents and the catalyst could not be recovered[21-23].Therefore,we next utilized TPT-Zn@MS to catalyze the synthesis of 2-aryl-1H-benzo[d]imidazoles.The experiments revealed that lots of 2-aryl-1H-benzo[d]imidazole derivatives could be synthesized with TPT-Zn@MS as a catalyst in water under base-free conditions(Scheme 6).
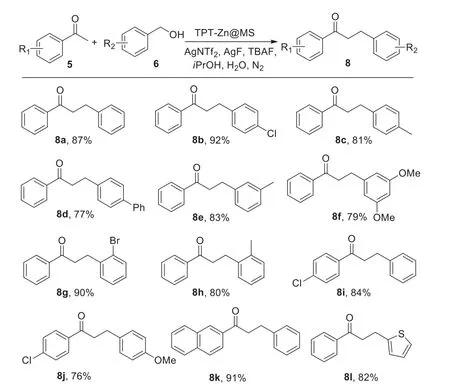
Scheme 5.Selective synthesis of phenylpropiophenones.Conditions:5(1.0 mmol),6(1.2 mmol),TPT-Zn@MS(10 mg),AgNTf2(0.3 mmol),KF(0.3 mmol),TBAF(0.3 mmol), iPrOH(3 mmol),water(3.5 mL),90 °C,N2,48 h.Isolated yields.
