Vectorcardiographic QRS area as a predictor of response to cardiac resynchronization therapy
Mohammed A Ghossein✉, Antonius MW van Stipdonk, Frits W Prinzen Kevin Vernooy,3
1.Department of Physiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht,The Netherlands; 2.Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, Maastricht, The Netherlands; 3.Department of Cardiology, Radboud University Medical Centre, Nijmegen, The Netherlands Correspondence to: mohammed.ghossein@mumc.nl https://doi.org/10.11909/j.issn.1671-5411.2022.01.003
ABSTRACT Cardiac resynchronization therapy (CRT) is a good treatment for heart failure accompanied by ventricular conduction abnormalities.Current ECG criteria in international guidelines seem to be suboptimal to select heart failure patients for CRT.The criteria QRS duration and left bundle branch block (LBBB) QRS morphology insufficiently detect left ventricular activation delay, which is required for benefit from CRT.Additionally, there are various definitions for LBBB, in which each one has a different association with CRT benefit and is prone to subjective interpretation.Recent studies have shown that the objectively measured vectorcardiographic QRS area identifies left ventricular activation delay with higher accuracy than any of the current ECG criteria.Indeed, various studies have consistently shown that a high QRS area prior to CRT predicts both echocardiographic and clinical improvement after CRT.The beneficial relation of QRS area with CRT-outcome was largely independent from QRS morphology, QRS duration, and patient characteristics known to affect CRT-outcome including ischemic etiology and sex.On top of QRS area prior to CRT, the reduction in QRS area after CRT further improves benefit.QRS area is easily obtainable from a standard 12-lead ECG though it currently requires off-line analysis.Clinical applicability will be significantly improved when QRS area is automatically determined by ECG equipment.
Intraventricular conduction disturbances lead to electrical dyssynchrony, a discoordinate contraction, and can ultimately lead to heart failure.Cardiac resynchronization therapy (CRT) is currently the hallmark of treatment for symptomatic heart failure patients with left ventricular (LV) systolic dysfunction in combination with intraventricular conduction disturbances.CRT aims to treat the intraventricular conduction disturbances by stimulating both the right ventricle (RV) and LV, i.e., biventricular pacing.Large landmark trials have shown the effectiveness of CRT in patients diagnosed with what is currently referred to as dyssynchronous heart failure.Dyssynchronous heart failure is diagnosed based on a low left ventricular ejection fraction (LVEF) (< 35%), and on a prolonged QRS duration.CRT seems especially effective in the presence of a left bundle branch block (LBBB) QRS morphology.[1-3]
Factors that are recognized to determine the benefit of CRT are patient selection, general heart failure treatment, the position of the LV lead, and optimization of device programming after implantation.Of these four, patient selection appears to be the most dominant factor.In this regard, recognition of the substrate that is amenable for CRT, the delayed activation of the LV lateral wall, is key.
The ECG criteria that are recommended in the current guidelines apparently are not optimal as many patients with prolonged QRS duration do not seem to improve from CRT when lacking the substrate of delayed activation of the LV lateral wall.This leaves around 30% of patients implanted with a CRT-device without improvement of cardiac function, relief in symptoms, and improved survival,[4]while being exposed to a 4% to 14% complication risk.[5]In addition the current ECG criteria may also miss patients who would benefit from CRT.[6]For these reasons, many studies have searched for better ECG criteria that could predict the benefit from CRT more accurately than the current ECG criteria do.
Recently, the vectorcardiographic QRS area has proven to be a promising new tool for prediction of the potential benefit from CRT.Vectorcardiography (VCG) contains 3D information about the electrical forces within the heart, integrating the information of the unidirectional conventional leads of the 12-lead ECG.From this 3D information, QRS area is determined as the sum of the area under the QRS complex in the orthogonal X, Y and Z leads(Figure 1).[7,8]Importantly, the vectorcardiogram can be easily constructed from a standard 12-lead ECG using relatively simple software, so far custom made, facilitating implementation in clinical practice.
