白洋淀喹诺酮类抗生素在底栖动物中的生物富集特征
付 雨,剧泽佳,陈 慧,赵鑫宇,宋圆梦,王广志,张璐璐,2*,崔建升,2
白洋淀喹诺酮类抗生素在底栖动物中的生物富集特征
付 雨1,剧泽佳1,陈 慧1,赵鑫宇1,宋圆梦1,王广志1,张璐璐1,2*,崔建升1,2
(1.河北科技大学环境科学与工程学院,河北 石家庄 050000;2.河北省污染防治生物技术实验室,河北 石家庄 050000)
利用超高效液相色谱串联质谱法(HPLC-MS)对白洋淀水体、沉积物以及7种底栖动物中喹诺酮类抗生素(QNs)进行检测,并探究QNs在底栖动物中的生物富集特征及其与环境因子的相关性.结果表明:在白洋淀水体中,ΣQNs浓度为0.7380~2004ng/L,其中氟甲喹(FLU)平均浓度最高(168.0ng/L),而在底栖动物中,氧氟沙星(OFL)和诺氟沙星(NOR)的检出率最高(Freq=100%),其次为FLU和恩诺沙星(ENR)(Freq³50%),其余QNs检出率小于40%(Freq£40%),底栖动物中ΣQNs浓度为23.70~289.4ng/g,其中FLU(29.43ng/g)和环丙沙星CIP(40.38ng/g)平均浓度最高;QNs在底栖动物中的生物富集系数(BCF)为219.6(BCFMAR)~1754L/kg(BCFENR),这表明QNs在底栖动物中的生物富集能力较高;检出率较高的OFL、恩诺沙星(ENR)和NOR的营养放大因子(TMF)为0.1299(TMFFLU)~6.426(TMFENR),其中NOR、ENR呈营养放大,而FLU、OFL呈营养稀释;相关性分析结果表明ENR、CIP、FLU和ORB的BCF与水深(WD)、温度()、透明度(SD)、溶解氧(DO)和沉积物总有机碳(TOCs)呈显著正相关,而与化学需氧量(COD)、总磷(TP)、总氮(TN)、NO3--N、PO43-、沉积物总碳(TCs)、沉积物总氮(TNs)和NH3-Ns呈显著负相关;TMFOFL与WD、、SD、DO和TOCs呈显著负相关,与COD、TP、TN、NO3--N、PO43-、TCs、TNs和NH3-Ns呈显著正相关.这表明生活污水和养殖废水对QNs的贡献最大.
白洋淀;喹诺酮类抗生素(QNs);优势底栖动物;生物富集;空间分异;环境因子
中国是世界上最大的抗生素生产和使用国[1].据估计,我国抗生素年使用量超过1.6×105t[2],抗生素因其分子较大,不能被机体完全吸收,约90%以母体化合物或代谢产物的形式随粪便或尿液排出体外[3-4],最终进入水环境[5].常见的抗生素包括β类酰胺类、喹酮类、大环内酯类、四环素类和磺胺类等.其中,喹诺酮类(QNs)药物[6-7]属于广谱抗生素,可有效预防和治疗多种传染病,在水产养殖领域中被广泛应用,在湖泊中含量更高.它可破坏水生环境中的微生物群落,引起抗菌基因的产生,同时干扰水体生态系统的物质循环与能量流动,打破原有生态系统的平衡[8-10],被水生生物吸收的抗生素还可发生生物富集和营养放大[11-12],进而对人类和动物健康以及生态环境造成极大的威胁.
QNs的生物降解率较低,在地表水[12-15]、地下水[16-17]、饮用水[18-19]和海洋[20]中广泛检出.有研究表明,QNs易富集于水生生物中[21-23],黄河[24]周边海域中QNs在虾和蟹体内具有生物累积作用,累积系数为46~766L/kg和35~2261L/kg.Bai等[25]的研究结果表明,恩诺沙星和环丙沙星在辽河海域亚洲海岸蟹中的生物富集系数分别为11192和150L/kg.北部湾水生生物体内依诺沙星、诺氟沙星、氧氟沙星和恩诺沙星的生物富集系数为83.18,57.54,66.07和67.61L/kg.海陵岛[27]周边海域环丙沙星和恩诺沙星在虾体内的生物富集系数为23,650L/kg[26].不同水环境中抗生素污染情况不同,不同底栖动物对抗生素的生物富集和传递作用也存在显著差异.然而此前的研究多集中于抗生素在生物体内的生物富集和营养传递行为,对抗生素生物富集和营养传递行为与环境因子的相关性研究较为缺乏.