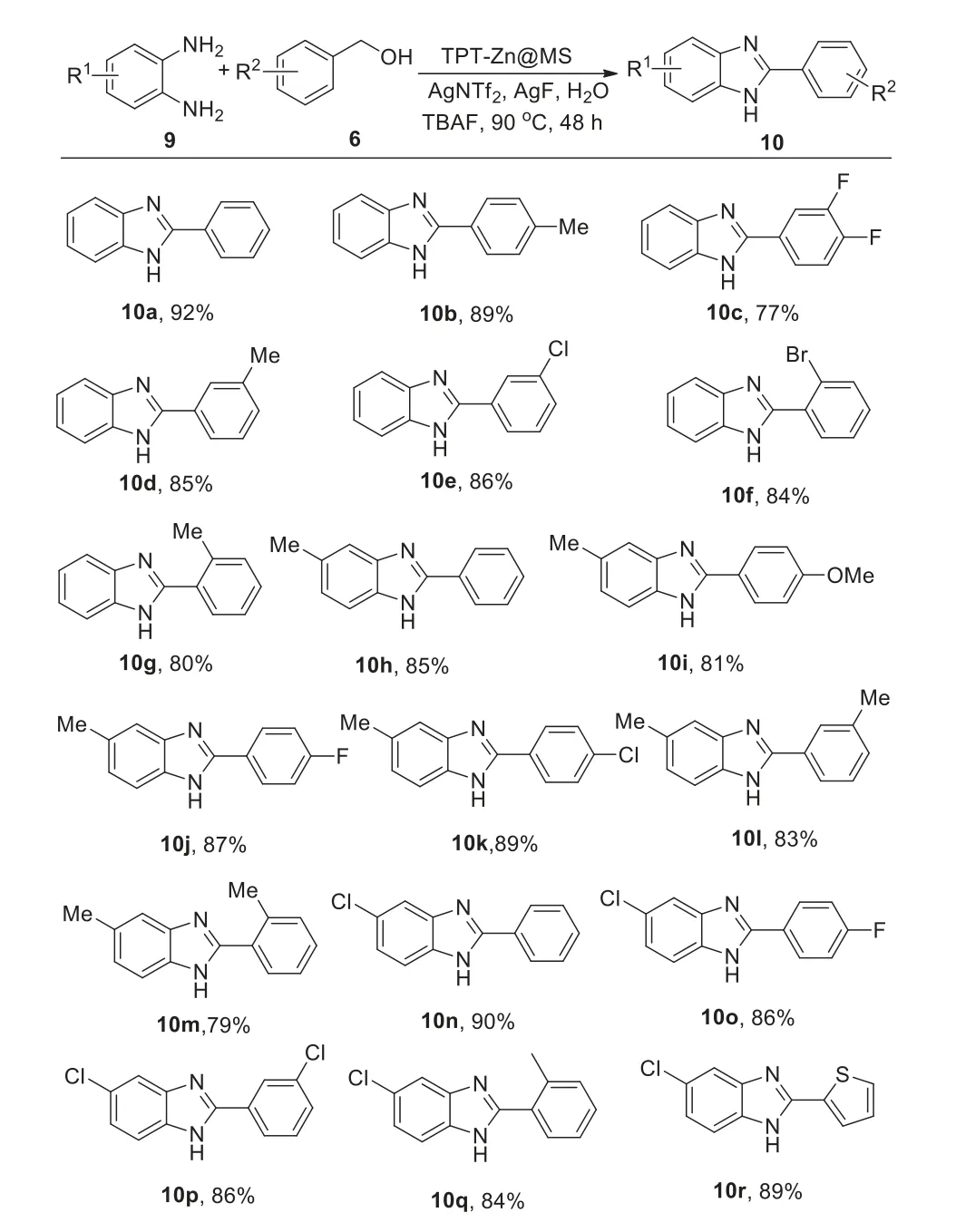
Scheme 6.Substrate expansion of 2-phenylbenzimidazole.Conditions:5(1.0 mmol),6(1.2 mmol),TPT-Zn@MS(10 mg),AgNTf2(0.3 mmol),KF(0.3 mmol),TBAF(0.3 mmol), iPrOH(3 mmol),water(3.5 mL),90 °C,N2,48 h.Isolated yields.
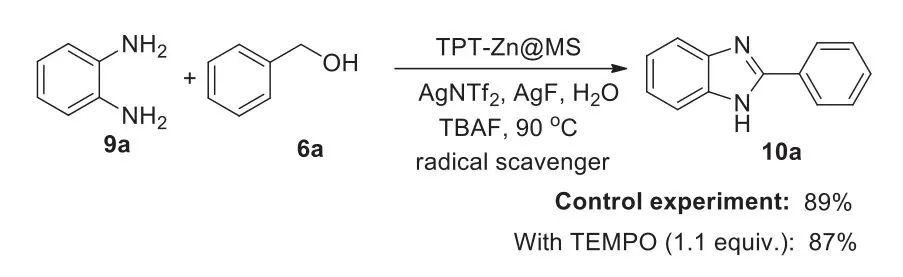
Scheme 7.The control experiments.
To better explore and understand what roles of TPT-Zn@MS and AgNTf2played in the synthesis of 2-aryl-1H-benzo[d]imidazole derivatives,the control experiments were introduced into this mechanism exploration and the results were listed in Table 1.