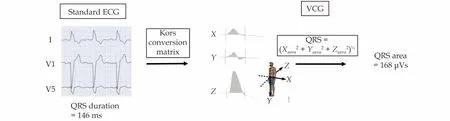
Figure1 From ECG to VCG.An ECG can be converted into the orthogonal X-, Y- and Z-leads through the Kors-conversion matrix.QRS area can then be calculated using the formula displayed.ECG: electrocardiogram; VCG: vectorcardiogram.
In this review, we set out why the vectorcardiographic QRS area could be an electrocardiographic parameter that has an important role in selecting patients for CRT.
THE IMPORTANCE OF THE ELECTRICAL SUBSTRATE
As mentioned before, the amenable substrate for CRT is considered to be the delayed activation of the LV lateral wall that results in mechanical dyssynchrony.[9]Importantly, mechanical dyssynchrony is not just a shift in contraction timing between regions, but it also leads to considerable discoordination.This discoordination exists of several phases of contraction and stretch in the septum during systole.In the LV lateral wall, myocardial shortening is delayed and prolonged into the relaxation phase.These pathophysiological shortening patterns also create “wasted” myocardial work, thereby decreasing the efficiency of contraction.In case of LBBB, activation and contraction of the interventricular septum precedes those of the LV lateral wall,causing less effective systolic function, increasing LV end-systolic volume and inducing cardiac remodeling, contributing to worsening of cardiac function and potentially leading to heart failure.[10,11]
CRT, by way of (bi)ventricular pacing, can restore the electrical synchrony and subsequent discoordinate coordination in patients with dyssynchronous hearts failure.[12]The improved coordinated contraction makes the heart work more efficiently leading to reversal of the pathophysiological processes, resulting in reverse remodeling, i.e.,reduced ventricular dimensions.
When deciding on CRT-device implantation, it is important to note that (bi)ventricular pacing is not able to correct the electrical ventricular dyssynchrony to the level of the physiological sequence of activation.Compared to normal physiological activation,(bi)ventricular pacing itself induces some dyssynchronous activation as it bypasses the intrinsic ventricular conduction system.[13]Therefore, in patients without evident ventricular dyssynchrony,(bi)ventricular pacing could worsen cardiac function which is associated with poor patient outcome.[13,14]As a consequence, benefit of CRT can only be reasonably assumed if ventricular dyssynchrony is considerable ánd treatable.
The importance of delayed LV lateral wall electrical activation has become clear from studies that revealed an association between a greater time delay from QRS complex onset to local intrinsic LVactivation, referred to as Q-LV which is measured by invasive electrophysiological evaluation, and outcomes to CRT.Studies have shown that a greater Q-LV time was associated with superior acute hemodynamic LV improvement after CRT.[15,16]In addition, studies have investigated the effect of CRT in patients without considerable electrical dyssynchrony, but with evidence of mechanical dyssynchrony.The Cardiac Resynchronization Therapy in Heart Failure with Narrow QRS Complex(ECHO-CRT) study revealed that in these patients CRT worsens LVEF and leads to a significant increase in mortality.[14]Therefore, we can conclude that the presence of an electrical substrate amenable to CRT is imperative for a patient to benefit from CRT.