白洋淀是华北平原最大的浅水草型湖泊,底栖动物群落是水生食物链的重要组成部分,对于维持水生态系统的健康具有重要意义.然而,白洋淀长期遭受保定市城市污水、养殖废水以及周边居民生活污水排放的影响,与其它湖泊相比,白洋淀抗生素污染水平相对较高[22,28-29].如:白洋淀水体和沉积物中QNs浓度分别为0.7400~2004ng/L和0.5100~ 1540ng/g,且QNs在水生生物体内也被检出[22,28,30-31].研究表明,白洋淀环境因子和QNs呈显著空间差异性[28,32],且生物体内抗生素的浓度与环境因子密切相关[33].因此,为了明晰典型抗生素在底栖动物中的生物累积和营养传递行为及其主要环境影响因子,本文以白洋淀为研究区,选取QNs为典型抗生素,分析优势底栖动物中QNs浓度,探讨QNs在优势底栖动物中的生物富集特征和营养传递行为,建立优势底栖动物中QNs与环境因子的相关性,以期为白洋淀生态修复和风险管控提供理论参考和数据支撑.
1 材料与方法
1.1 样品采集
2018年4月和8月,在白洋淀8个研究区25个采样点(图1)采集了水体、沉积物和底栖动物样品.依据白洋淀水体理化特征,采样点共分为3个生境,生境1(I、II)主要受上游保定市城市污水影响,生境2(V、VIII)主要受当地水产养殖废水和生活污水影响,生境3(III、VI、VII)受人类活动干扰较小.每个采样点采集4L表层水,做好标记,保存于冷藏箱中,运回实验室于-20℃冰柜内储存待用[29];在各个采样点使用干净的不锈钢铲采集足量的沉积物样品,并装袋标记,24h内运送至实验室,在-20℃的黑暗中冷冻保存,待实验[33];共采集10种优势底栖动物,包括中国圆田螺()、梨形环棱螺()、中华圆田螺()、扁蛭()、中华米虾()、日本沼虾()、萝卜螺()、克氏螯虾()、中华绒毛蟹()、马大头();每种底栖动物样品不少于8只,共采集80只,两批次样品混合后进行测定分析;水样进行理化分析(水深(WD)、水温()、pH值、溶解氧(DO)、透明度(SD)、化学需氧量(COD)、总磷(TP)、总氮(TN)、氨氮(NH4+-N)、硝氮(NO3--N)和PO43-等).
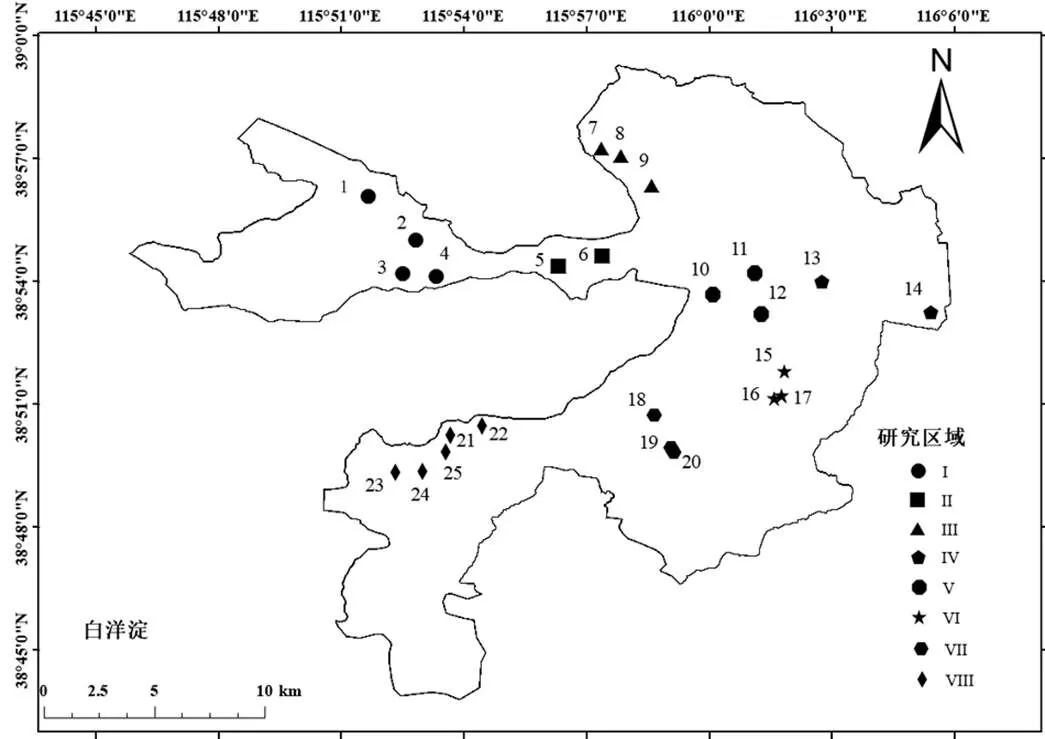
图1 白洋淀采样点示意
1.2 QNs测定
