猕猴桃细菌性溃疡病菌T3SS关键效应蛋白基因致病功能
张晋龙,赵志博,刘巍,黄丽丽
猕猴桃细菌性溃疡病菌T3SS关键效应蛋白基因致病功能
张晋龙,赵志博,刘巍,黄丽丽*
西北农林科技大学植物保护学院/旱区作物逆境生物学国家重点实验室,陕西杨陵 712100
【目的】由丁香假单胞菌猕猴桃致病变种(pv.,)引起的猕猴桃细菌性溃疡病是全球猕猴桃产业最具毁灭性的病害。病原细菌主要通过III型分泌系统(type III secretion system,T3SS)将多种效应蛋白(T3SS effector,T3SE)注入寄主植物细胞,进而促进病菌侵染和致病。本研究旨在解析基因组中T3SE的信息并对其T3SS和T3SE的致病功能进行系统分析,为溃疡病菌致病机制的研究和防治策略的制定提供依据。【方法】利用marker-free同源重组基因敲除技术获得M228菌株的T3SS功能缺陷突变体Δ和Δ,观察突变体在寄主上的致病力,同时检测突变体诱导本氏烟产生细胞坏死的情况;随后利用从Pseudomonas-Plant Interaction数据库下载的T3SE数据库,本地BLAST多序列比对构建强、弱致病菌株M228和M227的T3SE库,并对二者的T3SE基因信息进行比对分析;另外,获得M228菌株T3SE单、多效应子突变菌株20株及2株基因回补菌株(共涉及19个T3SE),并将各突变体室内有伤接菌猕猴桃枝条,系统评价各突变体致病力变化并进行统计分析。【结果】通过对的和基因进行突变,证明T3SS是其在寄主上致病以及非寄主上过敏性坏死反应(HR)所必需的。通过数据库同源比对,发现在强毒株系和弱毒株系中有31个T3SE基因具有100%的同源性,选取一些基因进行缺失突变,发现/和是重要的毒性因子,且二者不存在功能冗余。另外,单独敲除或均能提高致病力。在缺失A-F-E基因簇和的菌株中,敲除/和也分别导致的致病力显著下降;而同时敲除/、、和A-F-E基因簇导致病菌完全丧失致病力。【结论】HopM1/AvrE1与同家族HopR1均为重要致病因子,且独立于其他效应子发挥作用;和基因缺失可以增强的致病力。
猕猴桃溃疡病;丁香假单胞菌猕猴桃致病变种;效应蛋白;III型分泌系统;致病力
0 引言
【研究意义】由丁香假单胞菌猕猴桃致病变种(pv.,)引起的猕猴桃细菌性溃疡病是猕猴桃产业最具毁灭性的病害,在全球猕猴桃栽培区均有发生,严重制约着猕猴桃产业的健康发展[1-2]。该病害可以攻击寄主猕猴桃地上的多个组织,包括枝干、叶片和花蕾,具有侵染迅速、危害严重、防治困难等特点。对于猕猴桃溃疡病这样一个新的病害体系,其致病机制研究尚处于初级阶段。因此,明确主要攻击‘武器’III型效应蛋白(type III effector,T3SE)的致病功能进而解析其对植物的识别机制,对该病害新型防治策略的开发具有重要意义。【前人研究进展】笔者实验室前期调查了陕西省的种群结构和致病力差异,并利用比较基因组学分析了同一亚群的强、弱致病菌株M228(登录号GCA_000344475.3)和M227(登录号GCA_002890365.1)的遗传差异,发现一个位于III型分泌系统(type III secretion system,T3SS)基因簇上游启动子-930 bp区域的致病分化关键差异位点,该位点很可能调控/转录水平进而影响“HrpR/S-HrpL-T3SS/ effecors”级联通路导致致病力下降[3]。T3SS在许多革兰氏阴性菌中普遍存在,尤其在许多动物和植物病原细菌中对致病力起着关键作用[4-5]。T3SS结构通常由染色体上T3SS相关基因簇表达装配成一个“针状”的注射器结构[6-8]。病原细菌通过T3SS这样一个精密装置将多种T3SE直接注入寄主细胞内,进而引起寄主细胞一系列反应如免疫应答、激素信号转导和细胞代谢等,最终促进病原菌增殖侵染[9]。T3SE存在功能冗余、相互作用和不同菌株间复杂多样性等特征[5,10-11]。