TPE based aggregation induced emission fluorescent sensors for viscosity of liquid and mechanical properties of hydrogel
N Wng,Hng Yo,∗,Qi To,Jing Sun,Ho M,Yng Wng,ChengCheng Zhou,Hongying Fn,Hongxi Sho,Aijin Qin,Dwei Su,Chenyin Wng,∗,Hui Chong,∗
aSchool of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou 225009,China
bTesting center of Yangzhou University,Yangzhou 225009,China
cMinistry of Education Key Lab for Avian Preventive Medicine,Key Laboratory of Jiangsu Preventive Veterinary Medicine,College of Veterinary Medicine,Yangzhou University,Yangzhou 225009,China
dCenter for Clean Energy Technology,School of Mathematical and Physical Science,Faculty of Science,University of Technology Sydney,Sydney NSW 2000,Australia
1These two authors contribute equally to this manuscript
ABSTRACT Two amphiphilic TPE E/Z isomers with aggregation induced emission(AIE)property have been synthesized and characterized.The logarithmic fluorescent intensity of the two molecules was in positive relationship with logarithmic viscosity of liquid.To note,the Z-TPE isomer exhibited more sensitivity in the viscosity of liquid sensing in comparison with the corresponding E-TPE counterpart(around 1.80 folds).Furthermore,two molecules could be used as fluorescent sensors for mechanical properties(viscosity and storage modulus)of hydrogel as well.In addition,two sensors displayed low cytotoxicity in normal tissue cell line(L929)within the concentration range of 2–10 μmol/L.These results potentially promised their applications as fluorescent sensors for mechanical properties in the fields of biological and biomedical.
Keywords:TPE AIE Viscosity of liquid Mechanical property of hydrogel Fluorescent sensing
Tetraphenyl ethylene(TPE)and the derivates were distinguished with their aggregation induced emission(AIE)property,which allowed fluorescent emission in aggregating state[1-2].The reason was documented to be restricted intramolecular rotation(RIR)effect[3].In solution,non-emissive decay pathway of TPE in excited state privileged and almost no fluorescence would be observed[4].Upon aggregation or in solid state,rotation of phenyl rings was blocked and TPE would regain its fluorescent emission[5].Due to this unique fluorescent property,various TPE derivates have been widely applied in the fields of fluorescent imaging,fluorescent sensing,disease diagnosis and organic optoelectronics[6-17].Currently,TPE has been recognized as versatile building block of functional materials with fluorescent property[18].
The mechanical property of cellular microenvironment and extracellular matrix(ECM)has been reported to influence a plethora of biological behaviors[19-23].For instance,the nano-viscosity of fluid could influence enzyme catalyzed DNA cleavage rate[24].ECM stiffness was reported to affect intracellular rheology of cancer cells and was related to cancer metastasis[25-28].Currently,biocompatible 3D hydrogels were regarded as one of promising systems for mimickingin vivoECM of cancer cell[29-31].Therefore,a probe for viscosity of liquid and mechanical parameters of hydrogel is of great significance in biological and biomedical fields.In this aspect,fluorescent molecular rotors have been applied as efficient liquid viscosity sensor[32-33].Yet,these probes generally require complicated synthetical procedures and expensive fluorescence life imaging microscope(FLIM)[34].Liquid viscosity could affect intramolecular rotation of phenyl rings on TPE core.Therefore,fluorescence intensity of TPE was in positive relationship with the viscosity of surrounding liquid[35].A handful TPE based liquid viscosity sensors with cellular organelle targeting capabilities have been synthesized and succeeded in revealing the relationship of viscosity and cellular function[11-36].These TPE based probes advanced in simple synthetical procedure and avoidance of expensive instruments.On top of the fluid viscosity sensing,TPE derivates were applied in the description of hydrogel degradation[37].In principle,the viscosity of fluid confined to micro network inside hydrogel could also be sensed by TPE derivates.To some extent,this micro fluid viscosity could reflect the macro mechanical properties of hydrogel[38].On base of that,TPE could potentially be applied in mechanical property sensing of hydrogel.