CURRENT ECG SELECTION CRITERIA FOR CRT
The initial clinical application of CRT was in 1994,when the first patients with severe congestive heart failure were implanted with a biatrio-biventricular pacemaker.[17,18]The first randomized trials (Figure 2)appeared years later (MUltisite STimulation In Cardiomyopathy (MUSTIC) study, Multi-centre Insync RAndomized CLinical Evaluation (MIRACLE)study, Comparison of Medical therapy, Pacing and Defibrillation in Chronic Heart Failure (COMPANION), and the CArdiac REsynchronization (CARE)-HF study) and showed that in severe heart failure patients (NYHA III, LVEF < 35%) with a broad QRS complex (first > 150 ms, later > 130 ms and > 120 ms), biventricular pacing improved the 6-min walking distance, peak oxygen uptake, quality of life score,NYHA-class, reduced LV-volumes, reduced HF hospitalizations, and prolonged event-free survival.[19-23]
The consistency of these results led to the first CRT-recommendations for patients with NYHA IIIIV, LVEF < 35%, and a QRS complex > 120 ms and on optimal medical treatment (Figure 2).[24]Later studies investigated the effect of CRT in less symptomatic patients (NYHA II) (the Resynchronization Reverse Remodelling in Systolic Left Ventricular Dysfunction (REVERSE),[25]Multicentre Automatic Defibrillation Implantation Trial (MADIT)-CRT,[26]and Resynchronization/defibrillation for Ambulatory heart Failure Trial (RAFT) trials.[1]These studies confirmed echocardiographic and clinical benefit after CRT in these patients as well, but additional subgroup analyses of these trials revealed that the CRT-benefits were mostly confined to patient with a QRS duration ≥ 150 ms, resulting in the addition of a Class I recommendation for patients with a QRS duration ≥ 150 ms in 2010 (Figure 2).[27]
Attention later shifted to QRS morphology.Substudies of the MADIT-CRT,[2]REVERSE,[3]and RAFT[1]found that most benefit was found in patients with an LBBB QRS morphology, while patients displaying right bundle branch block (RBBB)or non-specific intraventricular conduction delay(IVCD) morphology had less or no benefit.Subsequently, the CRT-guidelines changed and included LBBB as primary ECG criterion having a class I indication, while QRS duration ≥ 150 ms only became important for non-LBBB patients having a class IIa/IIb indication (Figure 2).[28,29]

Figure2 Timeline of CRT guidelines development.All studies included patients with LVEF ≤ 35%.The first studies leading to the 2007 guidelines found benefit from CRT with regards to quality of life, HF hospitalizations, echocardiographic improvement, and survival in patients with NYHA functional class III-IV and QRS duration ≥ 120 ms.Subsequent studies additionally found CRT-benefit in patients with NYHA II and sub-analyses of these studies found that CRT benefit was confined to patients with QRS duration ≥ 150 ms,leading to the 2010 guidelines.More sub-studies followed and found an important role of QRS morphology in determining CRT-response, leading to the 2013 guidelines.CRT: cardiac resynchronization therapy; HF: heart failure; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association.
The emphasis of current guidelines on QRS morphology have resulted in cardiologist’s quick referral of LBBB patients for CRT, while non-LBBB patients receive this treatment significantly less despite their frequent occurrence in the heart failure population.[30]This bias is potentially leading to under treatment of CRT-eligible patients with non-LBBB.Suboptimal outcomes and potential undertreatment stem from several issues that are related to the ECG criteria in general and the LBBB definitions specifically.