例如,在丁香假单胞菌番茄致病变种(pv.,)DC3000中有36个效应蛋白,其中至少存在2个功能冗余群[12-13];在缺失28个效应子基因的突变体D28E中,最少回补8个效应蛋白便可达到野生菌株的致病力水平[12]。因此,在一个病害体系中,仅有少数T3SE发挥关键致病作用。具有典型的/group I基因簇[14],同时具有约30个T3SE基因。Choi等[15]调查了T3SE的亚细胞定位及激发或抑制烟草过敏性坏死反应(hypersensitive response,HR)的能力;Jayaraman等[16]发现效应蛋白HopZ5是定位于质膜的乙酰转移酶,可在非寄主本氏烟()和拟南芥()中激发依赖于SGT1的HR;随后,Choi等[17]发现拟南芥SOBER1可抑制HopZ5激发的HR;Yoon等[18]发现烟草Rpa1,而非RPM1,介导了T3SE AvrRpm1激发的HR。这些研究借助非寄主植物探索了部分T3SE的功能,但不清楚其在侵染猕猴桃致病中的作用。近期,Jayaraman等[19]发现AvrE1和HopR1参与了对寄主的致病过程,而HopM1可能由于伴侣蛋白shcM的不完整而不发挥致病功能。【本研究切入点】是一个新的病害体系,具有典型的T3SS基因簇和约30个效应蛋白基因,关于攻击植物的主要T3SE“武器库”只有少量报道,尚不清楚中大多数T3SE的致病功能。【拟解决的关键问题】通过生物信息学解析的T3SE库,通过构建单、多基因突变体,系统评价T3SE的致病力贡献,鉴定部分T3SE功能,为深入研究病原菌-寄主互作机制打下基础。
1 材料与方法
试验于2017—2019年在西北农林科技大学旱区作物逆境生物学国家重点实验室完成。
1.1 供试材料
供试M228和M227属于biovar3(3)的强、弱致病菌株,分离自中国陕西眉县‘红阳’猕猴桃[20]。供试菌株及其突变体菌株全部由西北农林科技大学植物保护学院果树病害综合防治研究团队保存,保存条件为-80℃,50%甘油。
1.2 培养条件及试剂
取保存的供试菌株及其突变体在LB平板上划线培养,25℃,48 h;大肠杆菌菌株DH5和S17-1/pir均培养于LB培养基,37℃,16 h。供试猕猴桃品种包括‘红阳’(var.cv. ‘HongYang’)、‘海沃德’(var.cv. ‘Hayward’)、‘翠香’(cv. ‘CuiXiang’)、‘亚特’(cv. ‘Yate’)、‘华优’(×cv. ‘HuaYou’),其枝条和叶片来自盆栽苗和周围农户。本氏烟来自于全天候的温室(14 h 22℃﹕10 h 20℃(L﹕D),相对湿度70%)。限制内切酶购自宝日医生物技术(北京)有限公司;抗生素包括卡那霉素(Kan)、氨苄青霉素(Amp)和其他生化试剂萘啶酮酸(Nal)等均购自北京索莱宝科技有限公司。
1.3 Psa M228和M277 T3SE分析
为了鉴定M228和M227菌株的T3SE,从Pseudomonas-Plant Interaction网站下载T3SE本地数据库(http://www.pseudomonas-syringae.org/),然后基于M228和M227菌株基因组数据进行本地BLAST比对并对T3SE注释。
1.4 回补和敲除突变体菌株的获得
质粒pDSK-GFPuv[21]用于构建基因回补表达载体,目标基因用各自特异引物进行PCR扩增回收得到目的基因片段,相关引物参考文献[20],将pDSK-GFPuv用H I和d III双酶切后与已回收目标基因片段按一定比例混合,通过一步克隆酶进行连接,然后转化大肠杆菌DH5,验证和测序正确后,电转至目标菌株获得回补突变菌株。同样,质粒pK18mobSacB用于构建基因敲除自杀载体[22],用R I和d III进行双酶切,然后与待敲除目标基因上下游同源臂片段进行多步克隆酶连接,同源臂片段扩增及检测引物参考文献[23],测序正确后的重组pK18mobSacB载体转入S17-1/pir,然后通过接合转移方式[20]将重组敲除载体转入至各自亲本菌株中,将接合子涂布于含15%蔗糖的LB培养基筛选敲除突变体,所有敲除突变体最终通过PCR电泳检测和Sanger测序验证,检测引物参照文献[20]。本论文中提到的敲除和回补突变体均在M228菌株及其突变体的遗传学背景下通过分子生物学技术构建,所有突变体为本实验室构建并保存,详细信息如表1所示。