1.2.1 底栖动物样品前处理 底栖动物样品采样时间分别为2018年4月(春季)、2018年8月(夏季).底栖动物取样采用1/16m2彼得森采泥器,在各采样点采集底泥2份,均匀混合,取其中一半当场用60目尼龙筛清洗后倒入样品瓶,瓶中加入质量分数为10%的甲醛溶液后密封保存,泥样带回实验室后用60目尼龙筛再次清洗,剩余物倒入解剖盘中,用镊子将动物活体逐一捡出,放入盛有质量分数为80%无水乙醇的离心管中,离心速率为1500r/min,时间为5min,随后在显微镜中进行鉴定、计数,并用滤纸擦拭干净后在万分之一的天平上称重,最终结果换算单位面积的生物量(g/m2)[34].
1.2.2 质量控制采用外标法定量QNs.以甲醇水(:=1:1)逐级稀释储备液(1μg/mL)制备0.1,0.5,1.0, 5.0,10.0,100.0ng/mL6个系列标准溶液,经HPLC- MS分析获得质量浓度与峰面积的标准曲线,相关系数均大于或等于0.99,各样品中QNs抗生素的回收率为72.8%~105.6%.
1.3 QNs在底栖动物中的富集与传递
底栖动物对QNs的累积效应用生物富集系数(BCF)表征[35],计算公式见式(1).
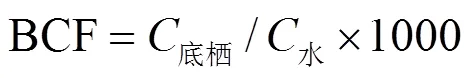
式中:底栖为底栖动物体内QNs含量,ng/g;水为水体中QNs的质量浓度,ng/L.
QNs在底栖动物中的营养传递水平用营养放大因子(TMF)表征,计算公式如下:
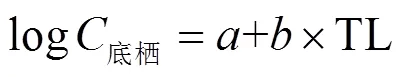
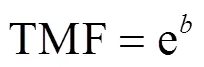
式中:TL为底栖动物的营养级;为轴的截距;为斜率.
底栖动物的营养级由文献[36]计算:

式中:15N底栖为底栖动物的同位素值;15N基准为基准生物(中国圆田螺)的平均同位素值,其值选择之前研究[37]中的数值(10.36‰(生境1)、9.43‰(生境2)和8.74‰(生境3))计算;2.98是每个营养级水平的预期15N值[38];2是基线生物(中国圆田螺)的理论营养级水平[39-40].白洋淀底栖动物同位素15N分析和结果详见文献[37].
1.4 数据统计与分析
采用Origin 2018软件进行理化参数、抗生素浓度分布以及底栖动物生物量分布制图;BCF和TMF与环境因子和生物量间的相关性采用SPSS 24.0软件和Canoco 5进行相关分析;各采样点分布利用ArcGIS 10.6软件进行绘图;利用Gephy绘制网络相关分析图.
