Near-infrared absorbing(>700 nm)aza-BODIPYs by freezing the rotation of the aryl groups
Yanyan Wang,Dongxiang Zhang,Kangming Xiong,Rong Shang,Xin-Dong Jiang
aShenyang Key Laboratory of Functional Dye and Pigment,Shenyang University of Chemical Technology,Shenyang 110142,China
bCAS Key Laboratory of Separation Science for Analytical Chemistry,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China
cDepartment of Chemistry,Graduate School of Science,Hiroshima University,Higashi-Hiroshima 7398526,Japan
1These authors contributed equally to this work.
ABSTRACT The typical aza-BODIPYs in the dye family are known for bright fluorescence,excellent stability,and tunable absorption wavelengths.Hence,these dyes are attracting the increasing attention.Aza-BODIPYs having the maxima absorption in the near-infrared(NIR)region(650–900 nm)are very favorable for bioimaging in vivo due to the less photo-damage,deeper tissue penetration,and less interference from background auto-fluorescence by biomolecules in the living systems.Many strategies have been employed to modify the structures of the aza-BODIPY core to provide the NIR absorbing dyes.Among these,the most effective method is the fusion of the aromatic rings in aza-BODIPY system.This review allsidedly summarizes the recent development of ring-fused aza-BODIPY dyes(λabs > 700 nm)focusing on the design,synthesis,and potential applications in the NIR region since 2002.
Keywords:Aza-BODIPY NIR Ring-fused Red shift Freezing rotation
1.Introduction
Near-infrared(NIR)absorbing(λabs>650 nm)dyes are found to have many especial applications.For example,fluorescence imaging techniques are widely used for visualizing and analyzing the localization of ions,cations and biomolecules[1].However,excitation wavelengths(λex<650 nm)have low-grade tissue penetration,and therefore,are applicable for superficial structures or small animal imaging.Fluorophores requiring excitation at about 600 nm results in excessive autofluorescence because the bulk of naturally occurring endogenous fluorophores,mostly hemoglobin and related molecules,are also excited in the same region.Therefore,the optimal excitation wavelength(λex>650 nm)for a fluorophore is advocated,due to the combined virtues of deep tissue penetration,minimum photo-damage to biological samples,and low autofluorescence[2].On the other hand,organic solar cells(OSCs)play an important role in the increase of the energy demand.However,one of the disadvantages of OSCs is the lack of light-harvesting in the NIR region beyond 700 nm,behaving 43%of the total energy flux[3].
Aza-borondipyrromethenes(aza-BODIPYs)in the dye family have long-wavelength absorption(λabs≥650 nm),high molar extinction coefficient,high fluorescent quantum yield,and narrow absorption and emission peaks[4].So,aza-BODIPY dyes can satisfy the essential requirements of an ideal NIR fluorophore.Therefore,aza-BODIPYs can be extensively applied to chemosensors[5,6],biological imaging[7–10],organic light-emitting[11],photosensitizers for photodynamic therapy[12–16]and other fluorescent functional dyes[17,18].Compared to the traditional BODIPY dye,by the introduction of an imine replacing a methene in BODIPY system to effectively narrow the HOMO-LUMO gap one gives aza-BODIPY that are well-known to be an attractive dye to achieve the NIR absorption[19,20].Importantly,π-πconjugated extension and freezing the rotation of the aryl groups in aza-BODIPY system are particularly promising and can effectively achieve the significant bathochromic-shift of absorption and emission bands into the NIR region[21,22].For instance,photoacoustic(PA)dyes,absorbing the NIR light to provide ultrasonic signals,can be probed at centimeter depths in tissues by fluorescence-based methods[23,24].Therefore,NIR absorbing(λabs>700 nm)aza-BODIPYs have received widespread attention over the last decade[25].
Recently,our group summarized the three syntheticisms for aza-BODIPYs firstly(Scheme 1)[26].Killoranet al.have explored novel routes to synthesize aza-BODIPYs by 1,3-diaryl-4-nitrobutanone or 3-methyl-4-nitro-1-arylbutan-one(Scheme 1a),which can smoothly synthesize the symmetric/asymmetric construction of aza-BODIPYs widespreadly[27].Aryl-fused aza-BODIPYs reported by Donyaginaet al.were prepared in one-pot reaction by the reaction of an aryl magnesium bromide with a phthalonitrile(Scheme 1b)[28].Utilizing 2,4-diaryl pyrrole or aryl-fused 2,4-diaryl pyrrole,Zhao and Carreira showed an efficient route of symmetric/asymmetric aza-BODIPYs(Scheme 1c)[29].Compared to the product yields of O’Shea’s and Lukyanets’methods,the product yield of Carreira’s method is higher(beyond 40%).Moreover,compared to O’Shea’s molecular structures,Lukyanets’and Carreira’s molecular structures have larger conjugated surface of the parent molecules.In the past decade,our group focused on the design of aza-BODIPYs bearing the restricted rotation of the aryl groups in order to obtain NIR absorbing aza-BODIPYs.Very recently,Shiet al.reported a review paper from the perspective of rational structural design about bioapplications of micromolecule aza-BODIPYs derivated by O’Shea’s method[30].However,the design and synthesis of ring-fused aza-BODIPY dyes(λabs>700 nm)were not systematically introduced in those review papers.By the strategy of freezing the free rotation of the aryl groups,we concluded design and synthesis of ring-fused aza-BODIPYs in this review.We also described the detailed spectral and photophysical data to provide the meaningful guidance for further design of NIR organic fluorescent materials.

Scheme 1.Synthetic methods of the aza-BODIPYs.