LIMITATIONS OF CURRENT ECG CRITERIA
An important limitation when we want to evaluate the presence of LBBB—and therefore that of non-LBBB—is the problem that there is no clear or exact definition for LBBB.Defining LBBB is highly dependent on the criteria used.[31]As a consequence,the prevalence of LBBB in a general CRT population ranges from 29% when using the definitions from the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS),[32]to 47% using the European Society of Cardiology (ESC) criteria,[28]and to 61% when the definition is based on the criteria from Strauss,et al.[33,34]
Moreover, the multiple LBBB criteria significantly differ from each other with regards to their association with CRT outcome.[2,3,32,34,35]Interestingly,only the LBBB definitions that use the ESC and Strauss criteria were able to significantly distinguish for the combined study endpoint of heart failure and mortality in a large CRT cohort (hazard ratio (HR) = 0.61; 95% confidence interval (CI): 0.43-0.87, and HR = 0.57, 95% CI: 0.40-0.80, respectively).[34]Even more striking is the absence of a significant association between QRS morphology and CRT response, shown by a meta-analysis of data from 3,782 patient from the 5 aforementioned randomized key CRT trials (CARE-HF, RAFT, MIRACLE,MIRACLE-ICD, and LBBB REVERSE),[36]which can be explained by the use of different definitions in which each definition influences outcome differently.In addition, a recent study revealed that determining QRS morphology is prone to subjective interpretation in which there is significant interand intra-observer variability.It was shown that one in five ECG’s will be judged differently with respect to QRS morphology, not only when judged by different cardiologists, but even when judged by the same observer.[31]
Another important limitation is that non-LBBB patients are being considered as one homogeneous group, whereas in fact they represent a very heterogeneous group, most importantly consisting of patients displaying a right bundle branch block (RBBB)versus intraventricular conduction delay (IVCD),the latter not falling into the LBBB nor RBBB criteria.Within and between these two subgroups of non-LBBB patients, there exists considerable diversity of the underlying electrical substrate.[37-39]A sub-group of non-LBBB patients benefits from the treatment,[30,40-42]and it is important that this subgroup is recognized.Current class IIa/IIb recommendations for the entire non-LBBB group without considering the heterogeneity hampers CRT decision making and potentially leaves patients undertreated.
IVCD display on the ECG is the result of complex activation patterns often with underlying myocardial disease, most likely explaining the lower response rates found in this group.[37,38,43,44]Studies that have mapped the ventricular activation sequence of IVCD patients found that conduction disturbances at a similar level of that in patients with LBBB may exist in 20% to 52% of IVCD patients.[37,38,44]Identifying CRT-eligible candidates in IVCD patients using the ECG has therefore been proven challenging.[37]Some studies have investigated the possibility that certain ECG features could detect the CRT amenable substrate within IVCD patients.[2,44]In their analysis of the MADIT-CRT, Zareba,et al.[2]found that IVCD patients displaying LBBB features (predominant negative QRS morphology V1-V3/V4, Q-waves V5 and V6) may derive more benefit from CRT than patients that don’t.Recently published data from 11,505 CRT-eligible patients with a non-LBBB QRS morphology found that CRT implantation is associated with better outcomes than an implantable cardioverter-defibrillator alone in IVCD patients with a QRS duration ≥ 150 ms.[45]However, the non-randomized retrospective design and the unclear LBBB definition in this study make it difficult to draw any firm conclusions.
The general absence of significant LV conduction delay in patients displaying RBBB[46]arguably explains the lack of CRT response in this group.In some RBBB patients however, both right and left ventricular conduction can be affected.[39]In these cases, an ECG displaying an RBBB could mask the presence of left ventricular conduction delay.Computer simulations have shown that in an RBBB model, stroke work only improves after CRT when sufficient concomitant LV activation delay exists.[46]In search of RBBB variants that could reflect the presence of LV activation delay, a recently conducted retrospective multicenter study showed that patients with an atypical RBBB (absent S-wave in leads I and aVL) displayed a greater delay in LV-activation time compared to patients with a typical RBBB.[41]The analysis continued by showing that two years after CRT these atypical RBBB patients also showed improved echocardiographic (71.4%vs.19.4%,P= 0.001) and clinical outcomes.[41]These findings however still need to be confirmed in prospective clinical trials.[47]Other investigated ECG criteria such as the presence of a left fascicular hemiblock led to conflicting results.[48,49]From the limited number of studies conducted, it is apparent that it only makes sense to treat an RBBB patient when concomitant leftward electrical delay is suspected.However, it remains uncertain how the ECG can detect this potential CRT-responder subgroup within RBBB patients.
The aforementioned limitations of recommended ECG criteria in the current CRT guidelines led to the investigation of alternative ECG measurements that could be of added predictive value for CRT response.In recent times, the vectorcardiographic QRS area has emerged as a biomarker that could fit that description.