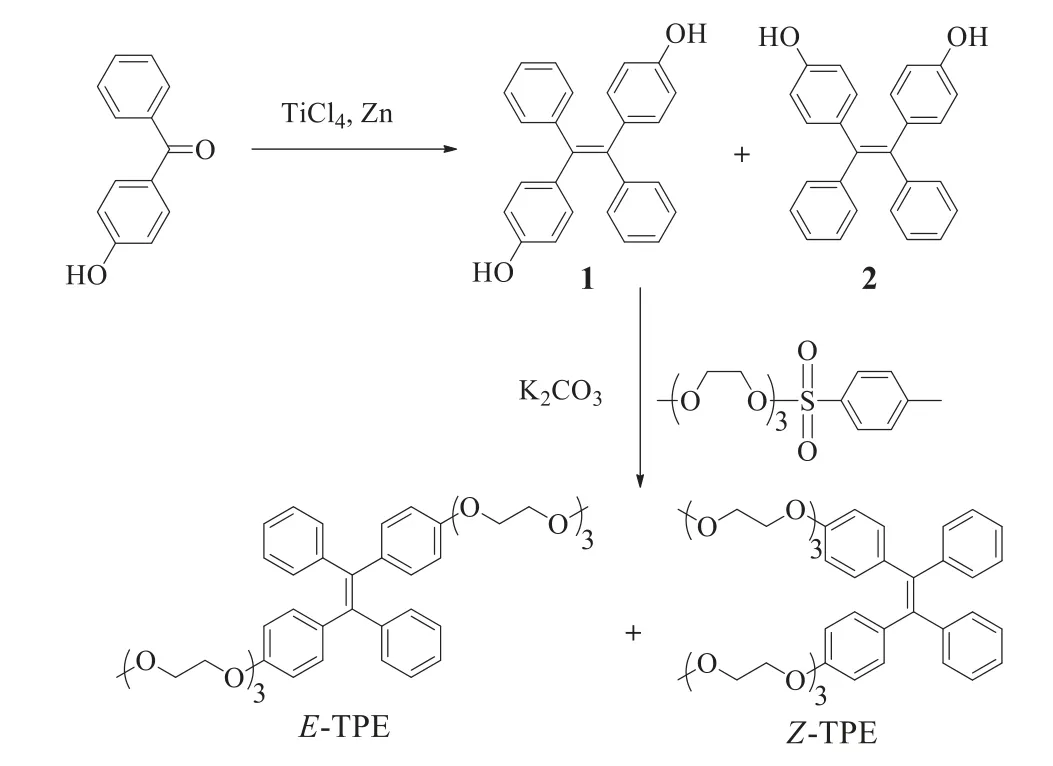
Fig.1.Synthetical route for E-TPE and Z-TPE.
Herein,we wish to report the syntheses of TPE based sensors for fluid viscosity and mechanical parameters of hydrogel.The resultingE/Z-TPE isomers succeeded in fluid viscosity and hydrogel mechanical parameters sensing.Noticeability,Z-TPE showed more sensitivity in viscosity of fluid sensing in comparison with the correspondingE-TPE(1.8 times).To best of our knowledge,this is the first report of TPE based sensor for both fluid viscosity and mechanical parameters of hydrogel.