Since the typical tetraphenyl-based aza-BODIPY 1 possessed a NIR absorption(λabs=650 nm)and emission spectra,the strategies by restricting the rotation of the aryl groups could directly achieve a longer wavelength(λabs>700 nm)for aza-BODIPYs(Scheme 2).Six kinds of synthetic strategies of the ring-fused aza-BODIPYs are shown in Schemes 2.For example,aza-BODIPY containing the phenyl-fused groups at 1,2/6,7-positions has a NIR absorbing spectra(λabs≥710 nm)(aza-BODIPY 3c)[28],and aza-BODIPY bearing the aryl-fused groups at 2,3/5,6-positions shows the maximum absorption beyond 700 nm(aza-BODIPY 18)[29].Moreover,the NIR fluorescent aza-BODIPY bearing all aryl-fused groups at 1,2,3/5,6,7-positions(aza-BODIPY 49)[31],even possesses a NIR absorbing spectra at 882 nm.The details are as follows.
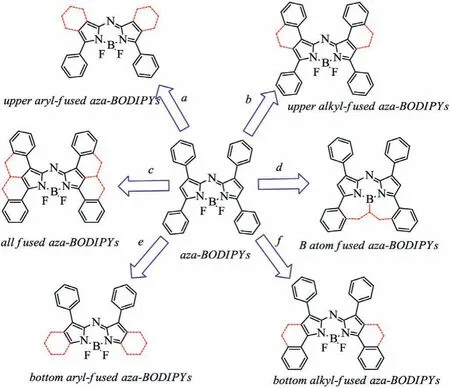
Scheme 2.Strategies for the NIR absorbing(λabs > 700 nm)aza-BODIPYs.
2.Ring-fused aza-BODIPYs
2.1.The upper aryl-fused aza-BODIPYs
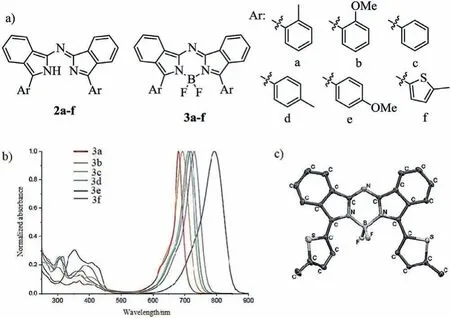
Fig.1.Molecular structures,absorption spectra and single crystal.(a)Structures of dyes 2a–3f.(b)Absorption spectra of 3a–f.(c)X-ray analysis of 3f.Reproduced with permission[34].Copyright 2011,John Wiley and Sons.
Owing to the direct fusion of the upper aryl-group with the pyrrole of aza-BODIPY core to extendπ-πconjugation(patha,Scheme 2),the upper aryl-fused aza-BODIPYs possess longer wavelength absorption in NIR region.In comparison with the typical dye aza-BODIPY 1,the upper aryl-fused aza-BODIPYs were found to have an apparent advantage of the longer absorption wavelengths(Fig.1 and Table 1)[32,33].In 2011,Gresseret al.reported that the precursors 2a–f and aza-BODIPYs 3a–f were obtained by reduce reaction and complex reaction with Grignard reagent and BF3.OEt2(Fig.1)[34].The corresponding aza-BODIPYs 3 show a weak absorption in the visible region and the maxima absorption are bathochromic-shift into the NIR region beyond 790 nm,leading to a transparent window in the NIR region.Molecular orbital(MO)calculations clearly confirmed that the bathochromic-shift in aza-BODIPYs 3a–f was caused by an increase of the HOMO levels.Based on the X-ray analysis of 3f,the coplane of the aza-BODIPY core was confirmed.
Viathe substitute of the fluorine atom in aza-BODIPY 3c(Fig.1),the functional aza-BODIPY derivatives 4 and 5 were developed to satisfy the practical applications of fluorescent dyes[32,34].Due to a highly thermal stability,CN-substituted aza-BODIPYs can be successfully purified by vacuum sublimation.This upgrades thin film of high purity and quality,suggesting them suitable as candidates for vacuum-processed NIR organic electronic devices(Fig.2b and Table 1).
In 2017,Liet al.extended theπ-πconjugated structures by introducing the heterocyclic moieties and synthesized the NIR aza-BODIPYsviathe heterocyclic organolithium reagents(Fig.3 and Table 1)[33].Then,aza-BODIPYs were further modified to re-place one of the fluorine atoms with a cyanide which has the strong electron-withdrawing effect.As the NIR absorbing dyes,these dyes present high molar extinction coefficients(65,100–104,500 L mol−1cm−1)with absorption maxima in the NIR region between 762 and 797 nm.Based on the vacuum-deposited thin films,the absorption bands are red-shifted,peaking at 830–849 nm and broadened,giving the NIR donor materials for the vacuum-processed solar cells(Fig.3b).Cyclic voltammetry(CV)measurements and MO calculations indicate that the HOMO-LUMO orbital levels are suited as donor materials in the solar cells while combined with the C60compound as an acceptor.

Table 1 Dyes and their optical properties.
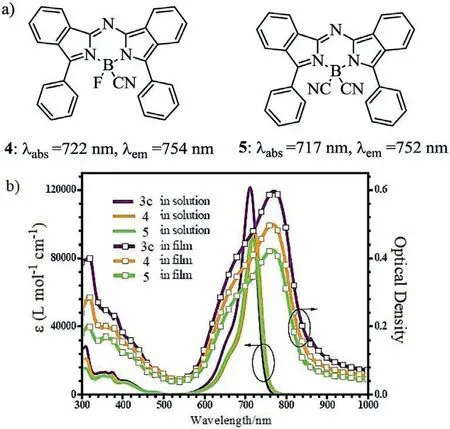
Fig.2.Molecular structures and absorption spectra.(a)Aza-BODIPYs with bearing the–CN group.(b)Absorption spectra of 3c,4 and 5 in CH2Cl2 solution and in film.Reproduced with permission[32].Copyright 2017,John Wiley and Sons.