1.5 致病力检测
采用室内枝条有伤接菌的方式评价各突变体的致病力差异。采集45 cm长的当年生猕猴桃健康枝条,用0.6% HClO表面消毒10 min,无菌水清洗3次,处理过的枝条晾干后剪切成5 cm左右的短枝条,两端石蜡封存备用。然后,无菌手术刀处理枝条切2 mm左右的伤口至木质部,调整菌液浓度OD600至2×108cfu/mL,接种10 μL至伤口处,每次10个重复,接种的枝条放置在全天候气候培养箱,培养条件16 h 18℃﹕8 h 14℃(L﹕D),相对湿度70%)。
叶盘真空渗透接种:叶片表面消毒后,使用打孔器(直径11 mm)制叶盘,避开叶脉;叶盘置于菌液(104cfu/mL),真空渗透(0.1 MPa,10 s,反复3次),无菌水清洗3次,置于0.5%水琼脂平板,每次试验至少10个重复,接种叶盘放置全天候气候培养箱16℃黑暗培养3 d后观察拍照[3]。
烟草叶片接菌法:摇菌培养后调整至浓度约为108cfu/mL,使用无针头5 mL注射器,从叶背面注射本氏烟叶片,至直径约为1 cm。每菌株至少注射3株植物的3个叶片[3]。每次试验至少3次独立试验。
1.6 统计分析
SPSS 19.0(IBM,Armonk,NY)用于数据统计分析,独立样本差异检验使用Student’s-test,多样本方差分析使用Duncan’s multiple range test。
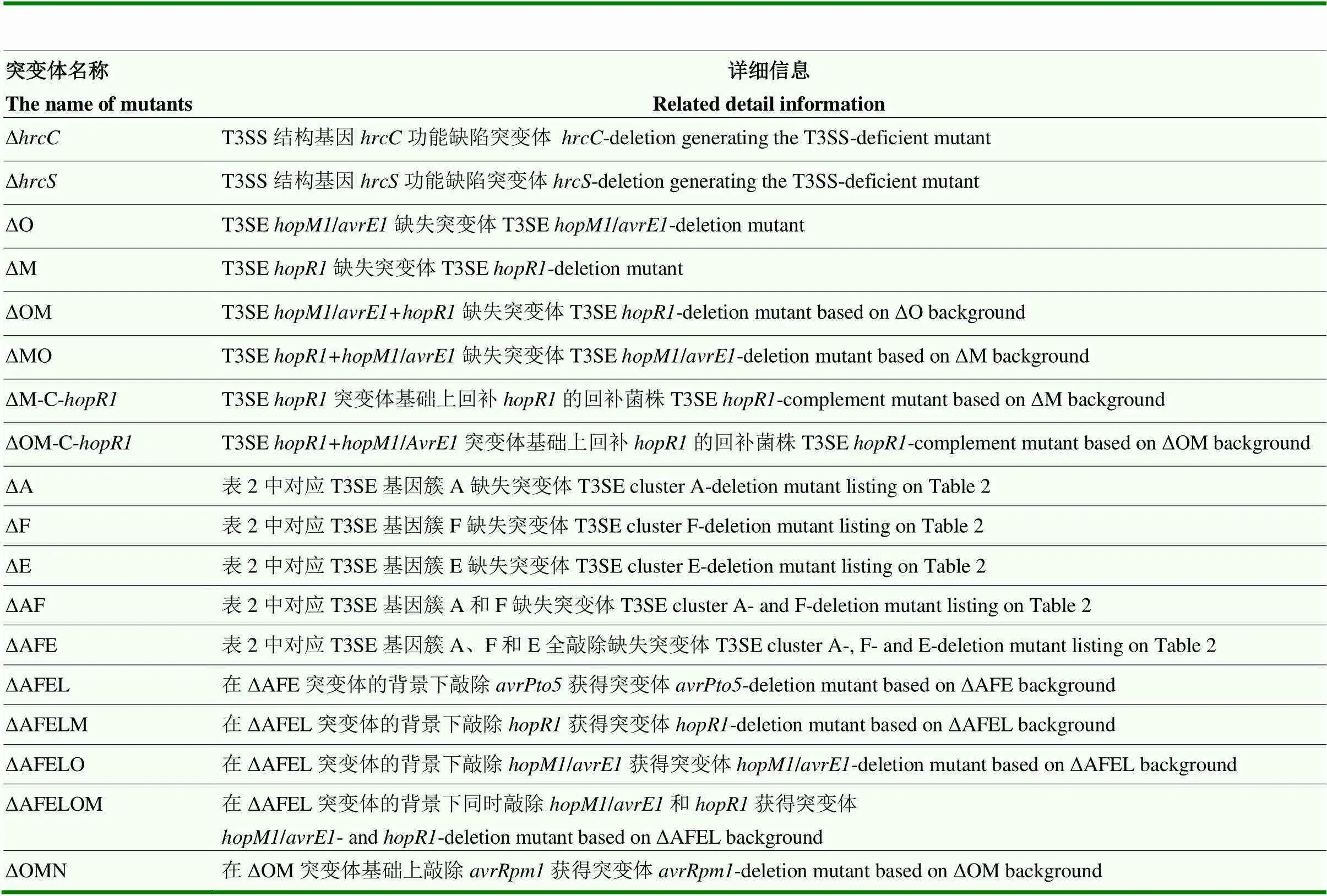
表1 敲除和回补突变体信息汇总
2 结果
2.1 T3SS为Psa致病及诱导非寄主HR所必需
T3SS是许多病原细菌攻击植物的主要武器,为了验证T3SS是否为致病所必需,将M228菌株的T3SS功能缺陷突变体Δ和Δ分别有伤接种猕猴桃枝条和真空渗透接种猕猴桃叶片,致病力分析结果显示,与对照M228相比,突变体Δ和Δ均不能引起猕猴桃枝条和叶片的坏死反应(图1-a、1-b)。进一步验证T3SS是否影响对非寄主诱导的HR反应,通过将突变体注射接种至本氏烟叶片后进行表型观察,与对照M228相比,突变体Δ和Δ同样也不能引起本氏烟叶片的HR反应(图1-c),综上结果表明T3SS是引起寄主上致病性以及非寄主上HR反应所必需的。
2.2 Psa M228和M227菌株中T3SE的鉴定
病原细菌主要通过T3SS直接将T3SE注射至寄主细胞内攻击植物,达到侵染定殖的目的,为了进一步探究M228和M227致病力差异是否由T3SE决定,利用生物信息学进行T3SE注释,结果发现M228和M227具有同等数量的T3SE,且序列100%相似,说明M228和M227菌株致病力分化与其他差异位点有关。另外,为了直观地了解中T3SE的种类、数目和特异性,Sawada等[23]构建3的33个T3SE基因库。本试验中通过本地blast同源库比对,在M228和M227菌株基因组中T3SE 100%相似,均可以找到31个完整的T3SE基因(其中HopAM1-1和HopAM1-2为100%同源双拷贝),约占3 T3SE基因库总数的94%,故M228强致病菌株中T3SE功能解析对于3整个种群致病力均有借鉴作用。此外,M228菌株中还有8个基因序列不完整或提前终止的T3SE(HopAY1、HopAW1、HopAA1-1、HopW1、HopA1、HopAV1、HopAA1-2和AvrRpm2)。在M228基因组上,Cluster A、O、E、F和M相关T3SE基因分别成簇存在,其中Cluster O中AvrE1和HopM1为core effectors,位于pathogenicity island侧翼CEL(conserved effector locus)。基因敲除标识字母栏A、O、E、F和M分别对应各自的T3SE基因簇,涉及19个T3SE(表2),后续将构建这些基因簇的敲除突变体和部分T3SE回补突变体,并进行致病力评估。