QRS AREA
Vectorcardiography (VCG) is a technique that records the size and direction of the electrical forces of the heart, and displays them in three directions with the use of three orthonormal X, Y, and Z-leads(Figure 1).This technique was invented by Williams in 1914 and is therefore almost as old as the ECG.[50]However, the VCG technique with its 3D vector loops was considered complicated and impractical because of the need of special equipment,while the easier to apply 12-lead ECG became the standard in clinical practice for diagnosing heart disease.Fortunately, interest in the VCG’s diagnostic value never subsided, and since today’s computer technology is able to synthesize the VCG from standard 12-lead ECG’s, the VCG has regained interest.In this regard, Engels,et al.[51]showed that using the Kors conversion matrix to convert 12-lead ECG’s into VCG’s is justifiable as it is as good as using the VCG from the orthogonal X, Y, and Z leads(Figure 1).
As previously explained, VCG derived QRS area contains 3D information about the electrical forces in the heart (Figure 1).Because QRS area could provide accurate information about the direction of the ventricular conduction delay, it was hypothesized that QRS area could detect the CRT-treatable substrate, defined as electrical conduction delay in the direction of the LV.Van Deursen,et al.[52]were the first to investigate the relationship between the vectorcardiographic QRS area and CRT-response.In 81 consecutive patients it was shown that a higher QRS area predicted echocardiographic CRT-response better than QRS duration and conventionally defined LBBB (Table 1).[52]As mentioned above,QRS area reflects the degree of unopposed electrical forces, and it could therefore be a measure of the extent of the dyssynchronous ventricular electrical activation.This notion is supported by the observation that QRS area is larger in LBBB patients compared to IVCD patients.[52]In addition, QRS area is lower in patients with ischemic compared to nonischemic cardiomyopathy, probably explained by the significant non-active scar tissue that is present in ischemic hearts.[52]
Mafi-Rad,et al.[9]investigated the association between delayed LV lateral wall activation, defined as LV activation time > 75% of QRS duration and measured by coronary venous electro-anatomical mapping (EAM), and QRS duration, QRS morphology, and QRS area.In this study, QRS area identified delayed LV activation better than QRS duration (AUC = 0.89, 95% CI: 0.79-0.99vs.AUC = 0.49,95% CI: 0.33-0.65), and a QRS area > 69 μVs diagnosed delayed LV activation with higher sensitiv-ity and specificity (87% and 92%, respectively) than any of the LBBB definitions (Table 1).[9]Notably, the associations with QRS area were also applicable to RBBB and IVCD patients, showing QRS area’s superiority over ECG criteria in predicting LV delayed activation.[9]

Table1 Summary of QRS area, QRS duration and morphology in relation to echocardiographic response and delayed LV-activation.
QRS AREA, ISCHEMIC ETIOLOGY, AND SEX
The aforementioned observations suggest that the QRS area reflects the extent of delayed LV activation and the presence of excitable (non-scarred)myocardium, two factors associated with CRT outcome.Smaller studies have investigated the relationship between myocardial scar size and QRS area, for the reason that myocardial scar size, ischemic cardiomyopathy, and LV-lead position in or close to myocardial scar are negatively associated with CRT outcome.[53]Nguyen,et al.[53]showed in 33 patients that QRS area was significantly smaller in ischemic cardiomyopathy (median: 62 μVs, IQR =27-83), compared to patients with non-ischemic cardiomyopathy (median: 106, IQR = 58-145,P=0.046).Also in this study, QRS area predicted echocardiographic CRT-response (AUC = 0.737,P=0.012), and did so better than QRS duration (Table 1).These findings highlight the relationship between the presence of an ischemic cardiomyopathy (CMP),low QRS area, and poor CRT-outcome.