In 2020,Diaz-Rodriguezet al.prepared Cl-aza-BODIPY 9 and pH-aza-BODIPY 10(Fig.4)[35].Cl-aza-BODIPY scaffold 9 facilitates the substitutions at the boron atom to provide 10 by the treatment with phenyl Grignard reagent.pH-aza-BODIPY 10 was found to have a lower quantum yield,and this is mainly due to effi-cient non-radiative relaxation pathways offered by the free rotation of the numerous phenyl groups,particularly of the B-phenyl moieties.The maxima absorption of the pyrazine-fused aza-BODIPY 11 is 685 nm[36],which blue-shifts 24 nm,compared to that of 3c.The hypochromatic-shift is mainly attributed to the higher electronegativity of the nitrogen atoms in pyrazine,which enhances the HOMO-LUMO band gap.Aza-BODIPY 11 can be applied for a colorimetric and fluorometric sensor for NH4+(Fig.4 and Table 2).
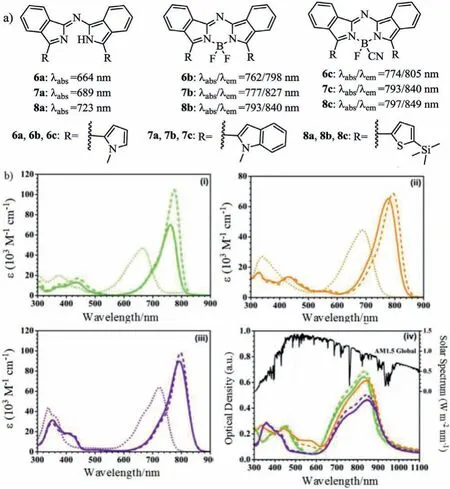
Fig.3.Molecular structures and absorption spectra.(a)Structures of dyes 6–8.(b)Absorption spectra of 6a–c(i),7a–c(ii)and 8a–c(iii)in CH2Cl2(1 × 10−5 mol/L)and(iv)the absorption spectra in solid state as 50 nm thin film on glass.Reproduced with permission[33].Copyright 2017,Royal Society of Chemistry.
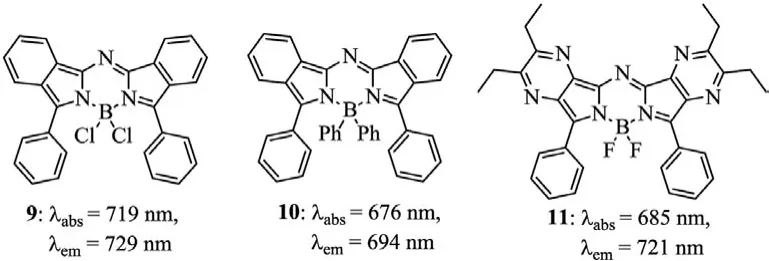
Fig.4.Structures of aza-BODIPYs 9–11.
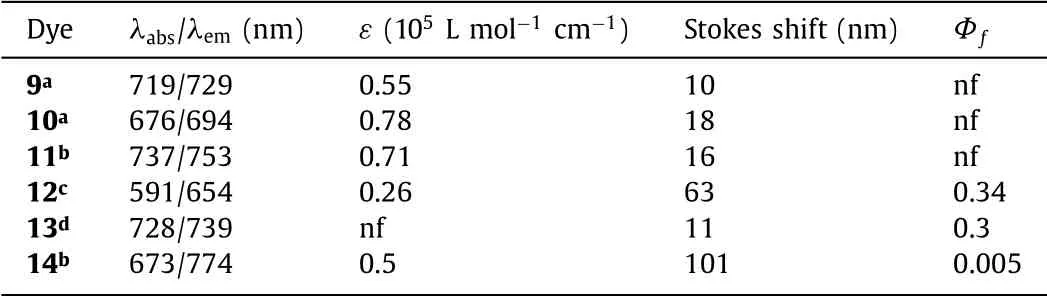
Table 2 Dyes 9–14 and their optical properties.
In 2017,Zhenget al.showed the novel aza-BODIPY 12 from phthalonitrile int-BuOK solution in a facile manner on large scale(Fig.5)[37–39].The asymmetric aza-BODIPY 12 prompts the weak B-N bond breakage in the presence of TFA,giving a sharp color change from red to colorless(Fig.5c and Table 2).

Table 3 Dyes 15–17 and their optical properties.
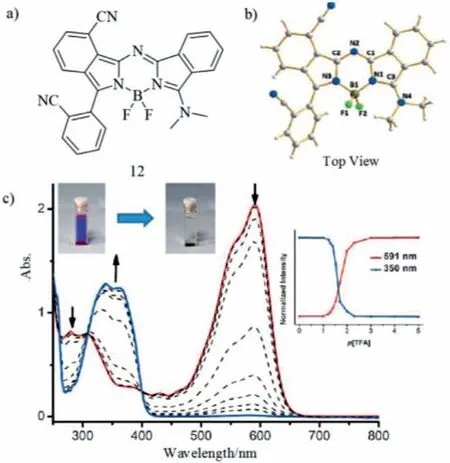
Fig.5.Molecular structures,single crystal and absorption change.(a)Benzo-fused aza-BODIPY 12 and(b)X-ray analysis of dye 12.(c)Absorption spectra of aza-BODIPY 12 with TFA.Reproduced with permission[37].Copyright 2015,John Wiley and Sons.
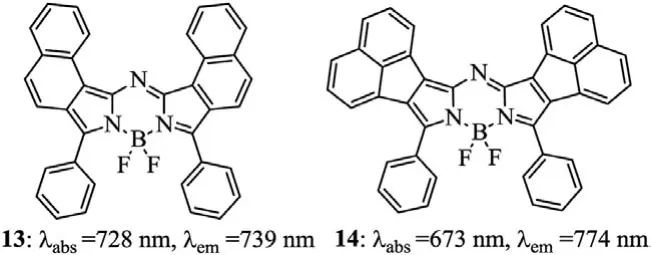
Fig.6.Structures of aza-BODIPYs 13 and 14.
The introduction of 1,2-naphtho-fused rings to produce dye 13 leads to a 25 nm bathochromic-shift of the maxima absorption relative to that of the parent dye 3c(Fig.6 and Table 2),along with a slight change in other optical properties[40,41].Majumdaret al.reported a NIR absorbing acenaphthalene-fused aza-BODIPY dye 14,which broad absorption band at the red terminal of the visible region,providing possibilities for the application in the field of solar cells[42].