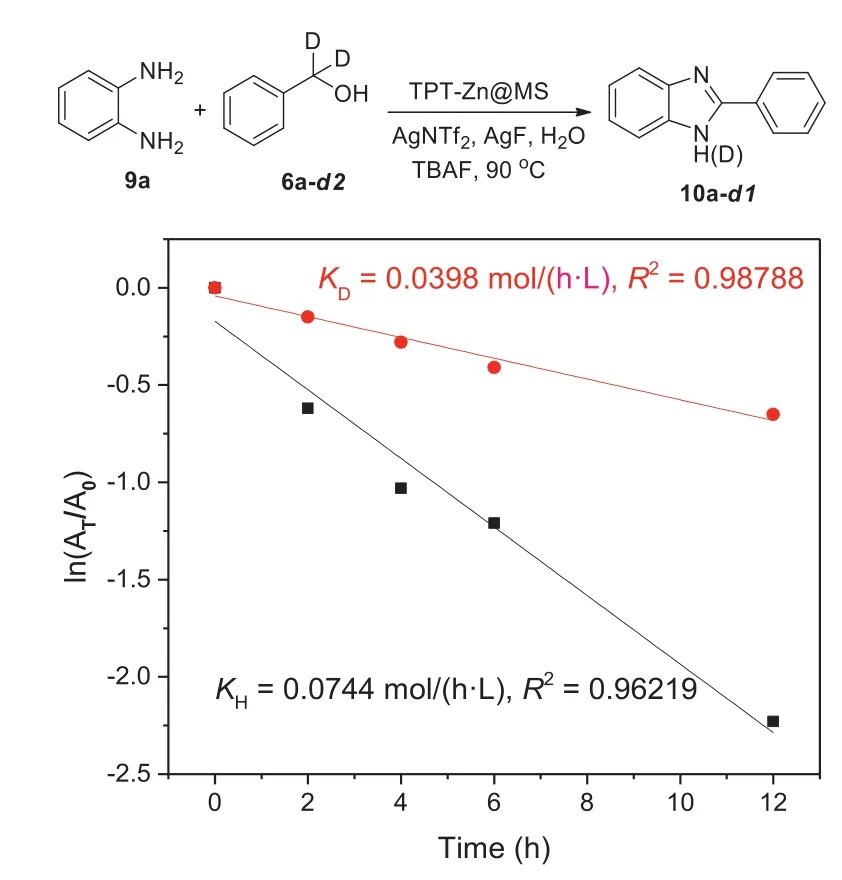
Fig.3.Kinetic plot of 2-aryl-1H-benzo[d]imidazole.A0:original concentration of substrate.At:concentration of substrate at time t. K:rate constant.
The experiments revealed that TPT-Zn@MS and additives played the important roles in 2-aryl-1H-benzo[d]imidazole synthesis process,which was not produced in the absence of TPT-Zn@MS.In addition,only TPT and MS could not catalyze this reaction.The control experiments were set up to exclude the possibility of a radical pathway including the single electron transfer process.The results showed that the yield of 2-aryl-1H-benzo[d]imidazole(10a)was almost the same by using TEMPO(1.1 equiv.)as a radical scavenger with TPT-Zn@MS as a catalyst(Scheme 7).As expected,the experiments disclosed that this process is not a single electron transfer one(SET).
Hammett plot equation was investigated and the results were concluded in Supporting information Meanwhile,to clearly explain this reaction,kinetic isotope effect vale(KIE)was studied to explore the kinetically relevant elementary steps and the experiments revealed that KIE value(1.86)was achieved through the first order reaction plot between ln[6a]and ln[6a-d2](Fig.3).This disclosed that the rate-determining step is the dehydrogenation of alcohol(6a)in the synthesis of 2-aryl-1H-benzo[d]imidazole derivatives.
TPT-Zn@MS was finally recovered and washed with water(10 mL × 3),ethanol(10 mL × 3)and water(10 mL × 3).After drying for 24 h,the recovered TPT-Zn@MS was reused to catalyze all the above four transformations and the recycled experiments were concluded in Scheme 8.It was demonstrated that yields of the desired products could be nearly maintained until the composite was recovered for even five times.In addition,the extent experiment of TPT-Zn@MS revealed that no copper was detected through ICP analysis,which exclude the effect of copper catalysis.Meanwhile,the ICP assessment of recovered TPT-Zn@MS showed that silver was not found.