2 结果与分析
2.1 白洋淀优势底栖动物空间分异特征
底栖动物最大生物量出现在生境3(1237g/m2),最小值出现在生境1 (337.4g/m2)(图2).中国圆田螺的生物量较高(250.5g/m2),而马大头的生物量较低(3.400g/m2);中国圆田螺的生物量在生境3较高(394.2g/m2),在生境1较低(28.00g/m2).中国圆田螺、梨形环棱螺、中华圆田螺、克氏螯虾和中华米虾在3个生境均有检出,中华绒毛蟹、扁蛭、日本沼虾和萝卜螺的检出为66.70%.而马大头的检出率最低(33.30%)最低.生境3底栖动物出现频率较高(100%),在生境3,中国圆田螺的生物量占比最高(33.20%);结果表明,白洋淀底栖动物的生物量和分布呈现显著的空间差异.

图2 白洋淀优势底栖动物的时空分异特征
2.2 白洋淀优势底栖动物的QNs浓度
选取白洋淀底栖动物中抗生素含量较高的5种,共检出10种QNs,包括环丙沙星(CIP),依诺沙星(ENO),恩诺沙星(ENR),氟罗沙星(FLE),氟甲喹(FLU),马波沙星(MAR),诺氟沙星(NOR),氧氟沙星(OFL),奥比沙星(ORB),吡哌酸(PIP),其中NOR和OFL检出率最高(100%),其次为FLU和ENR(³50%), QNs平均浓度为2.990 (ENO)~40.38(CIP)ng/g(图3).底栖动物中ΣQNs浓度为23.70~289.4ng/g.