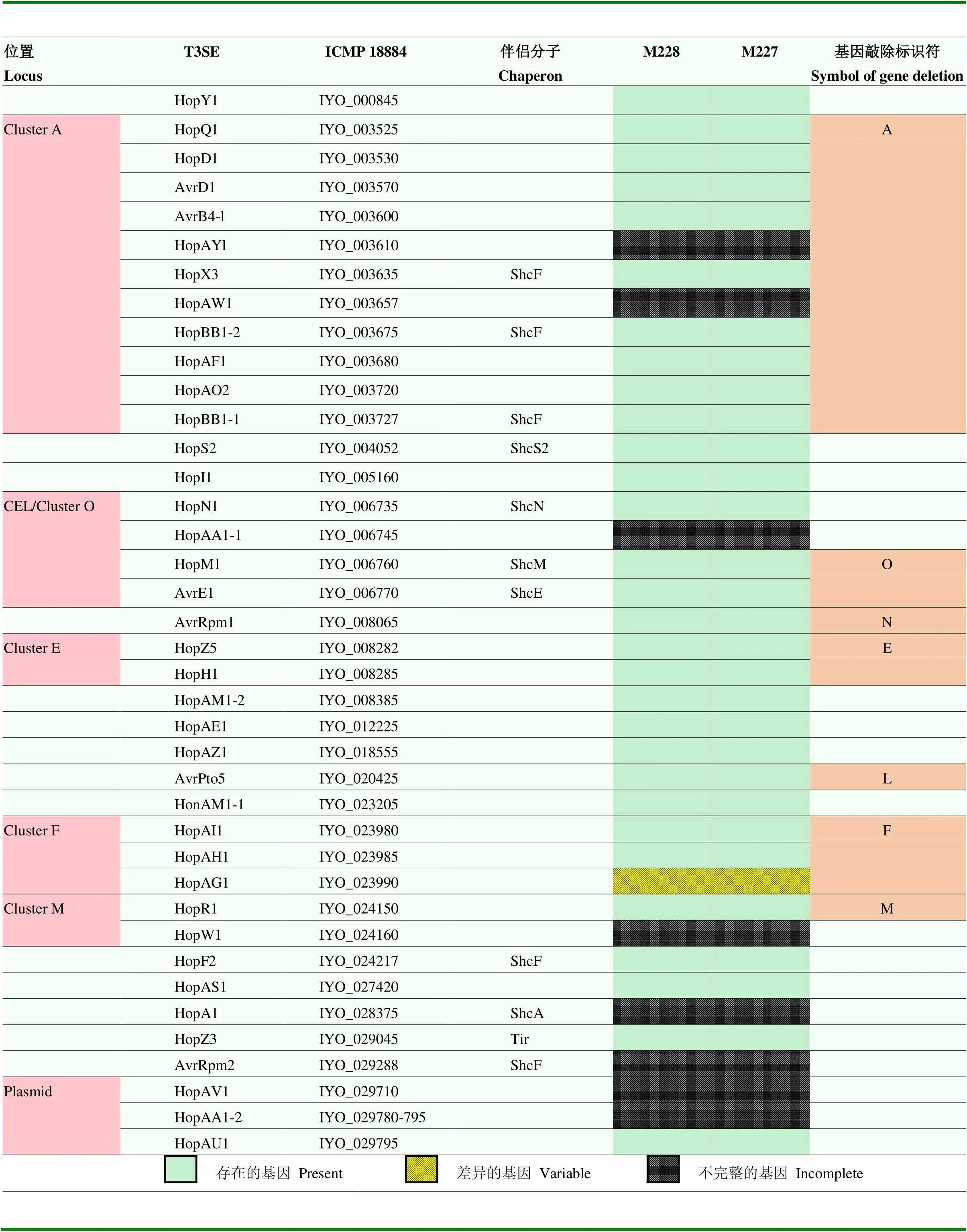
表2 Psa M228和M227菌株的T3SE库
根据同源比对结果建立该表,列出T3SE在M228菌株中的分布情况T3SEs were listed according to the BLAST results against to thehop database;3菌株ICMP 18884(GCA_000648735.3)为参考3 strain ICMP 18884 genome as reference;右侧基因敲除栏内的符号表示敲除的基因/基因簇The deletion of T3SE for each gene or cluster was designated as the capital letter listed in the right column;在M228栏中,绿色方块标识存在的完整基因,黄色方块表示在M228中完整基因但在部分3中不完整,黑色方块表示不完整或提前终止基因In M228 column, the green square represents the complete gene; the yellow square represents the complete gene in M228 but incomplete in partial stains of3; the black square represents the incomplete or early termination gene
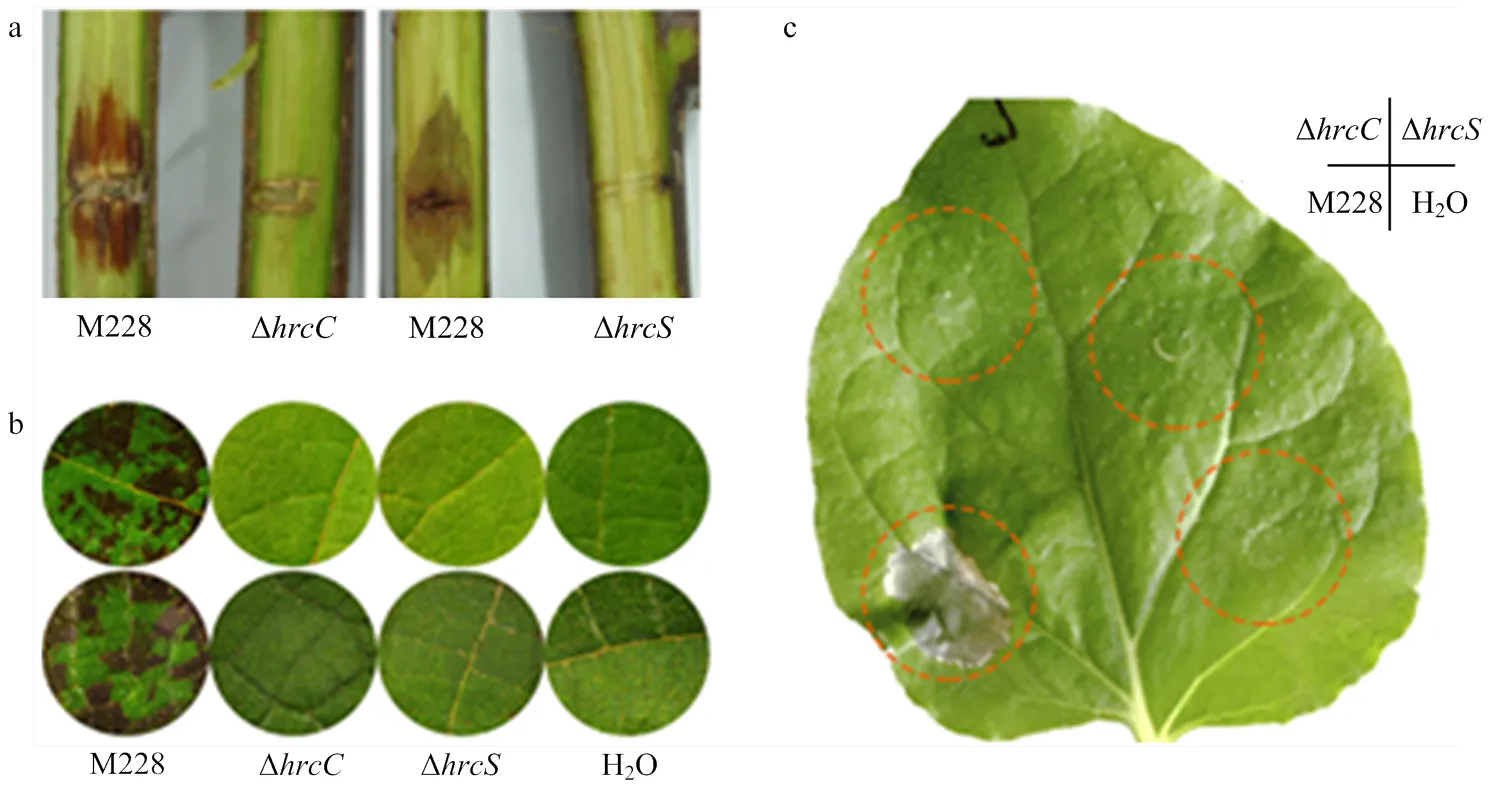
a:Psa菌株M228及其ΔhrcC、ΔhrcS突变体接种‘红阳’猕猴桃枝条,2×108 cfu/mL(15 dpi)Psa M228 strain and its T3SS-deficient mutants ΔhrcC, ΔhrcS were inoculated on canes of ‘HongYang’ with 2×108 cfu/mL concentration (15 days post inoculation);b:接种叶盘,上排为‘红阳’猕猴桃叶片,下排为‘翠香’猕猴桃叶片,104 cfu/mL(5 dpi)Leaf discs of ‘HongYang’ (upper) and ‘CuiXiang’ (bottom) with 104 cfu/mL concentration (5 days post inoculation);c:注射本氏烟叶片,108 cfu/mL(2 dpi)N. benthamina leaves with 108 cfu/mL concentration (2 days post inoculation)。每菌株接种至少10个枝条或叶盘,每菌株至少注射3株烟草的3个叶片。试验重复至少2次,得相似结果For the inoculation, at least 10 canes or leaf discs were used for each strain, and at least three tobacco leaves from three plants were treated with each strain. Experiments were repeated at least twice with similar results
2.3 M228菌株中关键致病T3SE鉴定
为了探究M228菌株发挥致病功能的关键T3SE基因,从核心效应蛋白HopM1/AvrE1出发,构建其缺失突变体ΔO。利用猕猴桃枝条室内有伤接菌方式评估各菌株的致病力,与对照M228相比,突变体ΔO致病力降低50%左右,且在不同寄主‘红阳’和‘海沃德’中致病力表现一致(图2),说明HopM1/AvrE1为M228的重要致病因子。