2.2.The upper alkyl-fused aza-BODIPYs
By the interlinkage of theσsingle bond(pathb,Scheme 2),a new substitution pattern of aza-BODIPYs were synthesized by the upper phenanthrene fusion by a key Pd-catalyzed intramolecular C–H activation reaction(Figs.7a and b)[31].Such phenanthrenefused aza-BODIPYs have strong red-shifted NIR absorptions and high fluorescent quantum yields(Table 3).Aza-BODIPY 15a has a planar structure of the phenanthrene-fused aza-BODIPY core by Xray analysis(Fig.7c).Aza-BODIPY 15a having low cytotoxicity,can stain the HepG2 cells,indicating a bright NIR bioimaging nature(Fig.7d and Table 3).
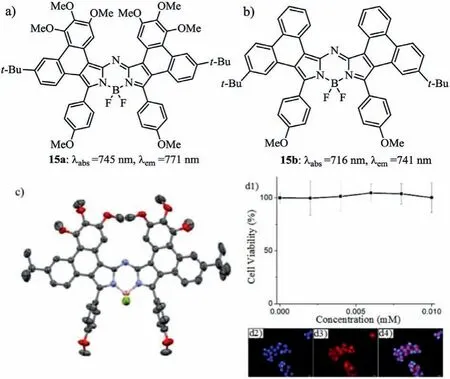
Fig.7.Structures of(a)aza-BODIPY 15a and(b)aza-BODIPY 15b.(c)X-ray analysis of 15a.(d1)Fluorescence images of HepG2 cells stained with 15a(5.0 μmol/L)and DAPI(1.67 μg/mL),d2)DAPI fluorescence,(d3)15a fluorescence,and(d4)merged images of parts d2 and d3.Reproduced with permission[31].Copyright 2017,American Chemical Society.
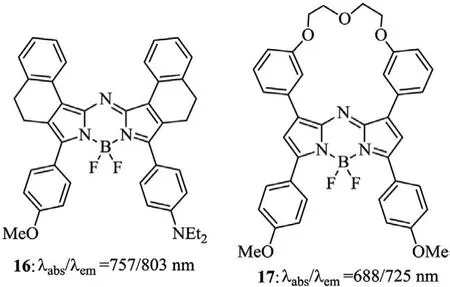
Fig.8.Structures of the upper phenyl-restricted aza-BODIPY 16 and 17.
Zhouet al.reported a restricted aza-BODIPY 16 for the NO probe with enhanced photoacoustic properties(Fig.8),by utilizing the upper phenyl-fused pyrrole with the -CH2-CH2- alkyl chain for the first time(pathb,Scheme 2)[43].Compared to 3c,aza-BODIPY 16 formed by connecting the 1,7-positions of the phenyl groups with the 2,6-positions of aza-BODIPY by the alkyl chain can further improve the spectral properties.To restrict the free rotation of the phenyl group in 1,7-positions similar to the molecule 16,aza-BODIPY 17 with two phenyl groups linked by oligoethylene glycol chains was synthesized and applied for intracellular imaging for HepG2 cells(Fig.8 and Table 3)[44].
2.3.The bottom alkyl-fused aza-BODIPYs
Zhao and Carreira reported the novel NIR conformational restricted aza-BODIPYs 18–29(Fig.9)prepared by an efficient process in 2006 for the first time(pathc,Scheme 2)[29,45].By tunable substitution and restriction,the bottom alkyl-fused aza-BODIPY dyes have the long-wavelengh fluorescence at 700–900 nm,high fluorescence quantum yield,narrow full width at half maximum and remarkable photostability(Table 4)[46–51].The morpholine-containing aza-BODIPY 29 as a probe can detect the pH value.Additionally,aza-BODIPY with the sulfur atom 21 as a photosensitizer(PS)could generate the single oxygen.
In 2018,Jianget al.prepared five-membered-ring fused aza-BODIPY 30(Fig.10)[52].Such aza-BODIPY has long absorption/emission wavelength,high molar extinction coefficient,and narrow excitation/emission peak.However,the planar structure of 30 could not obviously enhance the optical properties than these of six-membered-ring fused aza-BODIPY.In 2015,Jianget al.reported the symmetric pyrene-containing aza-BODIPY 31 in the NIR region(λabs=746 nm,λem=762 nm),based on the combined effect of the extension of theπ-πconjugation and the rigidization(Fig.10)[53].Restricting the free rotation and extending the conjugation by introducing the pyrenyl substituent in 31 effectively promote bathochromic-shift(110 nm),comparing to that of 1.The decrease in the HOMO-LUMO band gap for the lowest energy absorption bands was observed in the pyrene-fused aza-BODIPY(Table 4).

Fig.9.Structures of aza-BODIPY dyes 18–29.

Fig.10.Five-membered-ring fused aza-BODIPY 30 and pyrene-containing aza-BODIPY 31.
In 2012,Jianget al.synthesized a turn-on aza-BODIPY-based NIR fluorescent probe 32(Fig.11)[54].The maxima absorption and emission of probe 32 were 717 nm(ε=48,000 L mol−1cm−1)and 734 nm,respectively.Dye 32 displayed weakly fluorescent(Φf=0.03)(the solid curve in Fig.11c).When adding cysteine,the maxima absorption and emission wavelengths were bathochromicshifted to 735 nm and 755 nm,respectively,and markable enhanced quantum yield(Φf=0.14)was achieved(Fig.11 and Table 4).
In 2016,Jianget al.reported aza-BODIPY 34 containing the thiophene groups(Fig.12a)[55].Aza-BODIPY 34 has absorption/emission wavelengths of 760/782 nm in the NIR region.Aza-BODIPY 34 as a typical NIR chemical sensor is highly selective toHg2+.The mechanism of fluorescence quenching is the transfer of electrons from thiophene groups to the aza-BODIPY core by binding to Hg2+(Fig.12b and Table 4).