As shown in Fig.1,we employed a straight-forward synthetical route for compoundsE/Z-TPE.Mixed precursors 1 and 2 were afforded in the yield of 80% through typical titanium tetrachloride catalyzed Mcmurry reaction by using 4-hydroxybenzophenone as starting material.The mixture was subsequently reacted with tosyl activated oligoethylene glycol to afford desired productEandZ-TPE.After carefully purification using silica chromatography,the yield forEandZ-TPE amounted to 51% and 52%,respectively.The detailed synthetical procedures and corresponding1H NMR,13C NMR and high-resolution mass spectroscopies were shown in supporting information(Figs.S5-S12 in Supporting information).The structures ofEandZ-TPE were confirmed by1H NMR.As shown in Fig.2,doublets of protons of substituted phenyl ring(Ha,Hb,Ha′ and Hb′)indicated pureEandZisomers.In case of a mixture,the protons would be triplets.Haand HbinE-TPE slightly upfield shifted in comparison with that of Ha′ and Hb′ inZ-TPE(Δδ=−0.03 ppm).On the contrary,Hc,Hdand HeinE-TPE were observed to slightly downfield shift in comparison with that of Hc′,Hd′ and He′ inZ-TPE.The resonance signal pattern of individualE/Z-TPE isomer matched previously TPE isomers[39].
The photophysical properties were investigated.Both compounds displayed UV–vis absorption with maxima absorbance centered at 260 and 338 nm in ddH2O(Fig.S1 in Supporting information).These absorbances should be assigned toπ-π∗electronic transition of TPE[40].The amphiphilic nature of the two molecules facilitate self-assembling in water(10 μmol/L),thus a typical TPE fluorescent emission(centered at 491 nm,Fig.S1)has been observed.
TPE derivates were reported to be sensitive towards microenvironment,e.g.,fluid viscosity[10].Yet,the impact of configuration on sensitivity remained to be investigated.The currentE-andZTPE isomers(10 μmol/L)displayed different fluorescent emission intensity in relatively low viscous liquid(pure ethylene glycol,31 cp).In details,the fluorescent quantum yield ofE-TPE was around two times than that ofZ-TPE(data not shown).Tang reported similar oligoethylene glycol bearing TPE withZ-configuration has slightly lower fluorescent quantum yield in comparison with theE-configuration isomer due to different assembling behavior[41].In our case,E-TPE(10 μmol/L)showed an amorphous morphology(Fig.3a)andZ-TPE(10 μmol/L)was in a micelle-like morphology(Fig.3b)as characterized by TEM.The different assembly behavior together with fluorescent quantum yields in water was in good agreement with previous result,indicating the assembling behavior could be the reason for varied fluorescent emission properties of the isomers[41].
