Highly active electrocatalytic CO2 reduction with manganese N-heterocyclic carbene pincer by para electronic tuning
Can Huang,Jiahao Liu,Hai-Hua Huang,Xianfang Xu,Zhuofeng Ke
School of Materials Science &Engineering,School of Chemistry,PCFM Lab,Sun Yat-sen University,Guangzhou 510275,China
1These authors contributed equally to this work.
ABSTRACT Electronic tuning by para substitutions was explored to achieve a highly active manganese N-heterocyclic carbene pincer complex for the selective electrocatalytic reduction of CO2 to CO.[MnCNCOMe]BF4(L2-Mn)bearing an electron-donating group(–OMe)showed high activity with 63 × catalytic current enhancement,average Faradaic efficiency of 104%,and a TOFmax value of 26,127 s−1,which is 127 times higher than that of unsubstituted[MnCNCH]Br(L1-Mn)reported previously.In contrast,the electronwithdrawing group(–COOMe)in[MnCNCCOOMe]PF6(L3-Mn)inhibited the electrocatalytic activity.Ambient Brønstic acid,however,suppressed the activity of L2-Mn probably due to the protonation of the–OMe group.These findings indicate a potential electronic tuning strategy to improved manganese Nheterocyclic carbene catalysts for CO2 reduction.
Keywords:Pyridine N-heterocyclic carbene Electrocatalysis Carbon dioxide conversion Manganese Substituent effect
Carbon dioxide reduction is of particular interest in terms of greenhouse alleviation and the production of carbon feedstock.Therefore,the homogeneous and heterogeneous electrocatalytic CO2conversion is becoming more and more attractive recently[1–6].Various electrochemical CO2reduction can be involved in different pathways including two,four,six and eight-electron processes,leading to various carbon-based products such as,CO,HCOOH,CH3OH,HCHO,CH4,and other value-added chemicals or liquid fuels which could benefit our society sustainability[2,7,8].
Among different families of molecular catalysts for electrocatalytic CO2reduction,earth-abundant transition metal complexes,particularly the manganese-based bipyridine complexes,have attracted many interests[9–12].However,they displayed limited efficiency although extensive exploration has been performed on modifying pyridyl and bipyridyl ligands.N-Heterocyclic carbene(NHC)complexes exhibit unique selectivity and stability in catalysis[13–16],owing to the strongσ-donation and the weakπ-backdonation properties of the NHC ligands[17].Recently,manganese complexes supported by NHC ligands have been reported with potential electrocatalytic performances(Fig.1a)[18–21].
In 2018,Lucaet al.reported an NHC-based manganese(I)pincer complex[MnCNCH]Br(L1-Mn)for the selectively electrocatalytic reduction of CO2to CO[22].Inspired by this pincer framework,and guided by our previous theoretical understanding of theparaelectronic effect[23,24],herein,we developed new types of manganese pincer complexes to achieve highly active and selective electro-reduction of CO2by tuning theparaelectronic effect(Fig.1b).
By the introduction of pyridylparasubstituents on complex L1-Mn[22,25],we designed and synthesized new complexes L2-Mn and L3-Mn(Scheme 1)incorporating an electrondonating methoxy(-OMe)group and an electron-withdrawing methyl formate(-COOMe)group,respectively.Ligands L1,L2 and L3 were synthesized from dibromopyridine,2,4,6-trifluoropyridine,and methyl 2,6-dihydroxyisonicotinate,respectively(Schemes S1 and S2 in Supporting information).Complexes L1-Mn,L2-Mn and L3-Mn were obtained with conventional Mn(CO)5Br as starting materials by overnight reactions in the presence of excess Cs2CO3in MeCN under argon atmosphere(Scheme 1).1H NMR,13C NMR,infrared spectroscopy and single-crystal X-ray diffraction analyses(Scheme 1,Tables S1,S2,Figs.S1-S10 in Supporting information)verified the successful synthesis of this series of complexes.Single-crystal X-ray diffraction analysis confirmed the octahedral structures of L2-Mn and L3-Mn,with Mn-CNHCbond lengths of 1.999(5),2.009(9)˚A,and Mn-N bond lengths of 2.001(0),2.012(4)˚A,respectively(Scheme 1).The structures and bond lengths of both complexes are in accordance with the previously reported L1-Mn[22]with Mn-CNHCbond length of 1.989(3)˚A and Mn-N bond length of 2.017(3)˚A.