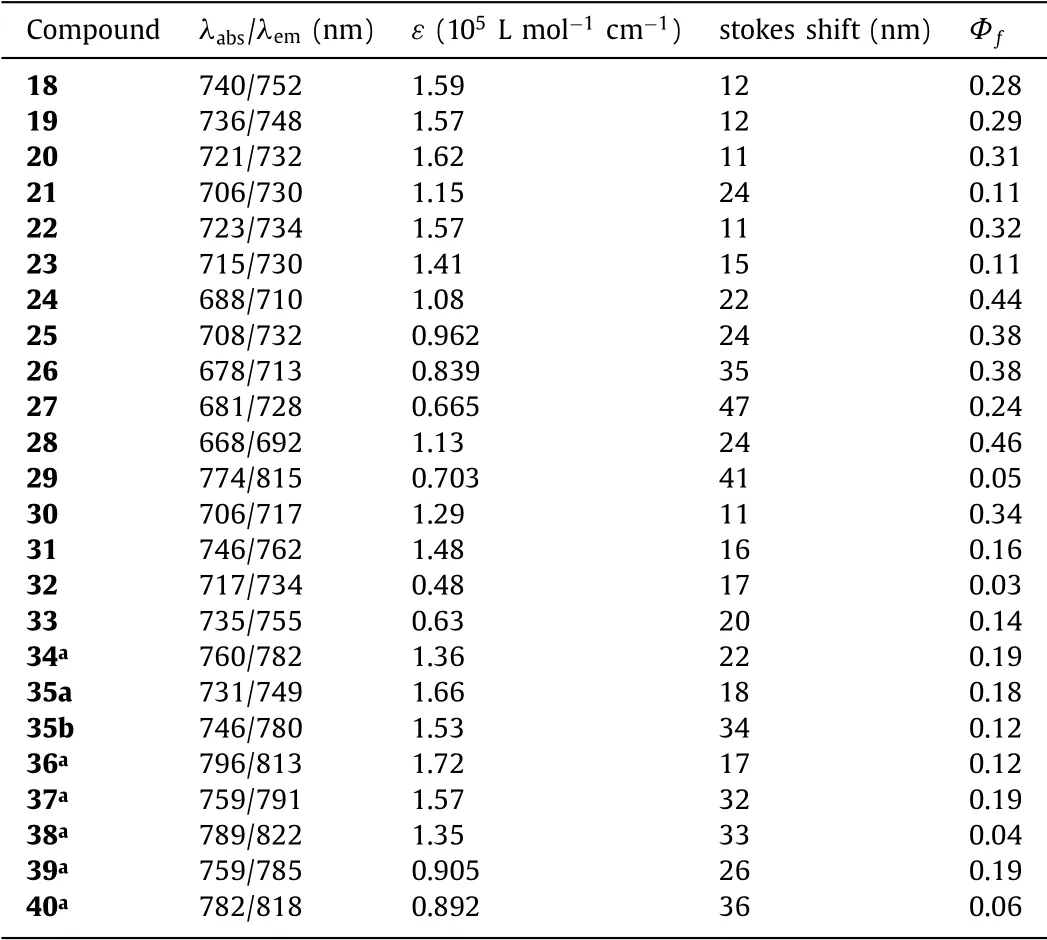
Table 4 Dyes 18–40 and their optical properties.
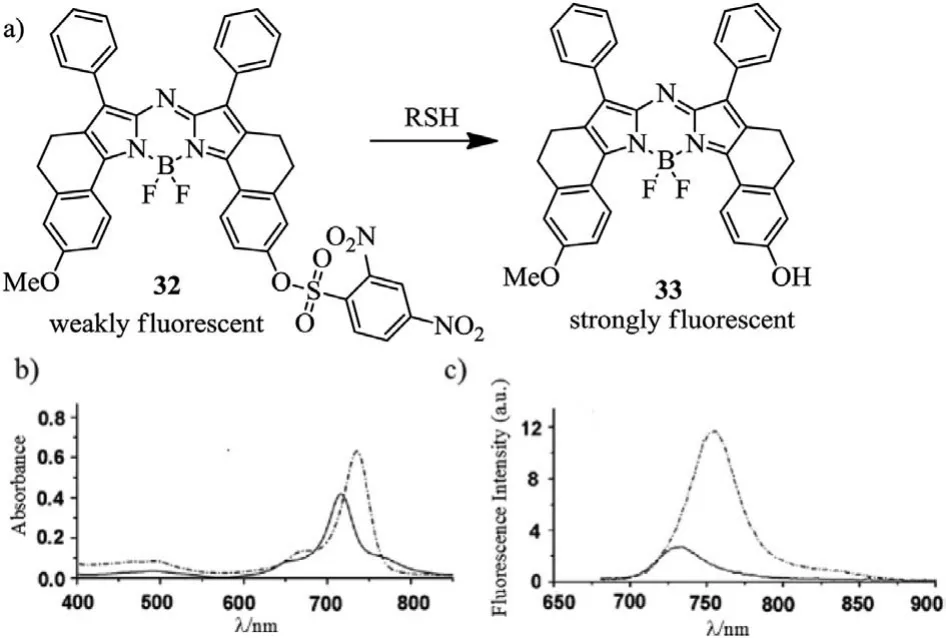
Fig.11.Reaction mechanism and spectral variation.(a)Reaction of aza-BODIPY with RSH.(b)Absorption and(c)Emission spectra(λex=670 nm)of probe 32 before(solid curve)and after(dashed curve)the addition of cysteine.Reproduced with permission[54].Copyright 2012,Royal Society of Chemistry.
In 2014,the styryl-containing asymmetric aza-BODIPYs 35 by Jianget al.were prepared in the NIR region for the first time,which was bright enough for labeling the living cells for fluorescence imaging assays(Fig.13 and Table 4)[56].In short,the asymmetric aza-BODIPYs enrich the quantities and categories of aza-BODIPYs which can be used to meet the purpose of the application.Compared to symmetrical aza-BODIPYs,asymmetric aza-BODIPYs are not easily synthesized,with lower yield.
