A Novel 3D CdII-CP Based on a Bi-functional (N-/O-Sites)Triazole-modified Carboxyl Ligand: Structure,Topology and Photoluminescence①
ZHAO Dan GUO Ting-Ting AN Yan-Yan YAN Juan-Zhi
(Taiyuan University, Taiyuan 030032, China)
ABSTRACT With triazole-modified derivative as link, one new framework [Cd5(μ5-HL2-)2(μ4-L3-)2(H2O)10]·2H2O(1, H3L = 2-(1,2,4-triazol-1-yl)benzene-1,3,5-tricarboxylic acid) has been hydrothermally synthesized and characterized by single-crystal X-ray diffraction, powder X-ray diffraction and elemental analysis. Complex 1 exhibits a 3D (3,4,6)-connected net cross-linked by binuclear [Cd2(COO)5]- cluster and mononuclear Cd(II)geometry via HL2-/L3- ligands. It crystallizes in triclinic system, space group Pwith a = 7.7411(11), b =9.8813(15), c = 20.177(3) Å, α = 85.547(4)°, β = 80.603(4)°, γ = 67.976(3)°, V = 1411.3(4) Å3, Z = 1, Mr = 1876.89 g/mol, Dc = 2.208 mg/m3, μ = 1.97 mm-1, F(000) = 918, GOOF = 1.03, the final R = 0.0869 and wR = 0.1950 for 3127 observed reflections with I > 2σ(I). The solid photoluminescent behaviour of 1 has been further functionally investigated.
Keywords: triazole, Cd(II) complex, crystal structure, fluorescence property;
1 INTRODUCTION
The structural diversity, controllability and post-modification of coordination polymers (CPs) as well as their potential applications in various fields (such as gas storage and separation[1-3], optics[4,5], catalysis[6], and magnetochemistry[7])still receive extensive attention. Fluorescent CPs are treated as an emerging optical material, exhibiting high application value in the fields such as photochemical sensors and electro-luminescent displays[8,9].
The formation of most coordination polymers (CPs) is self-assembly driven by coordination[10]. Many factors affect the assembly process, such as organic link, metal ion,temperature, pH, solvent and other weak interactions. Unfortunately up to now, the prediction and control for the final product are still a big challenge. During the self-assembly process of CPs, the ligand as an important component plays a direct impact on the final structure. A variety of typical bi-topic ligands, including pyridine carboxylate[11], amino polycarboxylic acid[12]and N-containing aromatic carboxylic acid[13], represent attractive constituents for cross-linked building blocks. 1,2,4-Triazole is a five-membered ring molecule containing three N atoms, and each N has a pair of donor electrons. By using substituents to modify the triazole,a variety of derivative ligands can be obtained. Triazolemodified aromatic carboxylic acid, as a bi-functional ligand containing multiple coordination sites (N/O), has been proved particularly successful[14-16]. Some obvious reasons for this success are briefly mentioned: (a) Aromatic and triazole rings can produce intramolecular conjugatedπbonds; (b) One or more carboxyl groups in the ligand tend to provide diverse coordination modes; (c) Neutral N atoms can easily coordinate with metal ions; (d) The different Pearson hardness[17]of N/O coordination sites favors cross-linking of different cations and conveys additional stability to metal coordination.
Given the above-mentioned background, we use 2-(1,2,4-triazol-1-yl)benzene-1,3,5-tricarboxylic acid (H3L) as ligand and Cd(II) ions as coordination centers to construct a new 3Dframework. Here, we report the synthesis, characterization and luminescence property of complex [Cd5(μ5-HL2-)2(μ4-L3-)2(H2O)10]·2H2O (1). The simplified grid of complex 1 is described into a (3,4,6)-conntopological net.
2 EXPERIMENTAL
2. 1 Materials and methods
All the reagents used in this work were commercially available. Elemental analyses for C, H, and N components were determined on a Perkin-Elmer analyzer. Powder X-ray diffraction patterns were collected on a D/Max-2500 X-ray diffractometer using Cu-Kαradiation. Solid fluorescent spectrum was measured on a Varian Cary Eclipse Fluorescence spectrophotometer.
2. 2 Synthesis of complex[Cd5(μ5-HL2-)2(μ4-L3-)2(H2O)10]·2H2O (1)
A mixture of CdCl2·H2O (0.102 g, 0.33 mmol), H3L (0.062 g, 0.25 mmol) and H2O/CH3CN (4 mL, 1:3 v/v) was placed into a 25 mL PTFE vessel sealed with a stainless-steel reactor and heated to 120 ℃ for 3 days. Colorless crystals with a yield of 65.2% were obtained through the cooling process(2 ℃/h). Elemental analysis found/calcd. (%) for 1: C,29.46/29.54; H, 2.14/2.24; N, 9.03/8.96.