构建基因敲除突变体ΔM,有伤接种至不同的寄主枝条,致病力分析发现,与对照M228相比,突变体在不同寄主品种上的致病力均显著下降30%左右(图3-b),当回补后,致病力均恢复至其亲本菌株水平(图3-a),表明HopR1也是的关键致病因子。
为了进一步探究HopM1/AvrE1与HopR1的关系,在缺失突变体ΔM的基础上,敲除/基因,获得突变体ΔMO,发现突变体的致病力仍然继续下降50%左右,且在不同寄主品种中表现一致(图2);此外,以ΔO为亲本菌株获得的缺失突变体ΔOM,并评价其致病力。结果显示,无论/是否存在,缺失后,菌株的致病力均显著下降30%,且在不同寄主品种上表现一致(图3);当回补后,致病力恢复至亲本菌株水平(图3-a),表明HopR1与HopM1/AvrE1不存在致病功能冗余。
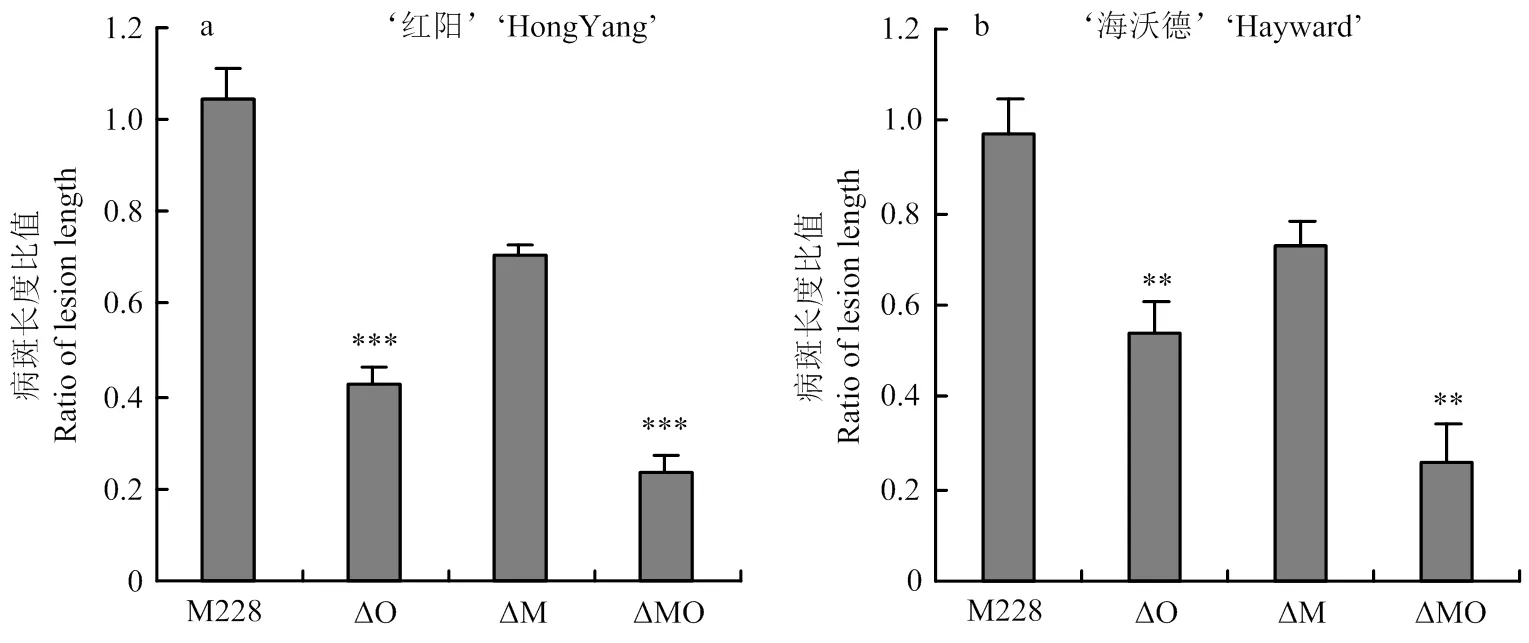
纵坐标为各菌株接种猕猴桃枝条后病斑长度与M228病斑长度的比值。下同The lesion length for each strain was normalized to that of the control strain M228. The same as below
2.4 T3SE致病功能冗余基因簇的鉴定
菌株M228的T3SE库中除了核心效应蛋白外,还存在T3SE基因簇,为了研究这些基因簇对致病力的影响,对基因簇A(包含11个基因)、E(和)和F(、和)分别进行单基因簇和多基因簇敲除,获得ΔA、ΔE、ΔF、ΔAF和ΔAFE 5株M228突变体菌株,并评估各突变体的致病力差异。结果表明,单独敲除每个基因簇后,对‘红阳’猕猴桃枝条致病力分别降低约15%,说明基因簇A、E、F均有效应蛋白参与致病;与∆AF相比,当基因簇A-F-E全部敲除时,对‘红阳’猕猴桃枝条致病力显著降低约40%,说明E基因簇在M228菌株致病过程中起着关键作用(图4-a)。上述突变体接种‘华优’猕猴桃枝条时,致病力测定结果表明仅基因簇A无显著致病功能(图4-b)。另外,单独敲除基因簇A或F与同时敲除基因簇A和F致病力下降水平无显著差异(图4),说明基因簇A和F中存在功能冗余的效应蛋白,且可能共同靶向同一关键免疫通路。

a:接种至‘华优’inoculation on cultivar ‘HuaYou’;b:野生型菌株M228及其hopR1突变体分别接种至‘亚特’‘翠香’和‘华优’M228 and the hopR1 mutant were inoculated on ‘Yate’ ‘CuiXiang’ and ‘HuaYou’。试验至少重复2次,得相似结果Experiments were repeated at least twice with similar results (Student’s t-test or Duncan’s multiple range test, P<0.01)
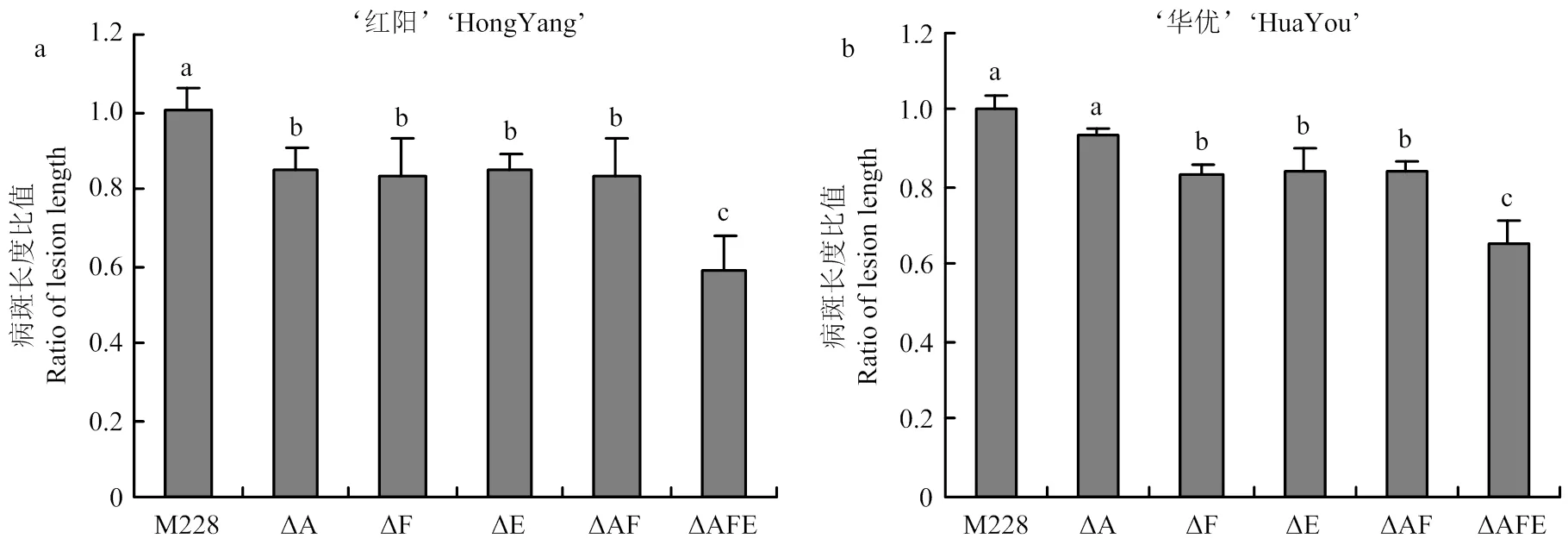
图中比较的是各菌株接种至同一品种后的致病力。图5同
2.5 AvrPto5和AvrRpm1可以激发猕猴桃抗病性
在突变体ΔAFE的基础上,敲除,获得突变体ΔAFEL。当接种‘红阳’猕猴桃枝条时,ΔAFEL的致病力较ΔAFE显著提升。在突变体ΔOM的基础上,敲除,获得突变体ΔOMN。当接种‘红阳’猕猴桃枝条时,ΔOMN的致病力较ΔOM显著提升(图5),表明当存在AvrPto5和AvrRpm1时,可能被寄主识别,激发了一定程度的抗病性。
此外,在ΔAFEL的基础上,敲除获得突变体ΔAFELM,敲除获得突变体ΔAFELO。在ΔAFELO的基础上敲除获得突变体ΔAFELOM。当接种‘红阳’猕猴桃枝条时,单独敲除或均能导致致病力显著下降,同时敲除和/,完全丧失致病力,表明HopR1和HopM1/AvrE1在致病中独立发挥关键作用,且与A/E/F基因簇靶向的抗病通路不同。