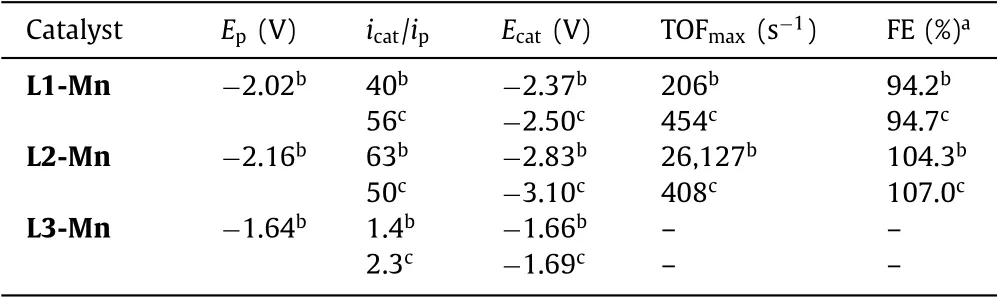
Table 1 Results of cyclic voltammetry and controlled potential electrolyses.
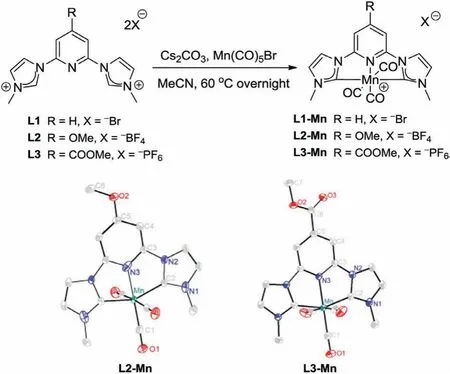
Scheme 1.Synthesis of Mn(I)NHC complexes and X-ray structures of L2-Mn and L3-Mn.
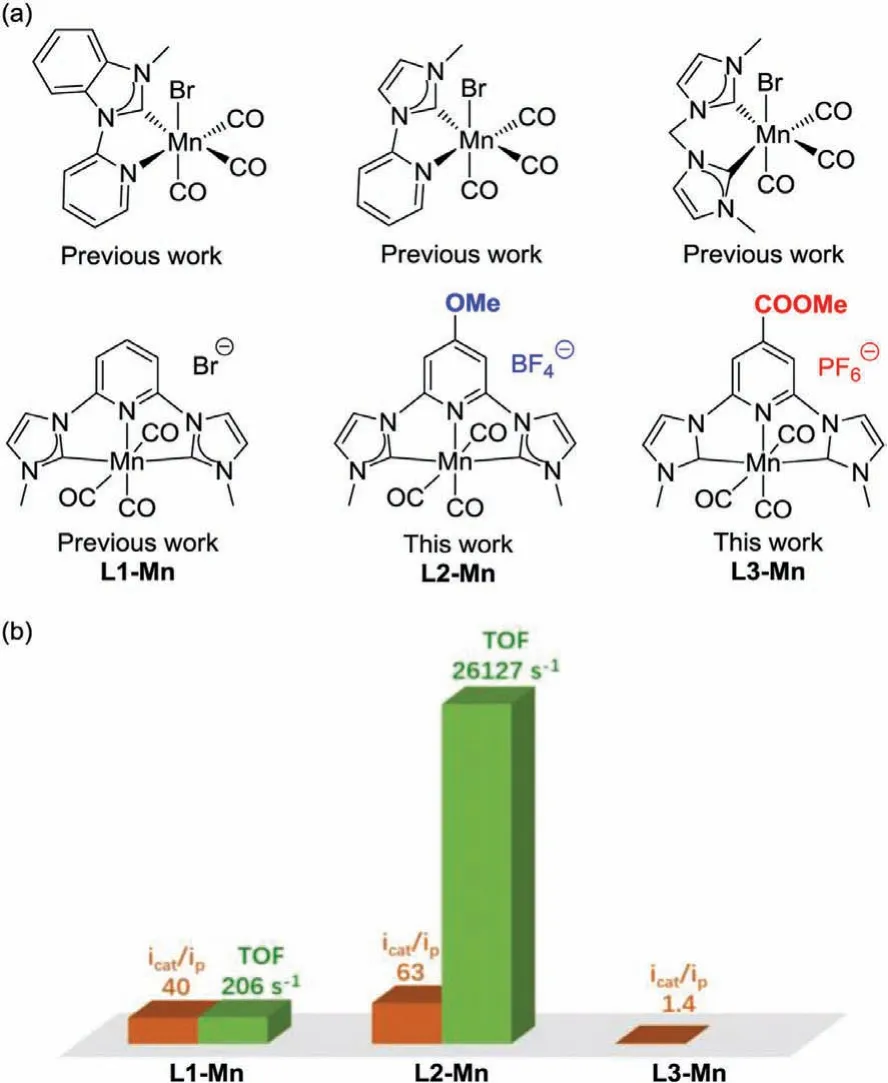
Fig.1.NHC Mn(I)complexes for electrocatalytic reduction of CO2.
Cyclic voltammetry analyses of the series of Mn(I)complexes were performed under argon and CO2atmosphere,respectively(Fig.2).Under Ar atmosphere,the cyclic voltammetry of L1-Mn showed an irreversible redox wave atEp1=−2.02 V(Fig.2a,all reduction potentials are reportedvs.Fc+/Fc),in agreement with previous studies[22].The redox wave in the CV of complex L2-Mn with -OMe shifted cathodically by approximately 200 mV(Fig.2b,Ep2=−2.16 V under Ar atmosphere)compared to the redox waves in the CV of complex L1-Mn,reflecting the strong electron-donating ability of the -OMe group.The CV of L3-Mn with-COOMe shows three pairs of redox waves at −1.64 V,−1.86 V,and −2.14 V,respectively(Fig.2c and Fig.S23 in Supporting information).Under CO2atmosphere,the catalytic current in the CV of L1-Mn increased by about 40-fold with the redox wave atEcat1=−2.37 V.Surprisingly,the CV of L2-Mn shows a dramatic current increase(ca.63-fold)atEcat2=−2.83 V,suggesting that the promising promotion of catalytic activity of complex