Related to QRS area and the presence of ischemic CMP is sex.Female sex is associated with improved CRT-outcomes, and this factor is also taken into account when selecting heart failure patients for CRT.[54]Part of this advantage is due to women more often having a non-ischemic heart failure etiology compared to men.Moreover, women have higher QRS area/LV end-diastolic volume ratio indicating a higher degree of dyssynchrony relative to the activated myocardial size, compared to men.[54]As will become apparent from the following paragraphs, QRS area is associated with clinical and echocardiographic response, independent from baseline variables known to affect CRT-benefit including the presence of an ischemic CMP and sex(Figure 3).
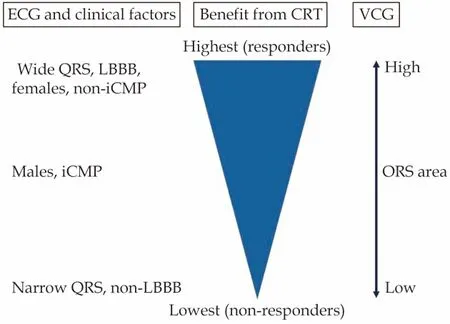
Figure3 ECG and clinical factors vs.QRS area.As depicted left, currently the combination of ECG criteria and clinical characteristics are being used to determine the likelihood of CRT-response.QRS area has been shown to be associated with all independent predictors, and could simplify prediction of benefit for the individual patient.CRT: cardiac resynchronization therapy;iCMP: ischemic cardiomyopathy; LBBB: left bundle branch block; VCG: vectorcardiography.
QRS AREA PREDICTS ECHOCARDIOGRAPHIC OUTCOME AFTER CRT
Subsequent studies continued investigating the value of QRS area in predicting echocardiographic(Table 1) and clinical response (Table 2) after CRT.Echocardiographic response after CRT is usually defined as a decrease in left-ventricular end-systolic volume (LVESV) ≥ 15%, or a > 5% increase in LVEF six months after implantation.[55]In the prospective Markers And Response to CRT (MARC) study,[56]QRS area was one of the few parameters to remain independently associated with LVESV reduction in a multivariable model.QRS area predicted LVESV reduction better than QRS duration.[56]In this study,the CAVIAR (CRT-Age-Vectorcardiographic QRS area-intraventricular mechanical delay-apical rocking) scoring system was found to be predictive of LVESV reduction.Of these significant markers QRS area appeared to be the greatest contributor to the scoring system, while QRS duration and morphology were not significantly predictive of LVESV reduction in multivariable analysis (Table 1).[56]Van Stipdonk,et al.[57]analysed 1,492 patients from the Dutch Maastricht-Utrecht-Groningen (MUG) cohort.Based on the magnitude of baseline QRS area,patients were stratified into four subgroups; the subgroups were significantly different in LVESV reduction, in which the higher QRS area groups had the greatest reduction in LVESV.[57]When patients without a traditional class IA (LBBB + QRS duration ≥ 150 ms) indication were divided into groups of baseline QRS area ≥ and < 109 μVs, LVESV reduction was significantly greater in the QRS area ≥109 μVs group (20%vs.7%) with corresponding echocardiographic response rates (54%vs.38%, OR =1.90, 95% CI: 1.19-3.03;P= 0.009).[57]Similar results were found when patients with an LBBB and QRS duration 130-149 ms were stratified in the same way.QRS area was able to identify echocardiographic responders better than QRS morphology and/or duration (AUC = 0.69vs.0.58 and 0.58, respectively, Table 1).[57]Also in patients without LBBB, QRS area and not QRS duration was significantly associated with echocardiographic response.A multivariable adjusted model for ECG parameters showed a significant independent association between QRS area and echocardiographic response,which was stronger than the combination of QRS duration and morphology (Table 1).[57]
QRS AREA IS ASSOCIATED WITH CLINICAL OUTCOME AFTER CRT
As could be expected from the echocardiographic data, various studies have also shown a strong association between QRS area and clinical outcomes after CRT in patients with a class I as well as a class IIa/IIb indication for CRT (Table 2).From the previously mentioned MUG-cohort, patients with a class I or class II CRT-indication—based on QRS morphology and duration criteria—were followed 3.4 ±2.4 years, and it was shown that there was a strong association with survival (free from heart transplantation and left-ventricular assist device implantation) and baseline QRS area.[57]The difference in survival was less pronounced when patients with LBBB were compared to patients with non-LBBB, and when patients with QRS duration ≥150 ms were compared to the patients with QRS duration < 150 ms.Interesting in this study was the finding that when using the cut-offs for QRS area ≥109 μVs and QRS duration ≥ 150 ms in non-LBBB patients (class IIa/IIb indication for CRT), only QRS area was able to differentiate survivors from nonsurvivors.[57]In a multivariable model with QRS area, QRS duration and morphology, QRS area consistently proved to have an independent association with survival (Table 2).[57]In a study with 705 CRT-eligible patients from Emerek,et al.,[59]QRSarea was significantly associated with survival in all subgroups; with and without LBBB, and with QRS duration ≥ 150 ms and < 150 ms.These findings show that QRS area is consistent in its significant association with survival across all classes of CRTindications (class I and II).