其中,中国圆田螺在生境1中ΣQNs平均浓度最高(34.34ng/g),扁蛭在生境3中ΣQNs平均浓度最低(4.450ng/g).CIP(40.38ng/g)和FLU(29.43ng/g)在底栖动物体内的平均浓度高于其它QNs(<25.00ng/g);除CIP、ENO和ENR外,其余QNs均呈现生境1>生境2>生境3,与水体中QNs浓度空间分布一致.
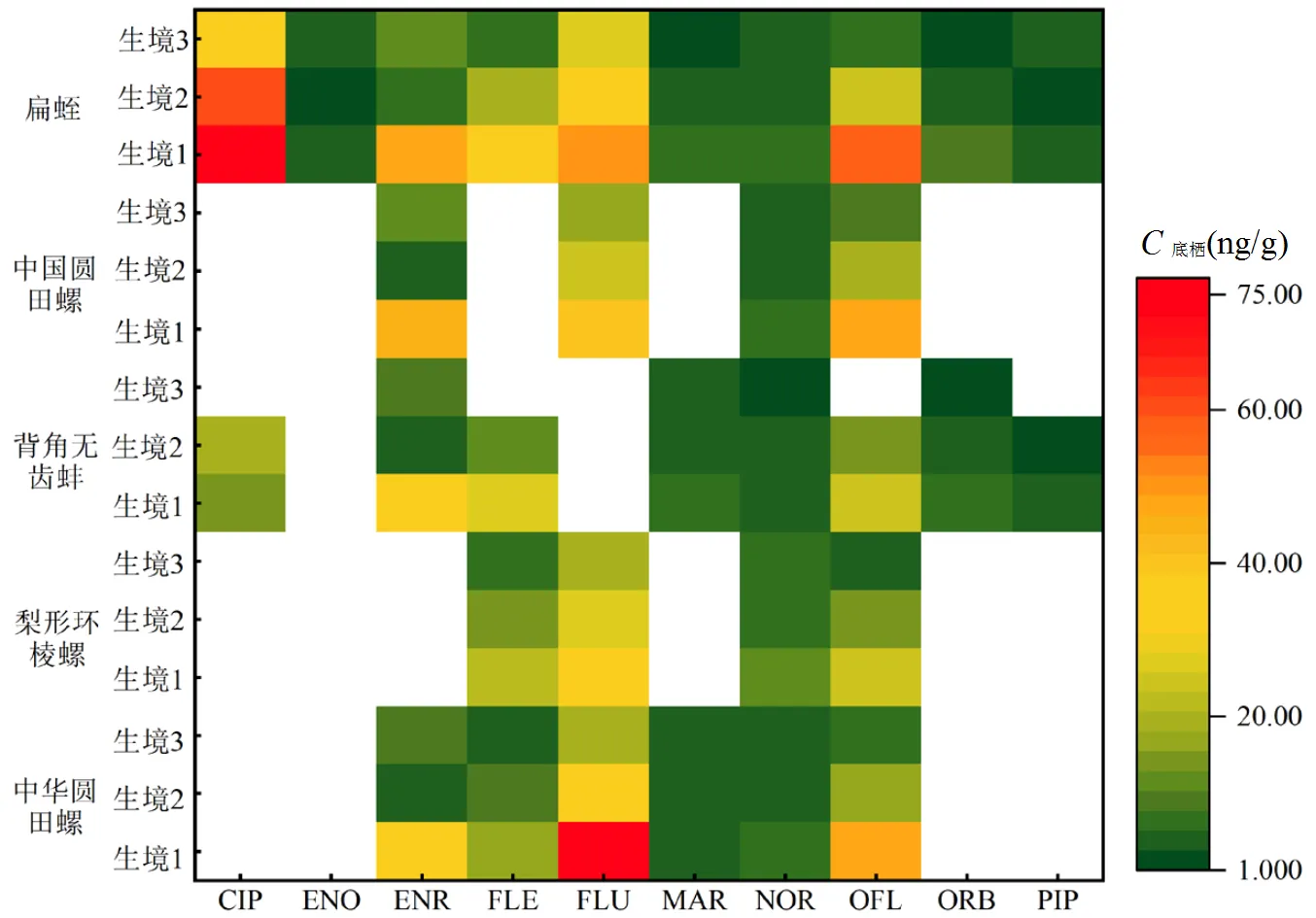
图3 底栖动物中QNs含量特征
空白部分表示未检出
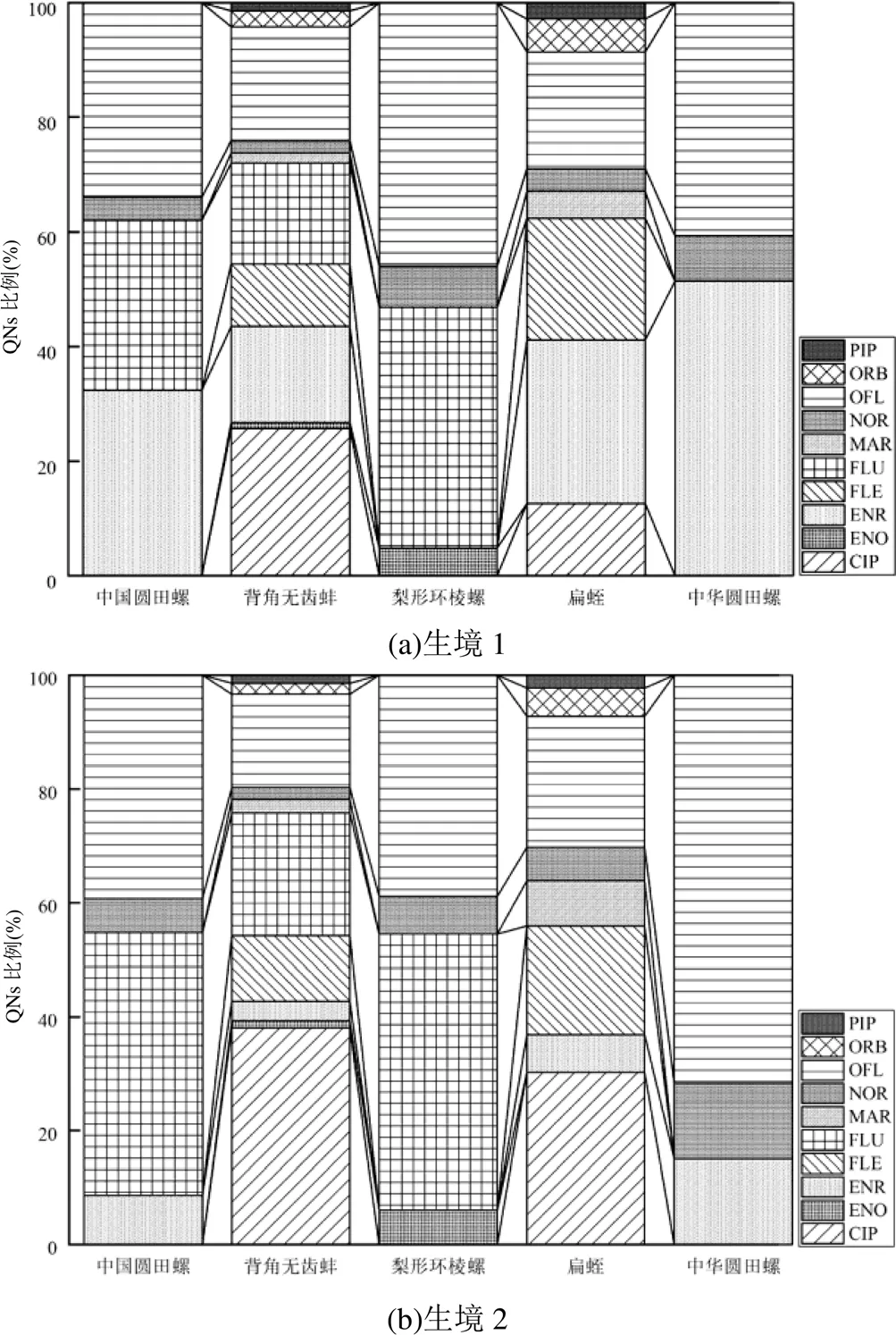
如图4所示,背角无齿蚌和扁蛭中QNs检出种类较多(>6种),其中背角无齿蚌检出种类为10种.底栖动物中FLU平均相对丰度最高(36%),其最高丰度(49%)在生境3的梨形环棱螺中,最低丰度(18%)在生境1的背角无齿蚌中;其次为OFL(31.20%),其最高丰度(72%)在生境2的中华圆田螺中,最低丰度(7%)在生境3的背角无齿蚌中;PIP的相对丰度最小.结果表明,白洋淀底栖动物中QNs丰度呈现显著空间差异,与水体中QNs质量浓度基本一致,表明底栖动物中与其生活的水环境中QNs浓度特征具有一定相似性.
2.3 白洋淀QNs在优势底栖动物中的BCF和TMF