L2-Mn for CO2reduction caused by the influence of the electron-donating effect.However,the CV of complex L3-Mn displayed only minor catalytic current increase(Fig.1c,ca.1.4-fold),which is in sharp contrast to the behavior of L1-Mn,indicating the negative effect of the electronwithdrawing group on the electro-reduction.
Cyclic voltammetry analyses of complexes L1-Mn and L2-Mn at scan rates of 8–12 V/s under both CO2and Ar atmosphere were performed(Figs.S11,S12,S17 and S18 in Supporting information).An ideally steady-state catalytic progress will lead to an S-shaped CV response,which is independent of the scan rate,with plateau current to determine the maximum turnover frequency(TOFmax).CVs of both complexes showed the S-shaped catalytic waves,indicating the ideally and purely kinetic CO2catalytic reduction progresses(Figs.S12 and S18).We operated theicat/ipanalyses to calculate the TOFmaxfor L1-Mn and L2-Mn from the reported equations[26–29].L1-Mn showed a TOFmaxof 206 s−1under the CO2atmosphere in anhydrous MeCN(Fig.S40 in Supporting information).Notably,the calculated TOFmaxof L2-Mn is 26,127 s−1,which is approximately 127 times higher than the value of L1-Mn under the same conditions(Fig.S42 in Supporting information).Reduction potentials,catalytic currents,and calculated TOFmaxunder different conditions are summarized in Table 1.
