High tumor mutation burden indicates a poor prognosis in patients with intrahepatic cholangiocarcinoma
INTRODUCTION
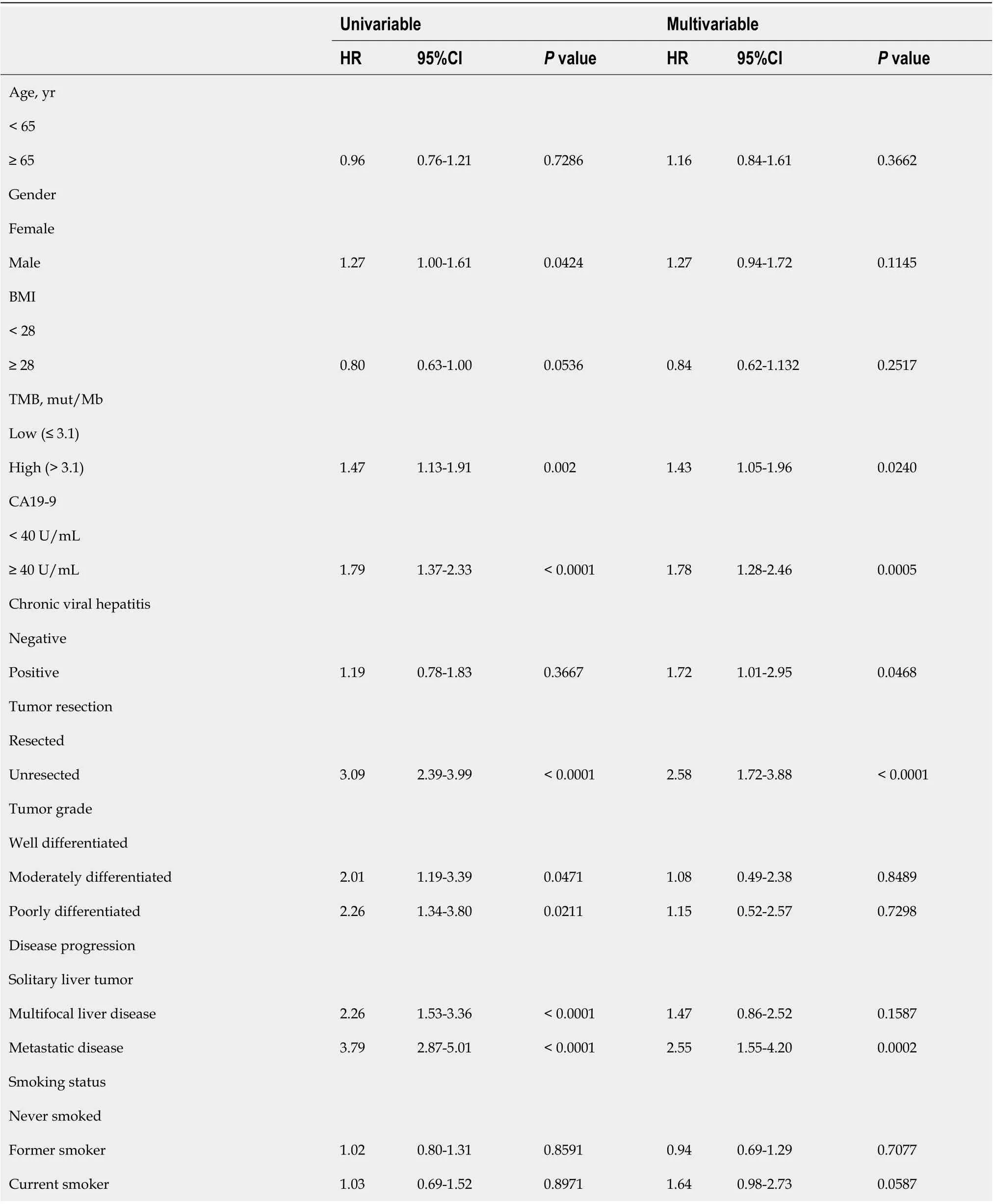
TMB was an independent risk predictor for ICC. Furthermore, independent prognostic factors of ICC included CA19-9, chronic viral hepatitis, tumor resection and disease progression (metastatic diseasesolitary liver tumor). The clinical characteristics and TMB data of some cases had missing. which increased the analysis error in our study.Using a single data source also increases statistical error. Further larger–cohort studies are necessary to confirm the predictive value of TMB in the prognosis of ICC patients.