Table2 Summary of QRS area, QRS duration and morphology in relation to clinical outcomes.
In addition to its independence from QRS duration and morphology, studies have also shown that QRS area is independent from baseline clinical characteristics—including ischemic etiology and sex—in its association with survival.From a subsequent study in the previously mentioned MUG-cohort as well as in the study of Emerek,et al.[59]QRS area remained significantly associated with survival in a multivariable adjusted model (Table 2).[58,59]Similar results were found in another study with regards to cardiac mortality.QRS area had a higher area under the curve (0.71) when compared to QRS duration, QRS morphology, or both combined (0.60, 0.53,0.66,P< 0.002 for comparison with QRS area).[60]Again, QRS area predicted cardiac mortality independently from QRS duration, morphology, and baseline clinical variables (Table 2).[60]These findings confirm QRS area’s value as a marker of response to CRT, independent from QRS duration and morphology, as well as from variables known to affect CRT-outcome including ischemic etiology and sex(Figure 3).
From the previously mentioned MUG-cohort, it was found that heart failure (HF) hospitalisations did not differ when patients were stratified according to QRS morphology or duration, while patients with a high QRS area did have fewer hospitalisations after CRT compared to patients with low QRS area.The beneficial association of a high QRS area with HF hospitalisations was found in both LBBB as well as non-LBBB patients.Additionally, a multivariable analysis on HF hospitalisations showed that only QRS area is independently and significantly associated with this outcome (Table 2).[57]Thus,in addition to its predictive value for a favourable echocardiographic response, a high QRS area prior to CRT is independently associated with survival and fewer HF hospitalisations after CRT.