2. 3 X-ray crystallography
Single-crystal X-ray diffraction data for complex 1 were collected on a Bruker APEX-II CCD diffractometer. Specific X-ray source and collection temperature are limited to Mo-Kαradiation (λ= 0.71073 Å) at 296(2) K. Unit cell parameters were determined by SMART program. Absorption was corrected by the multi-scan method using SADABS program[18]. Structure was solvedviadirect methods employed in the SHELXS-2018 program[19]and refined by full-matrix least-squares techniques againstF2. Non-hydrogen atoms were assigned anisotropically. Hydrogen atoms (C-H) were defined geometrically and refined using a riding model approximation, while H atoms (O-H) were located from Fourier maps and refined as riding with O-H bond lengths of 0.82~0.84 Å (Uiso(H) = 1.5Ueq(O)). One triazole ring shows conformational disorder. The PART instruction was used to split both conformers, and the sum of the occupancies was constrained to 1. Selected bond lengths, bond angles and H-bonds are shown in Tables 1 and 2.
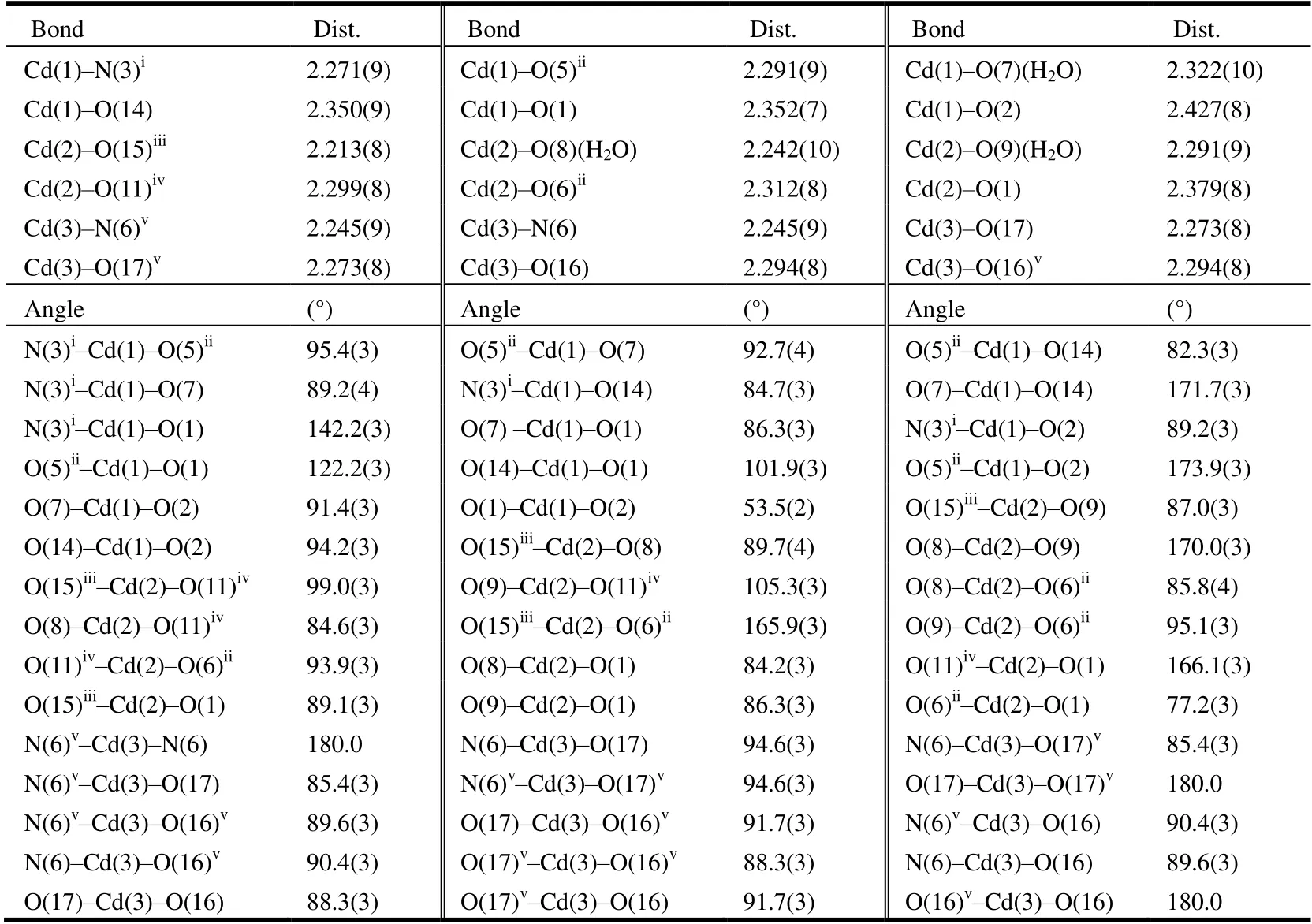
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)
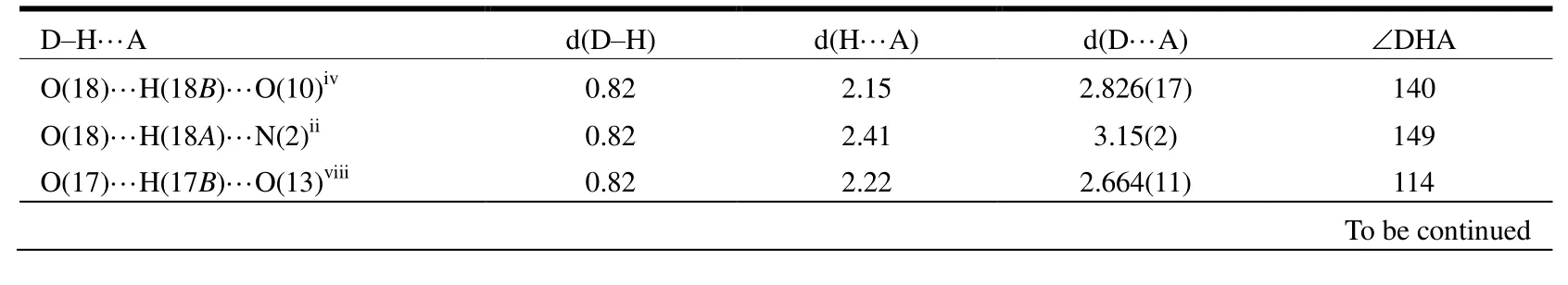
Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°)

Symmetry codes: (ii) x+1, y-1, z; (iv) x, y-1, z; (vi) x+2, y+2, z; (viii) x+1, y, z; (ix) -x+1, -y+2, -z; (x) -x+1, -y+1, -z+1
3 RESULTS AND DISCUSSION
3. 1 Structural description for 1
[Cd5(μ5-HL2-)2(μ4-L3-)2(H2O)10]·2H2O (1). Complex 1 crystallizes in triclinic space groupP, showing a 3Dframework. The asymmetric unit consists of two and a half crystallographically independent Cd(II) ions, oneμ5-HL2-anion, oneμ4-L3-anion, five metal coordination water molecules and one lattice water molecule. As depicted in Fig. 1,three distinct Cd(II) ions all adopt a distorted octahedral geometry. Cd(1) displays a seriously distorted octahedral geometry by coordinating as a [CdNO5] mode. Cd(2) and Cd(3) are also coordinated with six atoms, which promotes the conformation of octahedral geometry. Besides, Cd(3) is located on the inversion center contributing to half occupancy.All of the Cd-O bond lengths fall in the range of 2.213(8)~2.427(9) Å, and the Cd-N lengths are 2.164(8)~2.245(9) Å.