The addition of Brønstic acid,for example,2,2,2-trifluoroethanol(TFE)as the proton source,would generally result in the enhancement of catalysis,as reported previously[11,12,22,30,31].We performed cyclic voltammetry of complexes L1-Mn and L2-Mn at incremental TFE additions until the catalytic current no longer increased to study the optimum concentration for catalytic conditions(Figs.S13 and S19 in Supporting information).The addition of TFE promoted the catalytic activity of complex L1-Mn,as shown in Fig.S13.240 μL TFE was added to achieve the optimal catalytic conditions.Cyclic voltammetry of L1-Mn under Ar and CO2atmosphere in 0.1 mol/L TBAPF6/MeCN with 240 μL TFE added gave a 56-fold catalytic current enhancement(Fig.S14 in Supporting information).However,complex L2-Mn displayed poor catalytic property when TFE was added as the proton source.The value oficat/ipfor L2-Mn at the presence of TFE decreased to 50 ×(Fig.S20 in Supporting information).This phenomenon can be attributed to the protonation of the -OMe group,which thereby suppresses its electron-donation ability.L1-Mn and L2-Mn with 240 μL TFE added display S-shaped CVs response at scan rates of 8–12 V/s,which represented an ideally steady-state catalysis(Figs.S15 and S21 in Supporting information).With 240 μL TFE added,the calculated TOFmaxof L1-Mn is enhanced to 454 s−1(Fig.S41 in Supporting information).Owing to the possible protonation of -OMe mentioned above,the calculated TOFmaxvalue of L2-Mn decreased to 408 s−1with 240 μL TFE addition(Fig.S43 in Supporting information).Cyclic voltammetry of L3-Mn with 240 μL TFE added showed a low value of catalytic current enhancement(icat/ip=2.3),indicating its inefficient electrocatalytic properties for CO2reduction.Further control experiments with tetrabutylammonium bromide(TBABr)to introduce Br−anion into the catalytic system of L2-Mn were performed(Figs.S25 and S26 in Supporting information),indicating the counter anions did not affect the electrochemical properties[32,33].
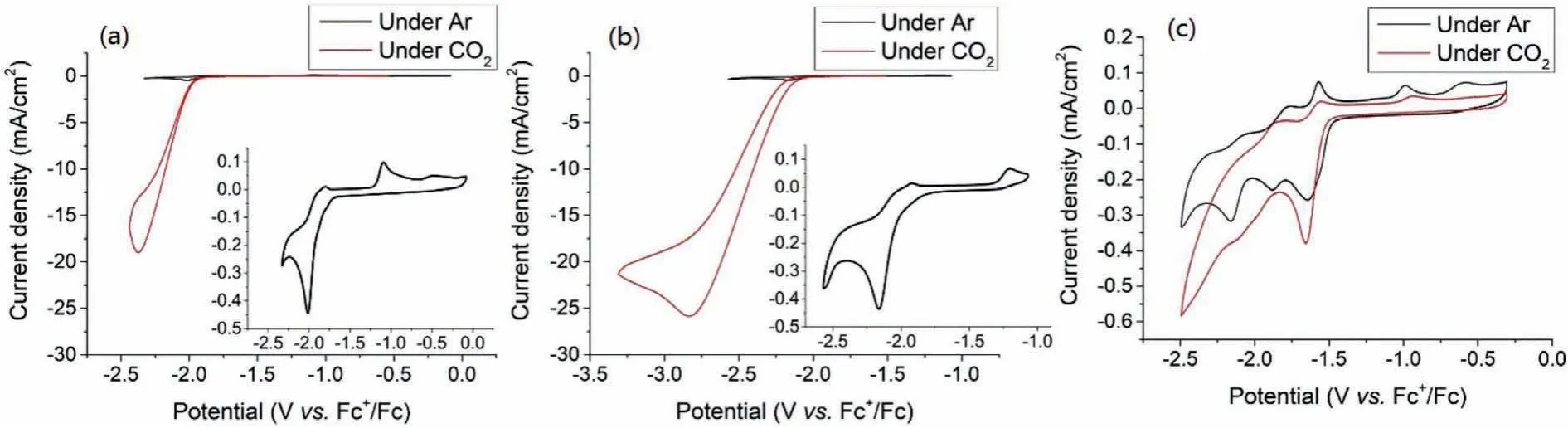
Fig.2.Cyclic voltammetry of L1-Mn(a),L2-Mn(b)and L3-Mn(c)under Ar(black)and CO2(red)atmosphere at 0.1 V/s in dry MeCN with 0.1 mol/L tetrabutylammonium hexafluorophosphate(TBAPF6)supporting electrolyte.All complexes were loaded at a concentration of 1 mmol/L.Glass carbon working electrode,platinum counter electrode,and Ag+/Ag reference electrode was used.