Gastroenterology and hepatology
Therefore, in this study, we used the ICC database from the Memorial Sloan Kettering (MSK) Cancer Center to investigate the impact of TMB on the prognosis of ICC patients in combination with other clinical features, confirming that TMB was an independent prognostic factor for ICC patients.
MATERIALS AND METHODS
Data collection and processing
Data of 412 ICC patients from the MSK Cancer Center cohort (MSK cohort:http://www.cbioportal.org/study/summary?id=ihch_msk_2021) were included[18].TMB was calculated as the total number of somatic, non-silent, protein-coding mutations divided by the coding region captured in each MSK-IMPACT panel (341 genes, 0.98 Mb; 410 genes, 1.06 Mb; 468 genes, 1.22 Mb). Ethics approval and patient consent were waived by the MSKCC Institutional Review Board and the need for informed consent has been waived by the MSKCC IRB per 45 CFR 46.116 and 45 CFR 164.512, since our data were retrieved from a public database. Clinicopathological information, including age, gender, BMI, TMB, CA19-9, chronic viral hepatitis, tumor resection, tumor grade, disease progression and smoking status, were all reviewed retrospectively.
Cox regression analysis and survival analysis
Cox regression analysis was performed to examine the correlation between TMB and patient’s overall survival (OS). According to the time-dependent receiver operating characteristic (ROC) curve, patients were divided into either the high (TMB > 3.1 mut/Mb) or low TMB (TMB ≤ 3.1 mut/Mb) group. Kaplan-Meier method was used to construct the survival curves of patients. The time dependent specificity and sensitivity of survival were analyzed by deploying timeROC and survival in the R package. The log-rank test was used to examine the differences between the curves,and avalue < 0.05 was considered to be statistically significant. The nomogram model and calibration curve were also analyzed using the rms package in R.
Statistical analysis
Statistical analyses were performed using the SPSS version 25.0 (IBM Corp.) software.The Kaplan-Meier curve was analyzed using the survival package in R version 3.6.3,and the time dependent ROC curve was analyzed using the timeROC package,wherein the picture was generated by the ggplot2 package in R version 3.6.3. All reportedvalues were two-tailed, and≤ 0.05 was considered statistically significant for all analyses in this study.
RESULTS
Overview of the MSK-IMPACT cohort
In this study, the MSK-IMPACT cohort included a total of 412 ICC patients who were mainly compared using TMB as an independent prognostic factor. Most patients in this cohort were examined using the 341- (IMPACT341) and 410-gene (IMPACT410)panels. In comparison to the latest 468-gene panel (IMPACT468), the unsequenced genes in the earlier versions were assumed to be wild-type or non-mutated. Clinical data in this study included age (< 65, ≥ 65), gender (male, female), BMI (< 28, ≥ 28),TMB (≤ 3.1, > 3.1), CA19-9 (< 40 U/mL, ≥ 40 U/mL), chronic viral diseases (negative,positive), tumor resection (resected, unresected), tumor grade (well differentiated,moderately differentiated, poorly differentiated), disease progression (solitary liver tumor, multifocal liver disease, metastatic disease), and smoking status (never smoked, former smoker, current smoker). Baseline clinicopathological features of the study cohort are summarized in Table 1 (median age: 63 years, range: 18-88; 46.1% of patients were females; median: TMB 2.5 mut/MB, range: 0-51.6).