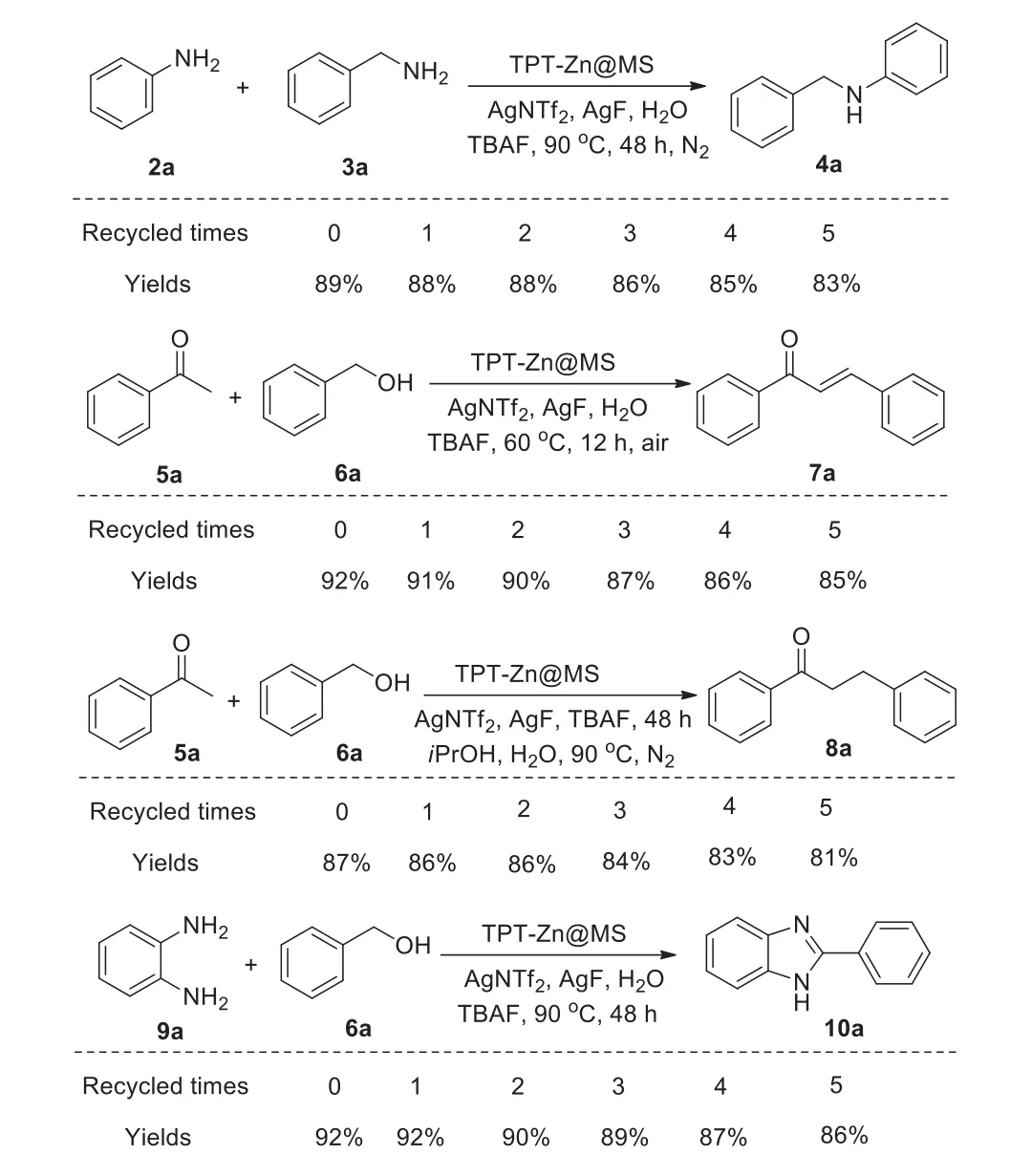
Scheme 8.Recycled experiments.

Scheme 9.The synthesis of 1-benzyl-2-aryl-1H-benzo[d]imidazole in gram scale.
Moreover,the summary of 2-aryl-1H-benzo[d]imidazole derivative derivatives synthesis[24-30]was listed in Table 2.It was observed that TPT-Zn@MS composite was a good catalytic system,which revealed a green method under base-free and water conditions with good recovery performance.This TPT-Zn@MS system offered an efficient methodology for the selective synthesis of saturated and unsaturated carbonyl compounds,2-aryl-1Hbenzo[d]imidazole derivative derivatives with only water(or hydrogen gas)as by-products for the first time[31,32].