Fig. 1. ORTEP view of the coordination environments of Cd(II) ions in complex 1
Interestingly, three carboxylate groups of H3L were deprotonated partially or completely to give two kinds of anions HL2-and L3-in the reaction system, resulting in HL2-and L3-anions which display specificμ5-κN:κOO′:κO′:κO′′:κO′′′ andμ4-κN:κO:κO′:κO′′ coordination modes (Fig. 2). The dihedral angles formed by triazole and phenyl rings in HL2-and L3-ligands amount to 73.796(0.620)° and 63.085(0.333)° in order to reduce steric strain. H3L as a typical bi-topic ligand possesses two slightly different Pearson hardness coordination sites (O and N).Negative carboxylate groups tend to bridge multiple metal centers to form cluster units. Neutral triazole nitrogen atom auxiliarily coordinated with above metals to expand the entire structure. As shown in Fig. 3a, atoms Cd(1) and Cd(2) are bonded to give a binuclear [Cd2(COO)5]-secondary building unit (SBU) by carboxylate groups, with the short inter-SBU separation Cd(1)···Cd(2) to be 3.8072(4) Å. Along theabplane, binuclear SBU units are linked through benzene carboxylate moieties to form a 2Dlayer ignoring the participation of N sites. By a self-assembly process, adjacent layers are connected by triazole N coordination sites andviaCd(3) atoms (purple color) to develop into a 3Dframework(Fig. 3b). In the simplified net, HL2-connects three binuclear SBUs. Unlike HL2-, L3-links three SBUs and one more extra Cd(3) atom to extend toward four directions. Considering the binuclear SBU as a 6-c node, HL2-and L3-ligands as 3-c and 4-c nodes, the whole structure can be simplified as a 3D(3,4,6)-conntopological network. The corresponding Schlafli symbol is determined to {4·62·83}2{43}2{44·68·83}2by topological analysis[20](Fig. 4).