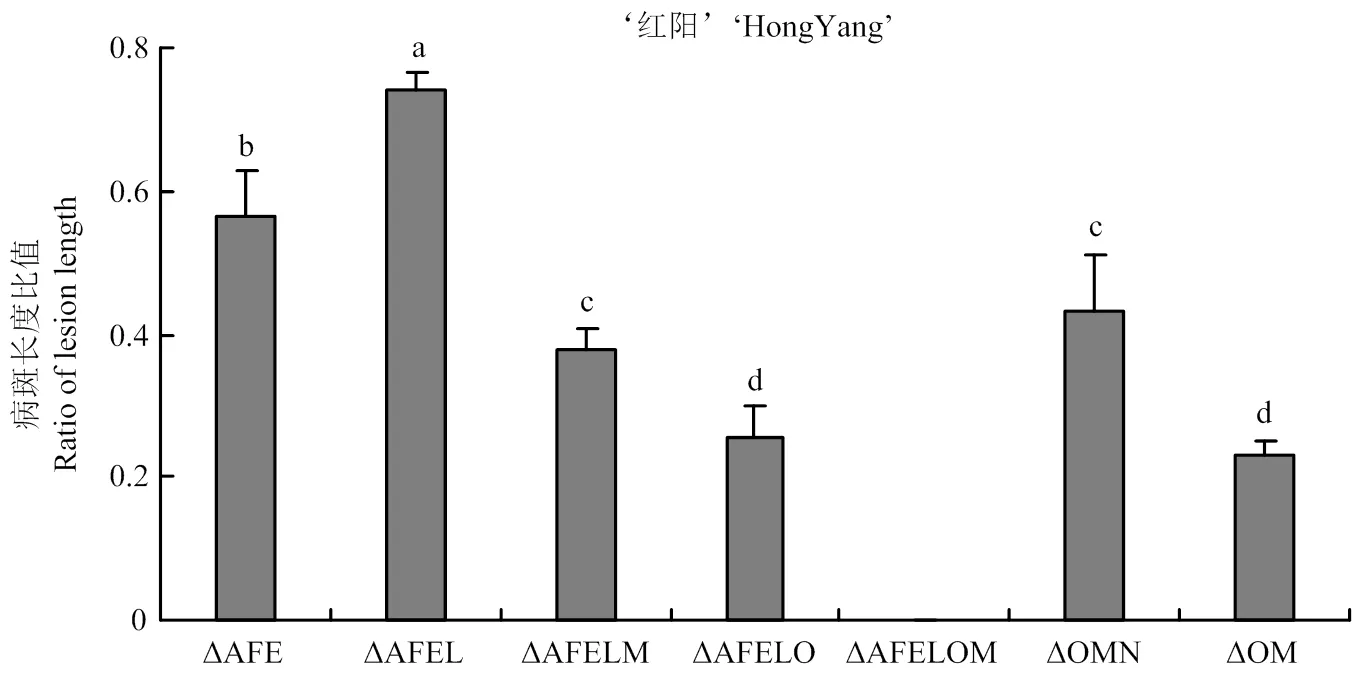
接种15 d后测量病斑扩展长度The length of lesion expansion of the above mutants was measured 15 days post inoculation
3 讨论
3.1 HopR1与HopM1/AvrE1为Psa发挥致病力的关键蛋白
本研究通过对菌株的T3SE进行预测,共找到31个完整的T3SE,系统地分析了M228中19个T3SE相关突变体致病力,从整体上明确了T3SE的种类和功能。结果表明,19个T3SE基因全敲除突变体ΔAFELOM接种寄主猕猴桃,其致病力完全丧失,说明M228菌株主要致病力蛋白集中在这19个T3SE内。通过对HopM/AvrE家族保守蛋白HopM1/AvrE1和HopR1功能进行研究,发现其均对菌株M228的致病力起重要作用,且两者不存在致病功能冗余。近期Jayaraman等[19]也发现了核心效应蛋白AvrE1和可变效应子HopR1对的重要致病作用,然而HopM1由于其伴侣分子shcM不完整而导致不发挥致病作用。在M228菌株中伴侣分子shcM基因也不完整,HopM1很有可能由于伴侣蛋白shcM的不完整而不发挥致病功能。与DC3000相似,利用AvrE1可能作用于抗病物质运输及创造胞间水环境,以及减少胼胝质的积累和活性氧的迸发[24-26]。HopR1属于HopM/AvrE蛋白家族,在DC3000中存在功能冗余,但对的致病力和适应性起着关键作用,关于其致病机理知之甚少,可能作用于抗病物质运输及关键的防卫路径,值得进一步深入研究。
3.2 T3SE基因簇间存在致病力功能冗余
进一步验证其他T3SE基因簇A、E和F的功能,发现这几个基因簇间存在功能冗余T3SE,且各基因簇对的致病力均有贡献。基因簇存在功能效应蛋白冗余,其原因可能存在不同效应蛋白靶向同一个关键PTI/ETI的识别与信号传递通路。另外E基因簇的HopZ5对致病功能显著,可能靶向其他关键的免疫信号通路。HopZ5属于YopJ serine/threonine acetyltransferase家族,在多种动植物病原菌中存在,例如(YopJ)、(PopP1和PopP2)、(ORFB)、(AvrRxv,AvrXv4,AvrBsT和XopJ)和(HopZ family),其分化程度较高、作用方式多样[27-33]。在3中T3SE HopZ5属于特异效应子,该基因可以作为3菌种鉴定的特异序列[34]。此外,研究发现在-基因簇E中,HopZ5而非HopH1对致病力有贡献[3];HopZ5可以激发模式植物拟南芥和烟草的HR反应[16-17],但HopZ5互作的寄主靶标仍然未知。
3.3 部分T3SE缺失可以增加Psa的致病力
敲除效应蛋白基因和后突变体对猕猴桃枝条的致病力上升,说明AvrPto5和AvrRpm1可以一定程度激发寄主的抗病性。据报道,Avr蛋白为一类无毒因子,可以与R蛋白互作激发植物的防御反应,缺失后可导致病原菌的致病力增强,如AvrPto5同源蛋白AvrPto是最早发现并鉴定的一类无毒蛋白,可以与R蛋白识别激活ETI途径从而增强寄主的抗性[35];同样,AvrRpm1也广泛存在于多种病原菌中,靶向R蛋白并激发寄主的抗性[36]。在中AvrPto5和AvrRpm1也可能为无毒因子,推测可能被寄主猕猴桃R蛋白识别并激发寄主抗病性,故和缺失导致病原菌的致病力增强。
4 结论
通过对M228菌株T3SE单、多基因敲除和突变体致病力评价,系统对其中19个T3SE进行功能分析与鉴定,发现HopR1与HopM1/AvrE1为主要影响M228致病力的功能蛋白,致病功能不冗余,且独立于其他效应子发挥关键致病作用;基因簇A、E和F对致病功能微效且存在功能冗余的效应蛋白;和缺失后可以增加对寄主的致病力。
[1] 秦虎强, 高小宁, 赵志博, 朱穗层, 李建民, 黄丽丽. 陕西猕猴桃细菌性溃疡病田间发生动态和规律. 植物保护学报, 2013, 40(3): 225-230.