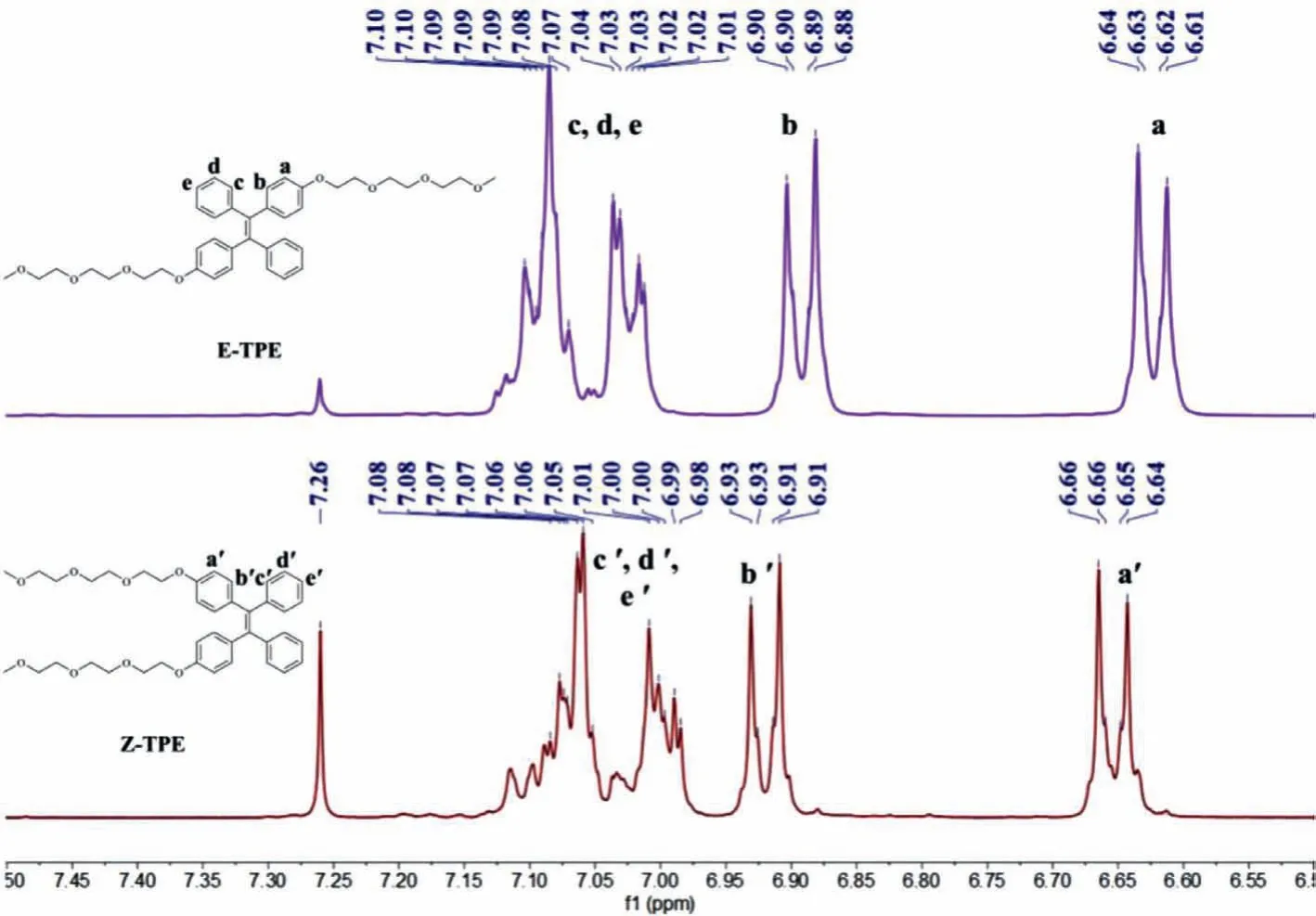
Fig.2.Partial 1H NMR spectra of E-TPE and Z-TPE(CDCl3,300 MHz).
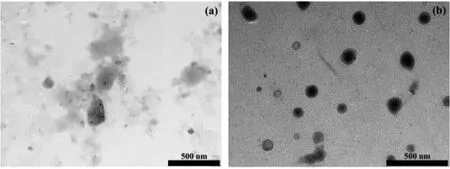
Fig.3.TEM images of E-TPE(a)and Z-TPE(b).Scale bar=500 nm.Acc.voltage was 100 kV.Magnificence for(a)and(b)were 25.0 K and 30.0 K.

Fig.4.Fluorescence emission spectra of E-TPE(a)and Z-TPE(c)in mixture of ethylene glycol and glycerol with different fraction of glycerol(0-99.90%).Plots of logarithmic fluorescent intensity(Log I)of E-TPE(b)and Z-TPE(d) vs. logarithmic mixture viscosity(Log η).[E/Z-TPE]=10 μmol/L,excitation wavelength was 340 nm.
We tuned viscosity of liquid by changing glycerol fraction in ethylene glycol.Both isomers displayed viscosity dependent fluorescent increase.As shown in Fig.4a,E-TPE showed an approximately 11 fold increase of fluorescence intensity in 99.90% glycerol solution(609 cp)in comparison to the condition of pure ethylene glycol(37 cp).The logarithmic fluorescent emission intensity ofE-TPE displayed excellent linear relationship(R2=0.9953)with logarithmic viscosity of the testing solvent(Fig.4b).The enhanced emission intensity should be due to fluid viscosity induced restriction of phenyl ring on TPE that block the non-radioactive decay pathway[39].In addition,the fluorescence maxima ofE-TPE displayed a slightly blue shift in high viscous medium(around 12 nm in 99.90% glycerol).Similar phenomena were observed in other TPE based viscosity sensor,and was supposed to be the influence of vibrational energy level in viscous solution[34].Z-TPE was found to be more sensitive towards microenvironment viscosity.As shown in Fig.4c,increasing glycerol fraction to 99.90% resulted a 19.82-folds enhancement of the fluorescent intensity(around 1.8 times sensitive towards viscosity in comparison toE-TPE).The logarithmic fluorescent emission intensity ofZ-TPE also displayed excellent linear relationship(R2=0.9935)with logarithmic viscosity of the testing solvent(Fig.4d).The fluorescence from the agarose has been ruled out according to the spectra of hydrogel with different agarose concentration(Fig.S2 in Supporting information).