Controlled potential electrolysis(CPE)of L1-Mn,L2-Mn,and L3-Mn were performed under CO2atmosphere at an applied potential ofEappl=−2.37 V under the conditions of anhydrous MeCN and with the addition of TFE as a proton source to study their selectivity and durability(Figs.S27–S36 in Supporting information).Quantification of the gaseous products was conducted by an online gas chromatography thermal conductivity detector(GC-TCD)analysis of the headspace.After an electrolysis over 2 h in anhydrous MeCN,approximately 40.9% current density of the starting state was retained for L1-Mn(Fig.S27),which gave an average Faradaic efficiency(FE)of 94.2%(Fig.S28).L2-Mn displayed better selectivity and durability with approximately 86.6% current density retained over 2 h(Fig.S31)and an average FE of 104.3% was obtained(Fig.S32).These results demonstrate that the electron-donating effect also enhances the selectivity and durability of the Mn NHC pincer catalyst.With 240 μL TFE added,further promotion of selectivity and durability for L1-Mn were shown by the CPE results.77.2% current density retained over 2 h(Fig.S29)and an average FE of 94.7% was obtained(Fig.S30).Consistent with the results of CVs,L2-Mn gave a low level of current density probably because of the protonation of the -OMe.On the other hand,L3-Mn showed poor catalytic activity whether TFE was added or not(Figs.S35 and S36).Average Faradaic efficiencies are summarized in Table 1.
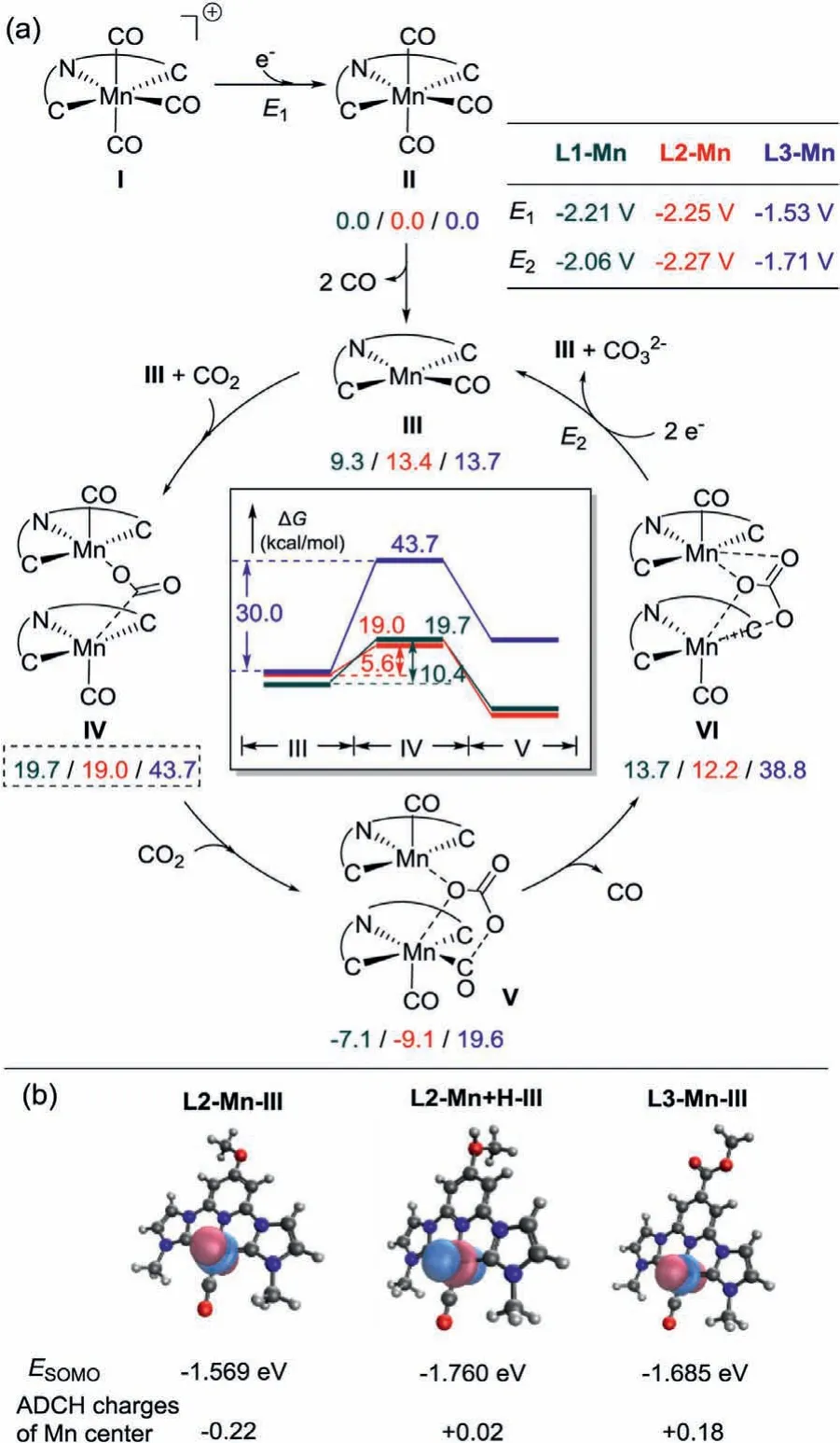
Fig.3.(a)Comparison of CO2 reduction cycle for L1-Mn(deep green),L2-Mn(red),and L3-Mn(blue)based on the binuclear proton-less reduction pathway.The Gibbs free energies and the calculated applied electro potentials are kcal/mol and V vs.Fc+/Fc,respectively.(b)SOMOs and ADCH charges of the Mn center of L2-Mn-III,L2-Mn+H-III,and L3-Mn-III.