In 2019,Zhouet al.developed aza-BODIPYs with the -CH2–CH2-alkyl chain and investigated their absorbance,fluorescence,and photoacoustic(PA)properties(Fig.14)[43,57].All conformationally restricted aza-BODIPYs 36–40 had the promotion in the molar absorptivity owing to the increased complanarity between the aza-BODIPY core and the aryl groups.MO studies indicated that the aryl groups at 3,5-positions in aza-BODIPY system efficiently enhance the HOMO levels than those at 1,7-positions(Fig.14).It was found that the free rotation of the aryl-groups was important to maximizingΔλ,while restriction of the aryl-groups was found to enhance the PA signal by increasing the extinction coefficient without promoting quantum yield markedly(Table 4).
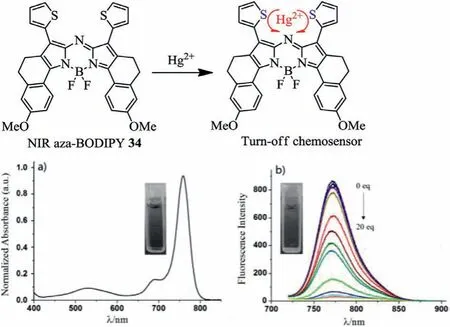
Fig.12.Spectral changes of(a)absorption spectrum of NIR fluorescent probe 34 with Hg2+.(b)Emission spectra of NIR fluorescent probe 34 with Hg2+.Reproduced with permission[55].Copyright 2015,Elsevier.
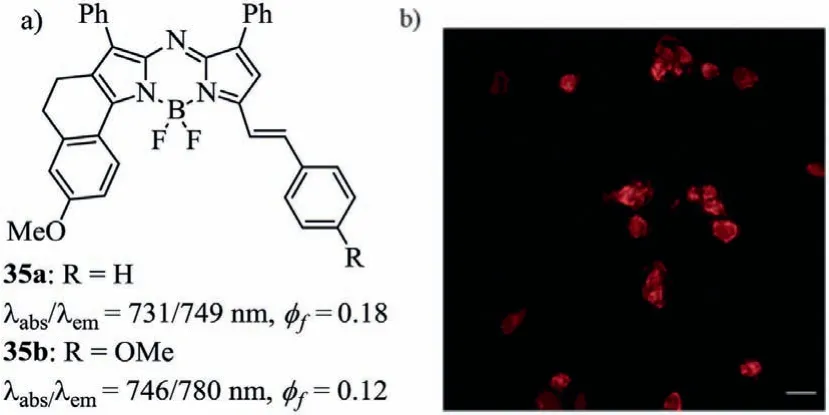
Fig.13.Molecular structures and cell staining.(a)Styryl-containing aza-BODIPYs 35.(b)Image of living cells stained by dye 35b.Reproduced with permission[56].Copyright 2014,Royal Society of Chemistry.
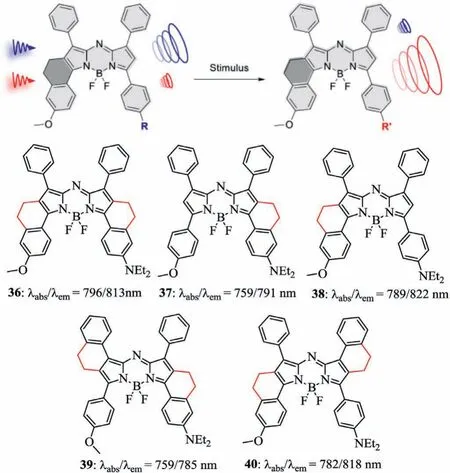
Fig.14.Restricted-aryl aza-BODIPY dyes 36–40.Reproduced with permission[43].Copyright 2019,American Chemical Society.

Fig.15.Molecular structures and optical spectra.(a)Thiophenyl-fused aza-BODIPYs 41.(b)Normalized UV−vis and(c)fluorescence spectra of aza-BODIPYs 41a(black),41b(red),41c(blue),41d(magenta)in chloroform.Reproduced with permission[58].Copyright 2014,American Chemical Society(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.).
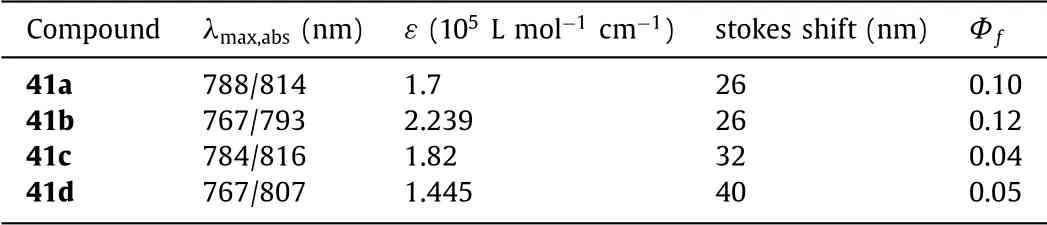
Table 5 Dyes 41a–41d and their optical properties.
2.4.The bottom aryl-fused aza-BODIPYs
To the direct fusion of the bottom aryl-group in 2,3/5,6-posions in aza-BODIPY system,in 2014 Kamkaewet al.reported a series of the bottom thiophenyl-fused aza-BODIPYs 41(pathd,Scheme 2)[44,58].Aza-BODIPYs 41 with the stronger and sharper absorption above 800 nm,absorbing weaker in the visible spectrum region from 380 nm to 700 nm were obviously observed(Fig.15 and Table 5).The excellent optical properties and simple synthesis of aza-BODIPYs 41 have led to further application in biotechnology and material science.