Prognostic impact of TMB in ICC patients
First, we analyzed the utility of TMB in prognosis, calculating a median TMB of 2.5 mut/Mb (range: 0-51.6 mut/Mb). To analyzed the predictive performance of TMB relating to OS, we generated a time-dependent ROC curve which showed the area under the curve (AUC) for TMB involving 1-, 3-, and 5-year survival was 0.545, 0.592,and 0.605, respectively (Figure 1A). Afterwards, we used the 1-, 3-, and 5-year ROC curve analysis with the corresponding maximum Youden index to calculate the TMB threshold values. As a result, when the TMB cut-off value was 3.1, the maximal AUC value was achieved (1-year sensitivity: 0.448, specificity: 0.656; 3-year sensitivity: 0.430,specificity: 0.742; 5-year sensitivity: 0.402, specificity: 0.767). Therefore, we defined 3.1 mut/Mb as the cut-off value. Patients with a TMB > 3.1 mut/Mb were clarified as the high group (= 140), and patients with a TMB ≤ 3.1 mut/Mb were clarified as the low group (= 239).
Following TMB classification, the Kaplan-Meier plotter of survival analysis showed that high TMB patients had a poor OS (HR = 1.47,= 0.002; Figure 1B) and RFS (HR =1.42,= 0.035; Figure 1C), as compared to low TMB patients. We then performed subgroup analysis of prognosis to assess the impact of TMB in different clinical subsets(Table 2). For tumor grade, high TMB patients had poor OS in moderately differentiated (HR = 1.46,= 0.026; Figure 1E) and poorly differentiated subsets (HR = 1.72,= 0.007; Figure 1F). In contrast, no definite results can be obtained in well differentiated subsets due to the small sample size (HR = 0.64,= 0.582; Figure 1D).
For disease progression, high TMB indicated poor OS in patients with multifocal liver disease (HR = 1.85,= 0.026; Figure 1H). However, no significant differences in survival between the high TMB and low TMB groups were found in patients with solitary liver tumor (HR = 1.42,= 0.140; Figure 1G) and metastatic disease (HR =1.17,= 0.357; Figure 1I).
For tumor resection, high TMB indicated a shorter OS in patients who underwent tumor resection (HR = 1.77,= 0.002; Figure 1J). Conversely, no differences in prognosis were observed between the high TMB and low TMB groups in patients without tumor resection (HR = 1.13,= 0.461; Figure 1K).
Construction of multivariate survival model
Finally, we would like to screen the independent prognostic factors and establish a prognostic model of ICC patients. Multivariate Cox regression analysis to was used to analyze the associations between OS and specific factors, including age, sex, and TMB.As a result, TMB was identified as an independent risk predictor for ICC patients [HR= 1.43 (1.05-1.96),= 0.0240]. Additionally, independent prognostic factors of ICC included CA19-9 [HR = 1.78 (1.28-2.46),= 0.0005], chronic viral hepatitis [HR = 1.72(1.01-2.95),= 0.0468], tumor resection [HR = 2.58 (1.72-3.88),< 0.0001], and disease progression [metastatic diseasesolitary liver tumor HR = 2.55 (1.55-4.20),= 0.0002](Table 3). Following this, we constructed a predictive nomogram based on the Cox regression coefficients of selected variables, and the predictive accuracy of every nomogram was evaluated using calibration plots (Figure 2A). The total score for ICC patients can be calculated to predict the 1-, 3-, and 5-year survival rates, which would help clinicians assess the risk level of ICC patients in clinical practice. Notably, the calibration curve indicated that the observed and predicted values were consistent in predicting OS (Figure 2B).
DISCUSSION
In this study, we investigated the role of TMB in predicting survival among patients with ICC. First, the clinical and mutation data of the 412 ICC patients were obtained from the MSK public database. Next, the best cut-off TMB value was determined using time-dependent ROC curve. Combined with other clinical features, univariate and multivariate Cox regression analyses were used to establish a risk model for prognosis prediction, showing that elevated TMB was associated with poor OS and RFS. In addition to TMB, CA19-9, chronic viral hepatitis, tumor resection, and disease progression (metastatic diseasesolitary liver tumor) were also found to be independent predictors of OS in ICC patients. Based on these risk factors, a reliablenomogram model was then constructed, demonstrating a satisfactory performance in predicting OS in ICC patients. Therefore, this study provided an effective indicator for the clinical prognostic evaluation of ICC patients, as well as contributed to the screening of high-risk ICC patients and the provision of individualized treatment.