QIN H Q, GAO X N, ZHAO Z B, ZHU H C, LI J M, HUANG L L. The prevalence dynamics and rules of bacterial canker of kiwifruit in Shaanxi. Acta Phytophylacica Sinica, 2013, 40(3): 225-230. (in Chinese)
[2] 高小宁, 赵志博, 黄其玲, 秦虎强, 黄丽丽. 猕猴桃细菌性溃疡病研究进展. 果树学报, 2012, 29(2): 262-268.
GAO X N, ZHAO Z B, HUANG Q L, QIN H Q, HUANG L L. Advances in research on bacterial canker of kiwifruit. Journal of Fruit Science, 2012, 29(2): 262-268. (in Chinese)
[3] ZHAO Z, CHEN J, GAO X, ZHANG D, ZHANG J, WEN J, QIN H, GUO M, HUANG L. Comparative genomics reveal pathogenicity- related loci inpv.biovar 3. Molecular Plant Pathology,2019, 20(7): 923-942.
[4] 孙思, 牛建军, 王岱. 细菌三型分泌系统效应蛋白转运的研究进展. 微生物学报, 2017, 57(10): 1452-1460.
SUN S, NIU J J, WANG D. Advances in studies of translocation of effector by bacterial type 3 secretion system. Acta Microbiologica Sinica, 2017, 57(10): 1452-1460. (in Chinese)
[5] LINDEBERG M, CUNNAC S, COLLMER A.type III effector repertoires: last words in endless arguments. Trends in Microbiology,2012, 20(4): 199-208.
[6] DOS SANTOS A M P, FERRARI R G, CONTE-JUNIOR C A. Type three secretion system in: the key to infection. Genes and Genomics,2020, 42(5): 495-506.
[7] 朱秀秀, 高必达, 赵廷昌, 张月娟. 植物病原细菌Ⅲ型分泌系统及pv.的信号分子分泌研究进展. 湖南农业科学, 2009(2): 19-22.
ZHU X X, GAO B D, ZHAO Y C, ZHANG Y J. Research progress of type III secretory system of plant pathogenic bacteria and signal molecule secretion ofpv.Hunan Agricultural Sciences, 2009(2): 19-22. (in Chinese)
[8] MARLOVITS T C, KUBORI T, LARA-TEJERO M, THOMAS D, UNGER V M, GALAN J E. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature,2006, 441(7093): 637-640.
[9] MACHO A P, ZIPFEL C. Plant PRRs and the activation of innate immune signaling. Molecular Cell,2014, 54(2): 263-272.
[10] 温晶. 猕猴桃溃疡病菌Ⅲ型效应蛋白的筛选及效应蛋白HopX3功能的初步研究[D]. 杨凌: 西北农林科技大学, 2016.
WEN J. Identification oftype III effectors and preliminary analysis of effector HopX3 in pathogenicity[D]. Yangling: Northwest A&F University, 2016. (in Chinese)
[11] BALTRUS D A, NISHIMURA M T, ROMANCHUK A, CHANG J H,
MUKHTAR M S, CHERKIS K, ROACH J, GRANT S R, JONES C D, DANGL J L. Dynamic evolution of pathogenicity revealed by sequencing and comparative genomics of 19isolates. PLoS Pathogens,2011, 7(7): e1002132.
[12] CUNNAC S, CHAKRAVARTHY S, KVITKO B H, RUSSELL A B, MARTIN G B, COLLMER A. Genetic disassembly and combinatorial reassembly identify a minimal functional repertoire of type III effectors in. Proceedings of the National Academy of Sciences of the United States of America,2011, 108(7): 2975-2980.
[13] KVITKO B H, PARK D H, VELASQUEZ A C, WEI C F, RUSSELL A B, MARTIN G B, SCHNEIDER D J, COLLMER A. Deletions in the repertoire ofpv.DC3000 type III secretion effector genes reveal functional overlap among effectors. Plos Pathogens,2009, 5(4): e1000388.
[14] TAMPAKAKI A P, SKANDILIS N, GAZI A D, BASTAKI M N, PANAGIOTIS F S, CHAROVA S N, KOKKINIDIS M, PANOPOULOS N J. Playing the “Harp”: Evolution of our understanding of hrp/hrc genes 1. Annual review of phytopathology, 2010, 48: 347-370.
[15] CHOI S, JAYARAMAN J, SEGONZAC C, PARK H J, PARK H, HAN S W, SOHN K H.pv.type III effectors localized at multiple cellular compartments activate or suppress innate immune responses in. Frontiers in Plant Science,2017, 8: 2157.
[16] JAYARAMAN J, CHOI S, PROKCHORCHIK M, CHOI D S, SPIANDORE A, RIKKERINK E H, TEMPLETON M D, SEGONZAC C, SOHN K H. A bacterial acetyltransferase triggers immunity inindependent of hypersensitive response. Scientific Reports,2017, 7(1): 3557.
[17] CHOI S, JAYARAMAN J, SOHN K H.SOBER1 (SUPPRESSOR OF AVRBST-ELICITED RESISTANCE 1) suppresses plant immunity triggered by multiple bacterial acetyltransferase effectors. New Phytologist,2018, 219(1): 324-335.
[18] YOON M, RIKKERINK E H A.mediates an immune response toavrRpm1and confers resistance againstpv.. The Plant Journal,2020, 102(4): 688-702.
[19] JAYARAMAN J, YOON M, APPLEGATE E R, STROUD E A, TEMPLETON M D. AvrE1 and HopR1 frompv.are additively required for full virulence on kiwifruit. Molecular Plant Pathology,2020, 21(11): 1467-1480.
[20] 赵志博. 猕猴桃细菌性溃疡病菌群体结构与致病机制研究[D]. 杨凌: 西北农林科技大学, 2016.