2.5.B atom-fused aza-BODIPYs
Linking the ortho-positions of the phenyl groups to the central atom B can establish aza-BODIPYs 43 and 44 with the six-member heterocyclic ring(pathe,Scheme 2,Fig.16)[59,60].Comparing to aza-BODIPY 42,the restricted dye 43 displays obviously absorption and emission bathochromic-shift of up to 86/58 nm(Table 6).The favorable emission wavelength and fluorescent quantum yield are strongly indicative of the future applications in biotechnology.
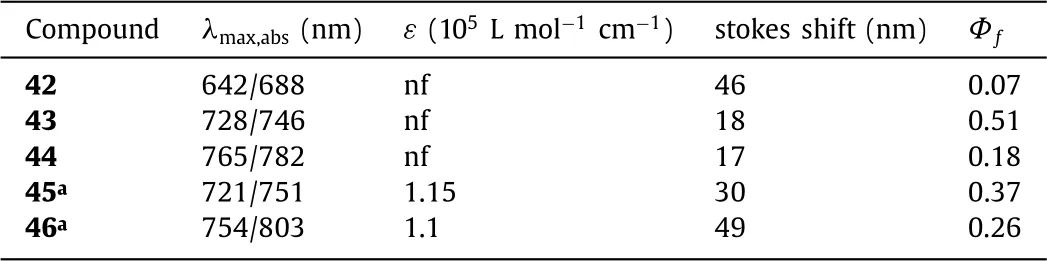
Table 6 Dyes 42–46 and their optical properties.
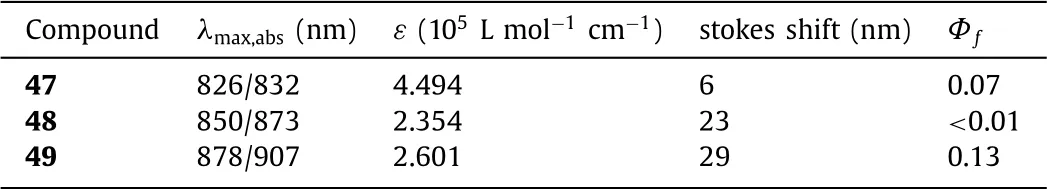
Table 7 Dyes 47–49 and their optical properties.
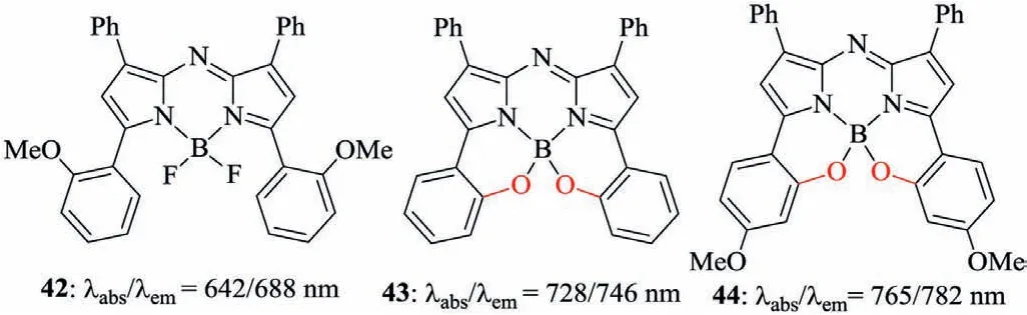
Fig.16.B,O–chelated aza-BODIPYs.
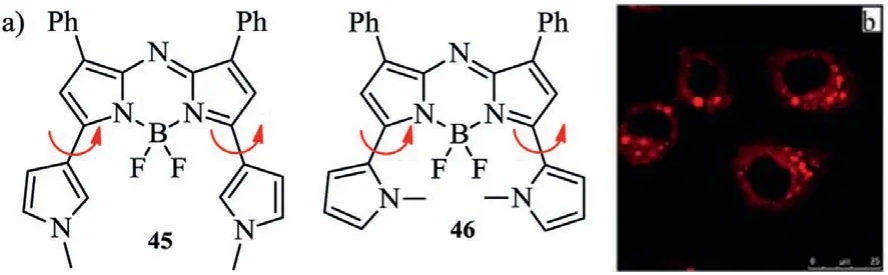
Fig.17.Molecular structures and cell staining.(a)Methylpyrrolyl-containing aza-BODIPYs.(b)Image of living cells stained by dye 46.Reproduced with permission[61].Copyright 2016,John Wiley and Sons.
To excavate the steric hindrance effect similar to aza-BODIPYs 42–43 with the six-member heterocyclic ring,in 2016 Jianget al.reported aza-BODIPY dyes bearing 1-methyl-1H-pyrrolyl groups at 3,5-positions(Fig.17 and Table 6)[61].Aza-BODIPY 45 absorbs at 721 nm and emits at 751 nm,respectively.However,aza-BODIPY 46 has long wavelengths of absorption and emission(λabs=754 nm,λem=803 nm)than these of the corresponding aza-BODIPY 45.Comparing to aza-BODIPY 45,the rotation of the pyrrolic group in 46 is be slowed or prohibited,due to the steric hindrance between the pyrrolic group and the fluorine atom at the boron center.These reveal the favourable consistency with the discrepant optical contrast between the restricted aza-BODIPY caused by the B-O bonds and its precursor by Burgess and O’Sheaet al.Aza-BODIPY 45 could stand pH 12 and is more stable than dye 1,and was suitable for labeling the living cells for fluorescence imaging assay in the NIR region.
2.6.All ring-fused aza-BODIPYs
In 2018,Shenget al.synthesized NIR fluorescent aza-BODIPYs 47,48 and 49 containing the aryl-fused group at 1,2,3/5,6,7-positions using FeCl3(pathf,Scheme 2,Fig.18a)[62].These aza-BODIPYs have unique structures(Fig.18b)and excellent optical properties with the NIR absorption and emission,high extinction coefficient up to 4.5 × 105L mol−1cm−1and good photostability(Table 7).These dyes reveal their potential applications in the construction of organic fluorescent clusters based on annular fused nitrogen-hetero-specific groups for the first time.Theoretically,all aryl-fused structures have the longest absorption maxima,however the complex molecular design and difficult synthesis limit their application.Therefore,for all ring-fused aza-BODIPYs,more convenient synthesis method and extensive applications are forward to be explored.