Recently, TMB has become a novel predictive biomarker with the potential to predict the therapeutic effect of ICIs and screen suitable patients for immunotherapy[19]. At present, the research on TMB has mainly focused on its ability to predict the efficacy of ICIs, with numerous studies showing its association with the survival rate of cancer patients. In particular, Xie[20] found that papillary thyroid carcinoma patients with high TMB reported a worse prognosis. A study by Zhang[21] also indicated that low TMB resulted in a better prognosis in patients with head and neck squamous cell carcinoma. Similarly, a study of 318 ICC patients showed that high TMB indicated a worse prognosis [HR = 1.500 (1.085-2.073)][22]. In the present study,the data of 412 ICC patients published by the MSK Cancer Center in March 2021 were used to determine the utility of TMB in prognosis prediction. Notably, the original researchers investigated the relationship between the mutation gene, clinical characteristics, and the prognosis of ICC patients; however, they did not explore the role of TMB in prognosis. Analyzing the aforementioned data, we found that ICC patients with high TMB had a poor OS and RFS, which was consistent with the findings of previous studies.
Clinically, CEA and CA19-9 levels are commonly used prognostic indicators in ICC[23,24]. However, their prognostic thresholds vary widely across different reports,with a lack of a large meta-analysis to consolidate these values[25]. Moreover, some studies have reported on other prognostic indicators associated with poor prognosis in ICC patients, including elevated C-reactive protein, circulating osteopontin, as well as KRAS and TP53 mutations in tumor tissues[26-29]. With the wide application of immunotherapy, TMB has also become a common clinical index. In order to detect TMB, common mutations in ICC patients were detected, which reflected the overall mutation of tumor tissue. Therefore, TMB is a convenient and crucial prognostic value in clinical practice.
Medical nomograms use biologic and clinical variables, including tumor grade and patient age, to graphically depict a statistical prognostic model that generates a probability of a clinical event for a given individual, such as cancer recurrence or death. Furthermore, nomograms are user-friendly, can incorporate continuous variables and relevant disease determinants into prognosis, and are superior to clinician judgment in estimating disease course[30,31]. In this study, we constructed apredictive nomogram according to the Cox regression coefficients of selected variables to help clinicians evaluate the prognostic risk of ICC patients, calculate their survival rate, and make correct clinical decisions. Particularly, TMB and CA19-9 were combined to construct a nomogram model to predict the prognosis of ICC patients,which was helpful for its clinical application. To ensure the accuracy of this nomogram model, we used a calibration plot, as it allowed us to determine how close the nomogram estimated risk was to the observed risk.


CONCLUSION
In conclusion, we explored the prognostic role of TMB in ICC patients. Multivariate analysis indicated that TMB and CA19-9 were among the identified independent prognostic factors in ICC. Although our study confirmed the prognostic value of TMB,our study had several limitations. First, the clinical characteristics and TMB data of the cases analyzed in this study were all extracted from the MSK Cancer Center, of which some cases had missing data. As a result, this increased the analysis error in our study.Second, using a single data source also increases statistical error. Thus, further larger.cohort studies are necessary to confirm the predictive value of TMB in the prognosis of ICC patients. For the benefit of future studies, we will continue to collect the clinical data of ICC patients and consolidate our conclusions by expanding the present study’s sample size.
Grade C (Good): 0
Intrahepatic cholangiocarcinoma (ICC) is malignancies of the biliary duct system and constitutes approximately 10%-20% of all primary liver cancers. Tumor mutation burden (TMB) is a useful biomarker across many cancer types for the identification of patients who will benefit from immunotherapy. This study collected the ICC database from the Memorial Sloan Kettering Cancer Center to investigate the impact of TMB on the prognosis of ICC patients.
The prognosis of ICC patients is very poor. Previous studies suggest that TMB can used to be a prognostic factor in many types of cancer. It is critical to analyze the prognostic value of TMB in ICC to help individual clinical treatment.