Fig.5.Fluorescence emission spectra of E-TPE(a)and Z-TPE(b)in mixture of ethylene glycol and glycerol with different fraction of glycerol(0-99.9%)in the presence of 1 mol/L acetic acid.[E/Z-TPE]=10 μmol/L,excitation wavelength was 340 nm.
Since the restriction of intramolecular rotation(RIR)effect contributes to fluorescence emission of TPE molecules,we suspectedE- andZ-TPE may have different rotational energy barriers.According to DFT calculations(Supporting information),only slight difference in rotational barrier is observed between the two isomers;the Gibbs free energy barriers at room temperature and ambient pressure are from 8.4 kcal/mol to 9.7 kcal/mol for both isomers(Fig.S3 in Supporting information),in good agreement with those for similar compounds[37].This suggests that there should be other reasons for the different sensitivity.Alternatively,we suspect hydrogen bonds between solvent and the oligoethylene glycol side-chains on TPE could serve as an external factor that influencing the rotation of the phenyl rings.In the situation ofZ-TPE,which the oligoethylene glycol side chains stay in close distance,a successive hydrogen bonds bridge mediated by solvent molecules may form between the two side chains.This successive hydrogen bonds bridge might produce extra rotational energy barrier of the phenyl rings.Whereas the oligoethylene glycol chains stay relative far apart inE-TPE,the corresponding successive hydrogen bonds bridge would be less stable or even hard to form.Therefore,E-TPE was supposed to be less sensitive towards hydrogen bond formation solvent molecules in contrast toZ-TPE.
In order to verify this hypothesis,we measured the fluorescent spectra of the two TPE isomers in solvents composed of different ratio of ethylene glycol and glycerol in the presence of 1 mol/L acetic acid to eliminate potential hydrogen bonds interactions.Both TPE isomers displayed fluorescence enhancement with increasing of glycerol in acidic solution.In 99.90% percentage of glycerol,E(Fig.5a)andZ-TPE(Fig.5b)showed 13.74- and 14.20-folds increase of fluorescence intensity enhancement,respectively.The similar fluorescence enhancement ratio in the presence of acid suggests hydrogen bond indeed played partial role in the RIR effect dominated fluorescence enhancement.
On top of liquid viscosity sensing,we further attempted to measure the mechanical properties of hydrogels using synthesized viscosity sensors.The hydrogels were fabricated straightforwardly by cooling the ddH2O solution of low-melting point agarose with varied concentrations(0.20%,0.30%,0.40%,0.50% and 0.60%)below 37°C.The mechanical properties of hydrogels were first characterized.The mechanical properties were first measured using a rheometer.The viscosity and storage modulus(G′)of the corresponding hydrogels were in positive relationship with the concentration of agarose.The viscosity of the hydrogel reduced with increase of shear rate,showing a pseudo-plastic behavior.