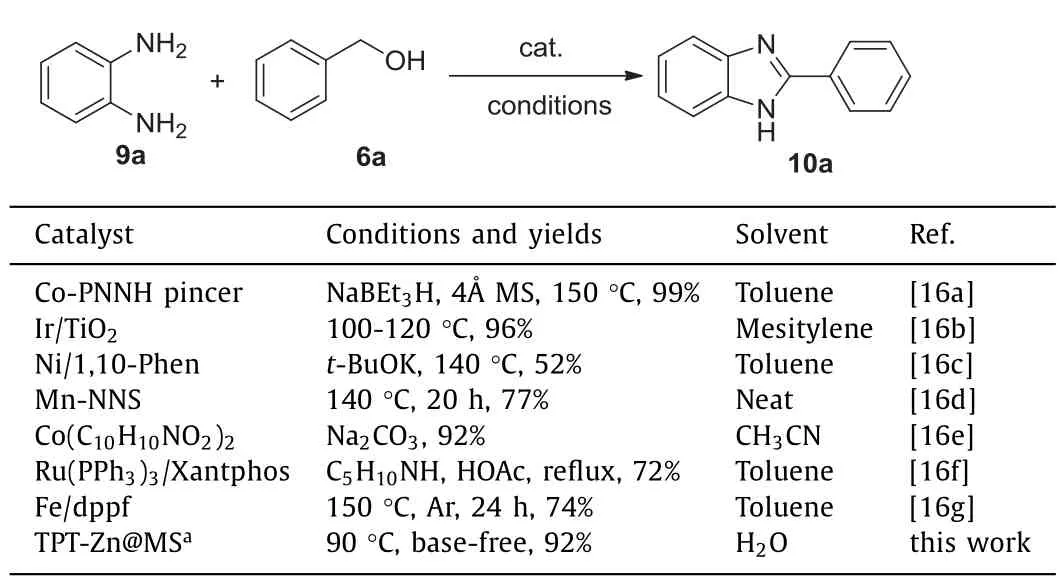
Table 2 The synthesis conditions of 2-aryl-1H-benzo[d]imidazole.
Finally,thegram-scalesynthesisof2-aryl-1Hbenzo[d]imidazole from the reaction of phenylenediamine(5a)and benzyl alcohol(2a)was carried out.As expected,the result showed that the desired product(10a)was achieved smoothly in 89% yield(Scheme 9).This gram scale experiment disclosed that TPT-Zn@MS system could promote the synthesis 2-aryl-1H-benzo[d]imidazole derivatives under base-free conditions in water.
In conclusion,we developed a new type of heterogeneous TPTZn@MS catalyst,which was proved to be effective for selective borrowing hydrogen reaction of ketones and alcohols.TPT-Zn@MS was efficient for theN-alkylation of amines with amines,and the synthesis of synthesis of 2-aryl-1H-benzo[d]imidazole derivatives from diamines and alcohols.This provided an easy method the synthesis of substituted amines,2-aryl-1H-benzo[d]imidazole derivatives with high yields in water.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledge financial support of this work by the National Natural Science Foundation of China(Nos.21776111,21861039),State Key Laboratory of Pulp and Paper Engineering(No.202001)and Central Laboratory,School of Chemical and Material Engineering,Jiangnan University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.06.060.
 Chinese Chemical Letters2022年1期
Chinese Chemical Letters2022年1期
- Chinese Chemical Letters的其它文章
- Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation:A review
- Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers
- Porphyrin-based heterogeneous photocatalysts for solar energy conversion
- Systematic evaluation of advance in application and discharge mechanism of solution electrode glow discharge
- Insoluble carbonaceous materials as electron shuttles enhance the anaerobic/anoxic bioremediation of redox pollutants:Recent advances
- Selective N-terminal modification of peptides and proteins:Recent progresses and applications