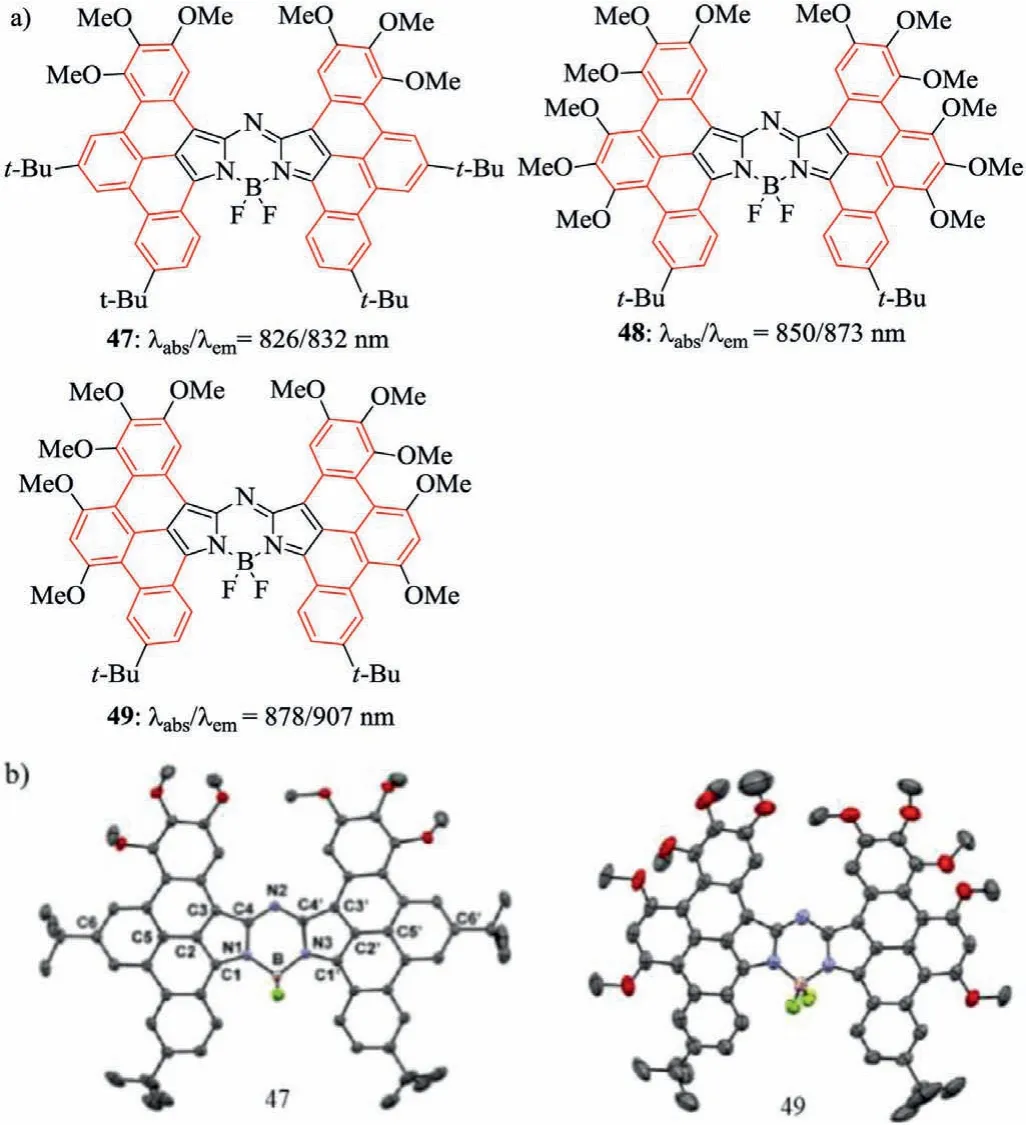
Fig.18.Molecular structures and single crystal.(a)Aza-BODIPYs 47,48 and 49.(b)X-ray crystal structures 47 and 49.Reproduced with permission[62].Copyright 2018,American Chemical Society.
3.Summary
Compared with the classical aza-BODIPY 1(λabs=650 nm),ring-fused aza-BODIPYs(Scheme 2)possess higher molar absorption coefficient,longer wavelength of absorption spectra(λabs>700 nm)in the NIR region.Aza-BODIPYs with the aryl-fused groups at 1,2/6,7-positions or 2,3/5,6-positions,aza-BODIPYs bearing the -CH2- link to the aryl group at the upper or bottom position,and B-fused aza-BODIPYs with the six-member heterocyclic ring at 4-position have the NIR absorbing spectra between 700 and 800 nm.Moreover,aza-BODIPYs bearing all ring-fused groups at 1,2,3/5,6,7-positions even possess an absorption maxima beyond 800 nm.The ring-fused aza-BODIPYs presented in this review are expected to give a useful guidance for further developing the efficient NIR organic fluorophores with suitable properties for biomedical and material applications and so forth.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Nos.22078201,U1908202),“Chunhui Program” cooperative research project of Education Ministry,Liaoning BaiQianWan Talents Program,Serving local project of Education Department of Liaoning Province(No.LZ2020005)and the Distinguished Professor Project Liaoning Province(No.20183532).
 Chinese Chemical Letters2022年1期
Chinese Chemical Letters2022年1期
- Chinese Chemical Letters的其它文章
- Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation:A review
- Recent advances in nanoscale metal-organic frameworks biosensors for detection of biomarkers
- Porphyrin-based heterogeneous photocatalysts for solar energy conversion
- Systematic evaluation of advance in application and discharge mechanism of solution electrode glow discharge
- Insoluble carbonaceous materials as electron shuttles enhance the anaerobic/anoxic bioremediation of redox pollutants:Recent advances
- Selective N-terminal modification of peptides and proteins:Recent progresses and applications