ZHAO Z B. Population composition and pathogenetic mechanism inpv.[D]. Yangling: Northwest A&F University, 2016. (in Chinese)
[21] WANG K, KANG L, ANAND A, LAZAROVITS G, MYSORE K S. Monitoringbacterial infection at both cellular and whole-plant levels using the green fluorescent protein variant GFPuv. New Phytologist,2007, 174(1): 212-223.
[22] Kvitko B H, Collmer A. Construction ofpv.DC3000 mutant and polymutant strains//Plant Immunity. Methods and Protocols, 2011, 712: 109-128.
[23] SAWADA H, FUJIKAWA T. Genetic diversity ofpv., pathogen of kiwifruit bacterial canker. Plant Pathology,2019, 68(7): 1235-1248.
[24] XIN X F, NOMURA K, AUNG K, VELASQUEZ A C, YAO J, BOUTROT F, CHANG J H, ZIPFEL C, HE S Y. Bacteria establish an aqueous living space in plants crucial for virulence. Nature,2016, 539(7630): 524-529.
[25] JIN L, HAM J H, HAGE R, ZHAO W, SOTO-HERNANDEZ J, LEE S Y, PAEK S M, KIM M G, BOONE C, COPLIN D L, MACKEY D. Direct and indirect targeting of PP2A by conserved bacterial type-III effector proteins. PLoS Pathogens,2016, 12(5): e1005609.
[26] DEGRAVE A, SIAMER S, BOUREAU T, BARNY M A. The AvrE superfamily: ancestral type III effectors involved in suppression of pathogen-associated molecular pattern-triggered immunity. Molecular Plant Pathology,2015, 16(8): 899-905.
[27] PALACE S G, PROULX M K, SZABADY R L, GOGUEN J D. Gain-of-function analysis reveals important virulence roles for thetype III secretion system effectors YopJ, YopT, and YpkA. Infection and Immunity,2018, 86(9): e00318-18.
[28] üSTüN S, KöNIG P, GUTTMAN D S, BöRNKE F. HopZ4 from, a member of the HopZ type III effector family from the YopJ superfamily, inhibits the proteasome in plants. Molecular Plant-Microbe Interactions,2014, 27(7): 611-623.
[29] LEWIS J D, LEE A H Y, HASSAN J A, WAN J, HURLEY B, JHINGREE J R, WANG P W, LO T, YOUN J Y, GUTTMAN D S, DESVEAUX D. TheZED1 pseudokinase is required for ZAR1-mediated immunity induced by thetype III effector HopZ1a. Proceedings of the national academy ofsciences of the United States of America,2013, 110(46): 18722-18727.
[30] LEWIS J D, LEE A, MA W B, ZHOU H B, GUTTMAN D S, DESVEAUX D. The YopJ superfamily in plant-associated bacteria. Molecular Plant Pathology,2011, 12(9): 928-937.
[31] ZHOU H B, LIN J A, JOHNSON A, MORGAN R L, ZHONG W W, MA W B.type III effector HopZ1 targets a host enzyme to suppress isoflavone biosynthesis and promote infection in soybean. Cell Host and Microbe,2011, 9(3): 177-186.
[32] MACHO A P, GUIDOT A, BARBERIS P, BEUZON C R, GENIN S. A competitive index assay identifies severaltype III effector mutant strains with reduced fitness in host plants. Molecular Plant-Microbe Interactions,2010, 23(9): 1197-1205.
[33] BARTETZKO V, SONNEWALD S, VOGEL F, HARTNER K, STADLER R, HAMMES U Z, BORNKE F. Thepv.type III effector protein XopJ inhibits protein secretion: evidence for interference with cell wall-associated defense eesponses. Molecular Plant-Microbe Interactions,2009, 22(6): 655-664.
[34] CHEN H, HU Y, QIN K Y, YANG X Z, JIA Z J, LI Q, CHEN H B, YANG H. A serological approach for the identification of the effectorofpv.: a tool for the rapid immunodetection of kiwifruit bacterial canker. Journal of Plant Pathology,2018, 100(2): 171-177.
[35] KRAUS C M, MUNKVOLD K R, MARTIN G B. Natural variation in tomato reveals differences in the recognition of AvrPto and AvrPtoB effectors from. Molecular Plant,2016, 9(5): 639-649.
[36] KIM M G, GENG X, LEE S Y, MACKEY D. Thetype III effector AvrRpm1 induces significant defenses by activating thenucleotide-binding leucine-rich repeat protein RPS2. The Plant Journal,2009, 57(4): 645-653.
The function of key T3SS effectors inpv.
Zhang JinLong, Zhao ZhiBo, Liu Wei, Huang LiLi*
College of Plant Protection, Northwest A&F University/State Key Laboratory of Crop Stress Biology for Arid Areas, Yangling 712100, Shaanxi
【Objective】pv.(),the causal agent of bacterial canker of kiwifruit, is the most devastating pathogen in global kiwifruit production. The pathogenic bacteria secrete a series of effectors (T3SEs) into host cell to promote infection and pathogenesis by the type III secretion system (T3SS). The objective of this study is to analyze the T3SEs information ingenome and systematically evaluate the pathogenicity function of T3SS and T3SEs, and to provide the basis evidence for the research of the pathogenic mechanism and the establishment of the control strategies.【Method】By marker-free homologous recombination knockout technique, the M228 deficiency mutants of T3SS, Δand Δ, were obtained for inoculating on host to evaluate the pathogenicity and injecting onto observe the cell death response. Then, based on the T3SEs database downloaded from Pseudomonas-Plant interaction, the T3SEs library of strong pathogenicity M228 and weak pathogenicity M227 was separately constructed against the database by local BLAST multiple sequence alignment program, and then the T3SEs information between them was compared. Moreover, 20 T3SE single- and poly-genetic mutants from M228 and 2-genetic complementing mutants were constructed, involving 19 T3SEs, and then the mutants were wound-inoculated on kiwifruit canes for assessing and statistical analyzing the pathogenicity.【Result】T3SS was proved to be essential forpathogenicity on host and hypersensitive response (HR) on non-host by theandmutants, separately. Further the BLAST results against database showed there were almost 31 complete T3SE genes and their sequences were displayed 100% similarity between the strong pathogenicity strain and attenuated strain. Then, some T3SE genes were selected for deletion mutants. The results showed that/andgenes were essential forpathogenicity and had no function redundant with each other. In addition, the- and-deletion mutant could in turn increase thepathogenicity. Based on- and T3SE group (cluster A, E and F) deletion mutant, single- or poly-genetic mutantofand/could still separately lead to a significant decrease inpathogenicity. However,simultaneous deletion of/,,andA-F-Ecluster resulted in complete loss of pathogenicity.【Conclusion】HopR1 and its homologous family HopM1/AvrE1, which don’t have a redundant function independent with others, are the unique key pathogenicity factors in, butand-deletion can enhancepathogenicity.
bacterial canker of kiwifruit;pv.(); effector; T3SS; pathogenicity
2021-07-14;
2021-08-26
陕西省特支计划杰出人才项目、国家自然科学基金(32102174)
张晋龙,E-mail:535268139@qq.com。通信作者黄丽丽,E-mail:huanglili@nwsuaf.edu.cn
(责任编辑 岳梅)