“打。”夏国忠见部队的行动已经日军被发现了,立即向战士们发出了战斗的命令。随着他的命令声,“呯”的一声枪响,跟在夏国忠身边的神枪手瞄准那盏探照灯开了枪,随着枪声响起,灯光一下灭了,眼前顿时漆黑一片。
This study aims to investigate the prognostic value of TMB in patients with intrahepatic cholangiocarcinoma ICC. In particular, we sought to confirm that TMB is an independent prognostic factor of ICC and construct a nomogram model to predict the prognosis of ICC patients, which was helpful for its clinical application.
This study is a retrospective cohort study of ICC patients. This is a study of large sample to investigate the prognostic value of TMB and other clinical characters in ICC.
2.3.3 解吸时间 从图4可见,AB-8大孔树脂吸附花青素后,在pH 1.0的70%乙醇溶液中解吸,解吸率随时间延长呈上升趋势。解吸2 h后,解吸率为74.3%。其后,随时间延长,解吸率升高幅度较小,解吸12 h后,解吸率仅为79%。因此,考虑时间成本及花青素的稳定性,解吸时间以2 h为宜。
实践教学是培养学生创新精神、创新能力和实践能力的重要环节,在创新创业人才培养中占有重要的地位。传统的实践教学强调专项技能或单学科训练,学生往往被动参与实践训练,很大程度上限制了自身创新思维的训练和实践能力的提高。有些高校在实践教学改革方面下了很大功夫,但由于实践条件尚不够完善,实践平台数量少,实践教学与工程实践联系较少,学生缺少实际训练和创新实践的机会,实践教学效果不佳。实践环节涉及大量实验操作和数据处理,部分学生缺乏科学严谨、细致认真的态度,不能按照国家标准和行业规范操作,不重视实验结果的准确可靠性,影响了工匠精神的养成。
Cholangiocarcinomas are malignancies of the biliary duct system, classified as being either intrahepatic or extrahepatic in origin. Particularly, intrahepatic cholangiocarcinoma (ICC) constitutes approximately 10%-20% of all primary liver cancers[1].Despite its increasing incidence rate worldwide, the etiology of ICC remains unclear[2]. Moreover, although surgery is the only potentially curative treatment for ICC,more than two-thirds of patients have been found to be unsuitable for surgery at the time of diagnosis, and more than 60% of patients who underwent surgery reported relapse of disease[3]. A previous study also showed that the 5-year survival rate and median survival time of patients with ICC (hereinafter, ICC patients) who underwent curative resection was approximately 30% and 28 mo, respectively[4]. Besides surgical resection, the standard treatment for ICC includes gemcitabine-based chemotherapy,liver transplantation, and local treatment, such as transarterial chemoembolization[5].Of the several prognostic factors of ICC, radical resection (R0), number of tumors(single or multiple), vascular invasion, and lymph node metastasis have all been recognized as the most important independent prognostic predictors for ICC patients[6].
These findings suggest that TMB was an independent prognostic biomarker in patients with ICC. Moreover, patients with ICC with high TMB had poor overall survival and relapse free survival as compared to those with low TMB.
从企业内部情况来看,大部分文献[11-12]都指出国有粮食企业依然背负沉重的历史包袱,政企尚未真正分开,内部组织结构、经营方式、管理模式落后[13];内部控制制度不健全、监管失控[14];产权制度落后,不适应市场环境[15-17]。童应[18]指出了国有粮企职工主观的问题,职工固步自封的消极情绪、难有作为的畏难情绪、安于现状的自满情绪导致国有粮食企业管理难度大。
We will continue to collect the clinical data of ICC patients and consolidate our conclusions by expanding the present study’s sample size.
 World Journal of Clinical Cases2022年3期
World Journal of Clinical Cases2022年3期
- World Journal of Clinical Cases的其它文章
- Lung injury after cardiopulmonary bypass: Alternative treatment prospects
- Acute myocardial injury in patients with COVID-19: Possible mechanisms and clinical implications
- Anemia in cirrhosis: An underestimated entity
- Does delaying ureteral stent placement lead to higher rates of preoperative acute pyelonephritis during pregnancy?
- Management of retroperitoneal sarcoma involving the iliac artery: Single-center surgical experience
- COVID-19 pandemic changed the management and outcomes of acute appendicitis in northern Beijing: A single-center study