REDUCTION IN QRS AREA AS A MEASURE FOR RESYNCHRONIZATION
The superiority and consistency of QRS area’s association with echocardiographic and clinical outcome strongly support the hypothesis that a high QRS area reflects an electrical substrate for CRT.From this idea, it was hypothesized that the reduction in QRS area (ΔQRS area) by CRT could be reflecting correction of the electrical substrate.In current practice, the ECG and echocardiogram are the best available tools to optimize CRT.Studies that investigated the value of the ECG for CRT-optimization led to conflicting results, and there is currently no evident proof that the investigated ECG markers including QRS duration could be used for CRT-optimization.[3,61]The reason for the conflicting results could lie in the inter- and intra-observer variability in measuring the QRS duration of a paced QRS complex.[62]The technique used to measure the paced QRS complex influenced the association with CRT response.[63]The same holds true for echocardiographic CRT-optimization.Whereas the ability to detail mechanical dyssynchrony and haemodynamic surrogates provide clear potential for echocardiographic parameters in CRT optimization,studies show conflicting results and its use for CRToptimization still needs to be investigated further.[64]
Because studies have shown that electrical substrate correction is a factor independent from electrical substrate presence[65,66]and QRS area’s value in detecting the CRT-treatable substrate, ΔQRS area as an additional predictive marker became the next target of investigation.A study by De Pooter,et al.[67]showed a relation between QRS area reduction and acute hemodynamic CRT-benefit.Later, Okafor,et al.[60]found in 380 patients that the change in QRS area was associated with long-term cardiac and total mortality, major adverse cardiac events, and ventricular arrhythmias in a univariable analysis.In 1,299 patients from the MUG-cohort, a strong and independent association was found between QRS area reduction and clinical and echocardiographic outcomes.[58]After correction for baseline QRS area,QRS duration and morphology, and patient characteristics known to affect CRT-outcome in multivariable regression analysis, ΔQRS area ≥ 62 μVs remained the strongest predictor of clinical and echocardiographic outcome.[58]In addition, there was no significant interaction between baseline and ΔQRS area, and ΔQRS area had the strongest predictive value in the high baseline QRS area subgroup.[58]The findings of these studies support the idea that ΔQRS area could be an independent marker that reflects correction of the electrical substrate, possibly applicable for CRT-optimization during and after device implantation.
CLINICAL IMPLICATIONS AND FUTURE DIRECTIONS
Current evidence suggests that QRS area reflects the CRT-treatable substrate better than QRS duration or morphology.Moreover, QRS area is a marker that fits the criteria for clinical application.First,QRS area is simple as it only requires standard ECG equipment and computer software (e.g., Matlab,Mathworks Inc.) to transform the ECG into a VCG.Second, QRS area is easily obtainable as it is automatically calculated from the VCG.Third, QRS area is an objective parameter which leaves little room for subjective interpretation, unlike QRS morphology.[31]And fourth, QRS area provides information about the status of viable myocardium and is lower in patients that are associated with poor CRT-outcome.
On top of baseline QRS area, a great reduction in QRS area further improves CRT benefit.QRS area and ΔQRS area could therefore play a central role in future patient selection and therapy optimization,respectively (Figure 4).Before definitive clinical application however, large prospective studies need to confirm QRS area’s value in predicting clinical outcomes after CRT.
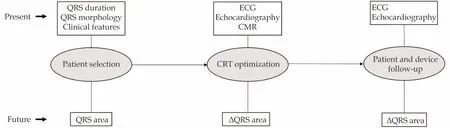
Figure4 Possible role of QRS area in the future.QRS area is a promising candidate to take a prominent role in future patient selection and CRT optimization.CMR: cardiovascular magnetic resonance; CRT: cardiac resynchronization therapy.
CONCLUSIONS
Evidence for QRS area’s value in patient identification and selection for CRT has mounted in recent years.QRS area detects the electrical substrate that is correctable by CRT, predicts improvement of LVpump function, and is independently associated with clinical outcomes including mortality.Patients with an ischemic cardiomyopathy have a lower QRS area and tend to have worse outcomes after CRT.Patient characteristics known to adversely affect CRT-outcome are as well associated with lower QRS area.For clinical application, QRS area is easily obtainable, and an easy to read and objective measurement.In the future, QRS area could take a prominent role in patient selection for and optimization of CRT.
Acknowledgements
None
 Journal of Geriatric Cardiology2022年1期
Journal of Geriatric Cardiology2022年1期
- Journal of Geriatric Cardiology的其它文章
- Atrial fibrillation in older adults with cancer
- Electrocardiographic markers of cardiac resynchronization therapy response: delayed time to intrinsicoid deflection onset in lateral leads
- Novel electrocardiographic dyssynchrony criteria that may improve patient selection for cardiac resynchronization therapy
- Evolving concept of dyssynchrony and its utility
- Alcohol consumption in relation to the incidence of atrial fibrillation in an elderly Chinese population
- Assessment of causal direction between thyroid function and cardiometabolic health: a Mendelian randomization study