According to the studies by Musgrave and Lucaet al.,a binuclear proton-less reduction pathway is suggested for the[MnCNC(CO)3]Br pincer system[34].Therefore,a comparison of the CO2reduction cycle for L1-Mn,L2-Mn and L3-Mn based on the bimetallic mechanism using density functional study(DFT)has been conducted to reveal the effects of theparasubstituents(Fig.3a).First,the Mn(I)species I are reduced to species II with the required potentials of −2.21 V,−2.25 V and −1.53 V for L1-Mn to L3-Mn,respectively,which are in agreement with the reduction potentials observed by the CV experiments(Fig.2).These results reveal the increasing reduction potential of electron-richer species with theparaelectron-donating effect.After the loss of two CO molecules from II,which was observed by Lucaet al.in the case of L1-Mn,complexes III adopting square planar geometry are generated with the calculated Gibbs free energies to be endergonic by 9.3–13.7 kcal/mol.Then,a complexation process of a CO2molecule sandwiched between two III intermediates generates complexes IV,which are calculated to be 10.4(9.3 kcal/mol to 19.7 kcal/mol),5.6(13.4 kcal/mol to 19.0 kcal/mol),and 30.0 kcal/mol(13.7 kcal/mol to 43.7 kcal/mol)endergonic,respectively.Such results indicate the disfavor of the complexation for L3-Mn bearing a pyridylparaEWG.Subsequently,the insertion of a second CO2molecule into species IV leading to species V with effective disproportionation into a carbonate anion sandwiched between two metal centers and a CO product bounded to one of them.After the release of the CO product,complexes VI will be formed.With the calculated potentials of −2.06 V,−2.27 V and−1.71 V for L1-Mn,L2-Mn and L3-Mn,respectively.Two III species can be regenerated with the release of the carbonate to furnish the catalytic cycle.