如图5所示,背角无齿蚌的BCF平均值最高(325.2L/kg),中华圆田螺的BCF平均值最低(81.90L/ kg);除了梨形环棱螺和扁蛭外,其余底栖动物体内QNs的BCF在生境3值最大,梨形环棱螺和扁蛭体内QNs的BCF在生境2值最大.
由表1可知,BCFENR平均值最高(1639L/kg), BCFMAR的平均值最低(240.7L/kg);BCF在生境1的范围为228.3L/kg(BCFMAR)~1569L/kg(BCFENR),生境2为219.6L/kg(BCFMAR)~1593L/kg(BCFENR),生境3为274.3L/kg(BCFMAR)~1754L/kg(BCFENR).结果表明,白洋淀优势底栖动物中QNs的BCF呈现显著的空间差异.
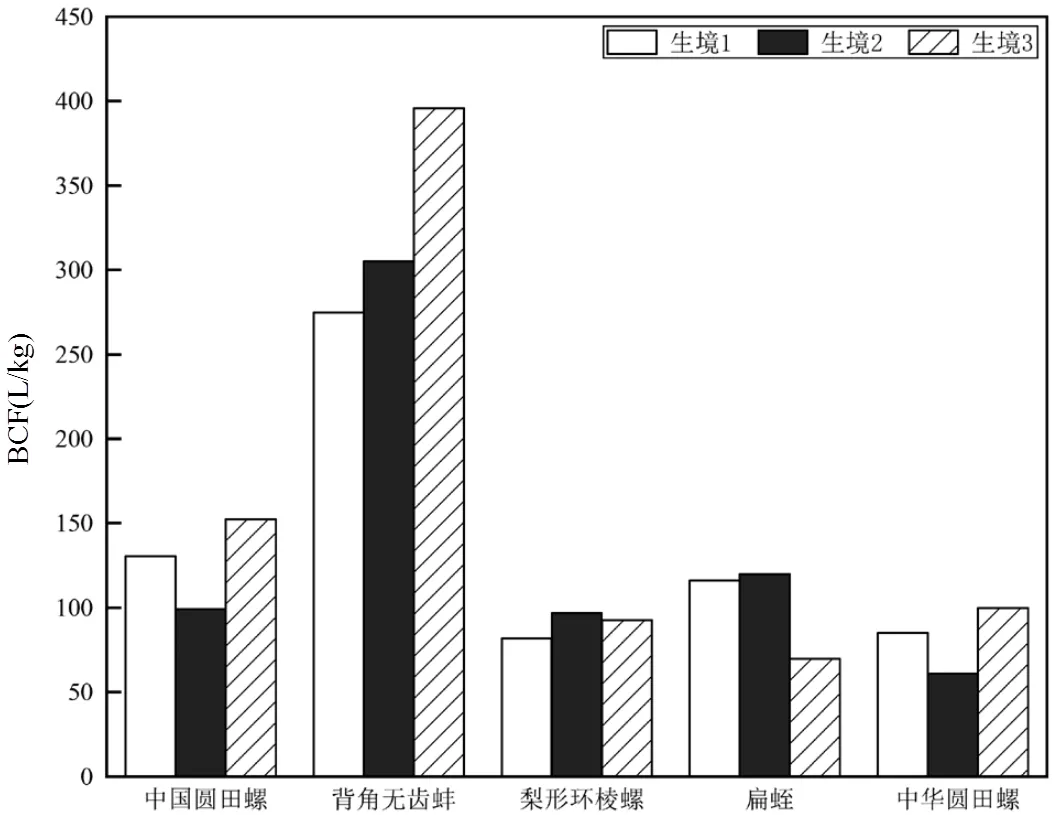
图5 优势底栖动物QNs的BCF
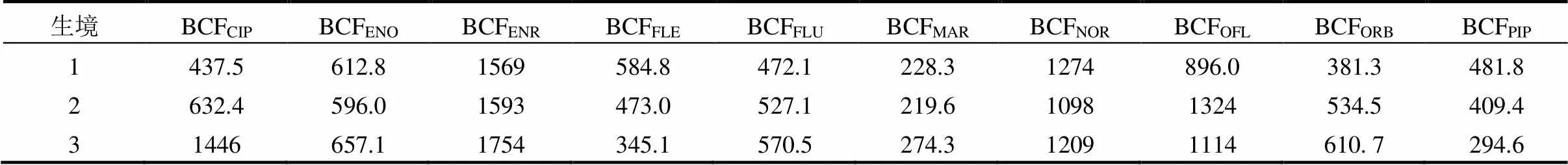
表1 白洋淀3个生境中优势底栖动物中QNs的BCF(L/kg)
方差分析结果表明,水生植物15N稳定同位素在不同生境存在差异性(表2),生境1(10.36‰(中国圆田螺)~11.45‰(扁蛭))和生境2(9.430‰(中国圆田螺)~10.93‰(扁蛭))的15N高于生境3(8.740‰(中国圆田螺)~10.14‰(扁蛭)).