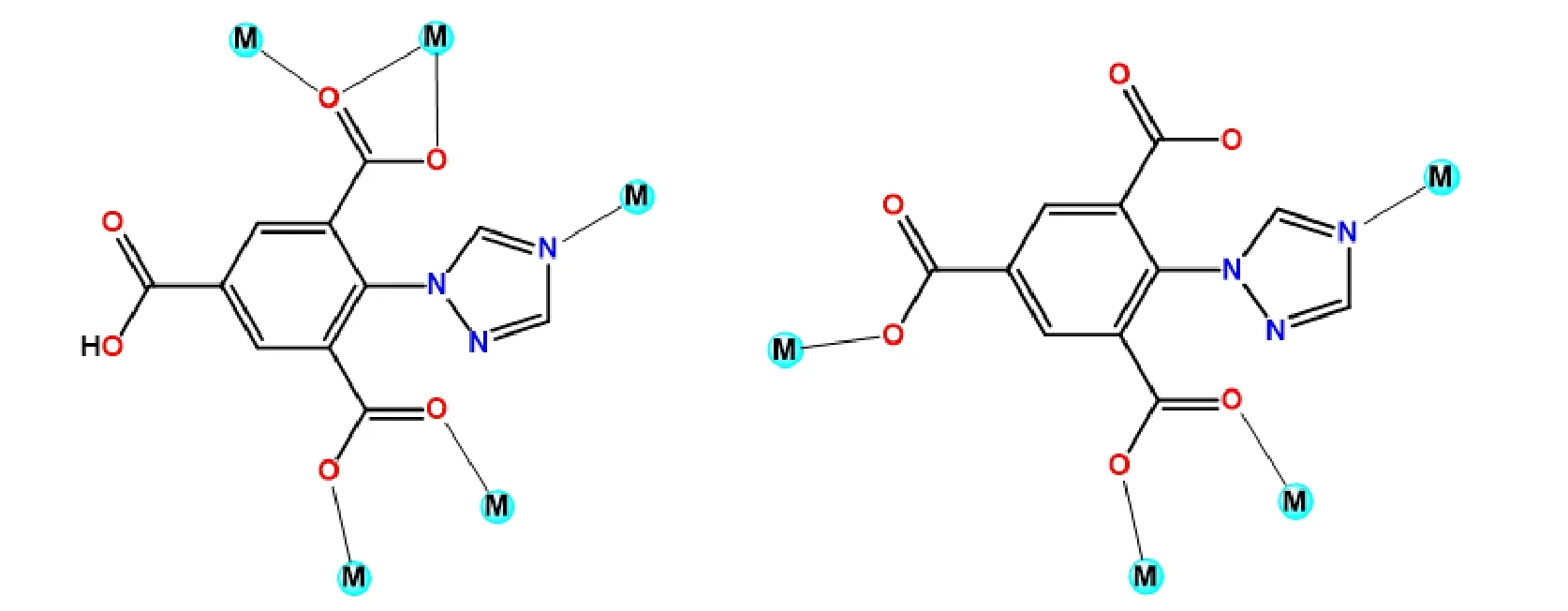
Fig. 2. Two kinds of coordination modes in H3L

Fig. 3. (a) 2D network built from O-coordination sites, (b) View of the 3D framework connected by triazole N-coordination sites

Fig. 4. Topologically (3,4,6)-connected network assembled by 3-/4-/6-conn nodes in 1
3. 2 Powder X-ray diffraction (PXRD) analysis
PXRD experiment of 1 has been carried out to establish its crystalline phase purity. The experimental patterns of bulk samples are consistent with the simulated patterns corresponding to single crystal data (Fig. 5). Therefore, we can confirm the identity of the bulk material with single crystal structures.