To further reveal the electronic effect of the pyridylparasubstituents,the molecular orbital analysis and the atomic dipole moment corrected Hirshfeld(ADCH)charges[35,36]analysis of the Mn center were performed for the reduced species III of L1-Mn(−0.19),L2-Mn(−0.22)and L3-Mn(+0.18)were analyzed with Multiwfn[36,37],as shown in Fig.3b.The ADCH charge of the Mn center increases to −0.22 in L2-Mn-III from −0.19 in L1-Mn-III,indicating the profound impact of the electron-donatingparapyridyl substituent on improving the nucleophilicity of the Mn center to activate the CO2molecule.The low electron density of the Mn center(+0.18)in L3-Mn-III and its relatively stable SOMO(−1.685 eV)can account for the low activity of L3-Mn for the electrocatalytic reduction of CO2due to the EWG.Notably,the protonation of the -OMe(species L2-Mn+H-III)significantly increases the ADCH charge of the Mn center to +0.02 from −0.22 in L2-Mn-III.And the energy of the SOMO of L2-Mn+H-III also decreases to−1.760 eV,which is in good agreement with the experimentally observed suppression of the catalysis due to the negative effect of the TFEs as a proton source.
We also performed Fourier-transform infrared reflectance spectroelectrochemistry(FTIR-SEC)to detect the main species involved in the catalytic reduction process of L2-Mn(Figs.S37 and S38 in Supporting information).Under Ar atmosphere,the spectral features at 2117 cm−1,attributing to a CO ligand released[38],corresponding to the report by Myrenet al.[34].Under the CO2atmosphere,the bands at 1649 cm−1and 1680 cm−1appeared,showing the formation of CO32−/HCO3−species,which is consistent with the DFT-calculated results.
In summary,a series of manganeseN-heterocyclic carbene pincer complexes were developed.Theparaelectron-donating strategy was achieved in a highly active complex(L2-Mn)having -OME group for the selective electrocatalytic reduction of CO2to CO,with a larger catalytic current enhancement(63 ×)and a TOFmax(26,127 s−1,127 times of unsubstituted L1-Mn).CPE study of L2-Mn gave an average Faradaic efficiency of 104%.While L3-Mn with electron-withdrawing -COOMe group inhibited the electrocatalytic activity.Ambient Brønstic acid suppressed the activity of L2-Mn probably due to the protonation of the–OMe group.These findings highlight the potential of electronic tuning for the design of efficient manganese NHC catalysts for CO2reduction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.21973113)and the Guangdong Natural Science Funds for Distinguished Young Scholar(No.2015A030306027),and the Fundamental Research Funds for the Central Universities.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.06.046.
 Chinese Chemical Letters2022年1期
Chinese Chemical Letters2022年1期
- Chinese Chemical Letters的其它文章
- Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation:A review
- Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers
- Porphyrin-based heterogeneous photocatalysts for solar energy conversion
- Systematic evaluation of advance in application and discharge mechanism of solution electrode glow discharge
- Insoluble carbonaceous materials as electron shuttles enhance the anaerobic/anoxic bioremediation of redox pollutants:Recent advances
- Selective N-terminal modification of peptides and proteins:Recent progresses and applications