We next addedEandZ-TPE(10 μmol/L)during the fabrication of these hydrogels,respectively.The hydrogels showed fluorescence emission centered around 490 nm,indicating existence of AIE effect in the hydrogels.As shown in Figs.6a and d,theG′ and viscosity of hydrogels was correlated with the fluorescent emission intensity centered around 490 nm.The logarithmic fluorescence intensity ofE-TPE containing hydrogel was displayed good linear relationship with logarithmic value of bothG′ and viscosity(R2=0.9487 and 0.9622,respectively,Figs.6b and c).Similarly,logarithmic fluorescence intensity ofZ-TPE containing hydrogel also showed good linear relationship with logarithmic value ofG′ and viscosity(R2=0.9912 and 0.9891,respectively,Figs.6e and f).The viscosity andG′ of the corresponding hydrogels were in positive relationship with the concentration of agarose.The fluorescence emission intensity of both TPE isomers increased with the concentration of agarose indicated the fluorescence intensity could reflect its mechanical properties.Given the fact that agarose constitutes the network structure inside hydrogels,increased concentration of agarose contributes to the enhanced viscosity andG′ of hydrogels.In the presence of more agarose,the grid size would become smaller.The liquid inside each grid might become less mobile and more viscous due to more hydrogen bond interactions.This might explain the enhancement of fluorescent intensity in the presence of more agarose loading.Finally,the two sensors displayed low cellular toxicity in mouse fibroblast cell line(L929)within the concentration range of 2–10 μmol/L.This promised their feasibility of future biomedical applications(Fig.S4 in Supporting information).

Fig.6.Fluorescence emission spectra of E-TPE(a)and Z-TPE(d)in hydrogels composed of different concentration of agarose(0.20%–0.60%).[E/Z-TPE]=10 μmol/L,excitation wavelength was 340 nm.Plots of natural logarithmic fluorescent intensity of E-TPE(b)and Z-TPE(e) vs. natural logarithmic storage modulus(G′)of hydrogel.Plots of natural logarithmic fluorescent intensity of E-TPE(c)and Z-TPE(f) vs. natural logarithmic viscosity of hydrogel.[E/Z-TPE]=10 μmol/L,excitation wavelength was 340 nm.
In conclusion,two oligoethylene glycol bearing TPE isomers(E-TPE andZ-TPE)have been synthesized and characterized.The fluorescent intensity of both compounds was in positive relationship with viscosity of liquid within the range of 31–690 cp(R2=0.9953 and 0.9935 forEandZ-TPE,respectively).In details,Z-TPE was more sensitive in viscosity sensing in comparison withE-TPE(around 1.80 times).Computational simulation suggests the rotational energy barriers for the two compounds are practically identical.Addition of high concentration of acid in viscous medium afford similar fluorescent enhancing ratio of two compounds,indicating the different hydrogen bond interacting strength might be the reason for different viscosity sensing performance.Furthermore,mechanical parameters(viscosity and storage modulus G′)of hydrogel were also in positive relationship with the fluorescent intensity of both TPE isomers.The reason was believed to be restricted intramolecular rotation effect as well.Finally,both compounds displayed neglectable cellular toxicity.These results allowed them to be potentially used as promising sensors of liquid viscosity and hydrogel mechanical parameters in biological and biomedical fields.
Declaration of competing interest
The authors declare no confliction of interest.
Acknowledgments
The authors thank National Natural Science Foundation of China(Nos.21375116,21978251,22073080),Nature Science Foundation of Jiangsu Province(Nos.BK20190903,BK20190905),and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions for financial support.H.Chong acknowledges The open funds of the Ministry of Education Key Lab for Avian Preventive Medicine(No.YF202020).Y.Wang.acknowledges the Thousand Talents Plan for Young Professionals of China.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2021.06.092.
 Chinese Chemical Letters2022年1期
Chinese Chemical Letters2022年1期
- Chinese Chemical Letters的其它文章
- Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation:A review
- Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers
- Porphyrin-based heterogeneous photocatalysts for solar energy conversion
- Systematic evaluation of advance in application and discharge mechanism of solution electrode glow discharge
- Insoluble carbonaceous materials as electron shuttles enhance the anaerobic/anoxic bioremediation of redox pollutants:Recent advances
- Selective N-terminal modification of peptides and proteins:Recent progresses and applications