3. 3 Luminescent property
As an emerging fluorescent material, fluorescent CPs have the advantages of simple synthesis, controllable structure,high luminous efficiency, and pure chromaticity[21-22]. As an important component, organic ligands usually contain largeπ-conjugated systems. They can coordinate with metal ions containing richd10electrons (such as Cd(II) or Zn(II)) to form fluorescent CPs[23]. The close packing of structure and the enhancement of system rigidity will induce the complex to generate excellent photoluminescence[24,25]. Thus, it has shown extremely high application values in the field of optical materials, such as photochemical sensors and electroluminescent displays[8,9]. In this line of argument, fluorescence monitoring represents a decisive analytical tool for exploring the photoluminescence of fluorescent CPs. Solid state fluorescence of 1 has been investigated at room temperature. Emission spectrum of 1 shows a maximum emission peak at 371 nm under the UV excitation of 285 nm(Fig. 6). Applying the CIE1931 program, the fluorescence chromaticity coordinates are calculated as (0.1521, 0.0257),which falls in blue light region (inset in Fig. 6). It is speculated that the reason for fluorescence may be the charge transfer from ligand to ligand (LLCT) or intra-ligand (n-π* orπ-π*) emission in the complicated network. The metalto-ligand MLCT or ligand-to-metal LMCT in Cd(II) complex can be completely ignored, because the full electron Cd(II)ion is difficult to oxidize or reduce[26].
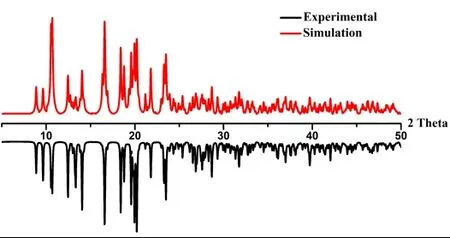
Fig. 5. PXRD patterns for the sample of 1
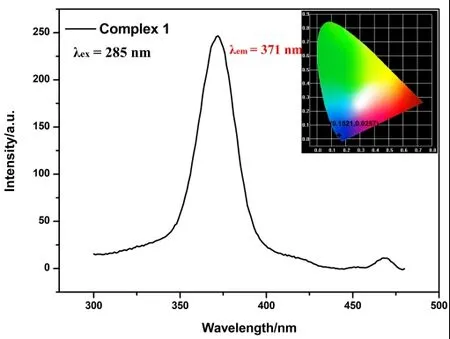
Fig. 6. Solid-state luminescence spectra of complex 1 at room temperature (inset: CIE chromaticity diagram)
4 CONCLUSION
We have employed 2-(1,2,4-triazol-1-yl)benzene-1,3,5-tricarboxylic acid (H3L) as organic link for the divalent Cd(II)cation to construct complex [Cd5(μ5-HL2-)2(μ4-L3-)2-(H2O)10]·2H2O (1). Owing to the specific N/O coordination sites, the structural results are partially predictable. As might have been expected, typical carboxylate groups bridge two Cd(II) ions to form a binuclear SBU unit. Furthermore, these units are linked by benzene carboxylate moieties to expand a 2Dlayer by ignoring the participation of N sites. From the view of 3Dspace, N-coordination promotes the development of the 3Dframework. Besides, complex 1 has outstanding photoluminescence, specifically exhibiting blue fluorescence.
- 结构化学的其它文章
- Synthesis, Crystal Structure and Fungicidal Activity of 3,4-Dichloro-5-(6-chloro-9-(4-fluorobenzyl)-9H-purin-8-yl)isothiazole①
- Synthesis, Crystal Structure and Antifungal Activity of New Furan-1,3,4-oxadiazole Carboxamide Derivatives①
- Syntheses, Crystal Structures, Anticancer Activities and DNA-Binding Properties of the Dibutyltin Complexes Based on Benzoin Aroyl Hydrazone①
- Synthesis, Crystal Structure, Fungicidal Activities and Molecular Docking of Acyl Urea Derivatives Containing 2-Chloronicotine Motif①
- Molecular Design and Property Prediction of High Density 4-Nitro-5-(5-nitro-1,2,4-triazol-3-yl)-2H-1,2,3-triazolate Derivatives as the Potential High Energy Explosives①
- A New Borate-phosphate Compound CsNa2Lu2(BO3)(PO4)2:Crystal Structure and Tb3+ Doped Luminescence①