就底栖动物种类而言,中国圆田螺的15N值最低(8.740‰),扁蛭的最高(11.45‰).3个生境中各底栖动物营养级(TL)为2.000~2.500;扁蛭的营养级最高(2.500),为2.370(生境1)~2.500(生境2);中国圆田螺的营养级最低(2.000);底栖动物营养级的空间分布特征为:生境3(2.000~2.470)>生境2(2.000~2.500)>生境1(2.000~ 2.370).结果表明,白洋淀同种底栖动物的营养级存在显著的空间差异.
在本研究中,优势底栖动物中QNs的浓度和检出率均呈现较大差异.因此,为了计算TMF数据的准确性,选择检出率>50%的QNs(ENR、NOR、FLU和OFL)来计算优势底栖动物的TMF值(图6),3个生境中QNs的TMF为0.1299(TMFFLU,生境3)~6.4261 (TMFENR,生境1).OFL和ENR的TMF值都是在生境1最大,其值分别为0.7648、6.4261;而TMFOFL呈现生境1>生境2>生境3的趋势,其值分别为0.7648、0.7470和0.1326;而TMFFLU和TMFNOR呈现生境2>生境1>生境3的趋势;就QNs种类而言,TMF呈现出TMFENR>TMFNOR> TMFOFL>TMFFLU的趋势,其中TMFENR为1.3290~ 6.4261,TMFNOR为0.8028~1.0576,TMFOFL为0.1326~ 0.7648,TMFFLU为0.1299~0.1704.

表2 白洋淀底栖动物δ15N稳定同位素和营养级(TL)空间差异
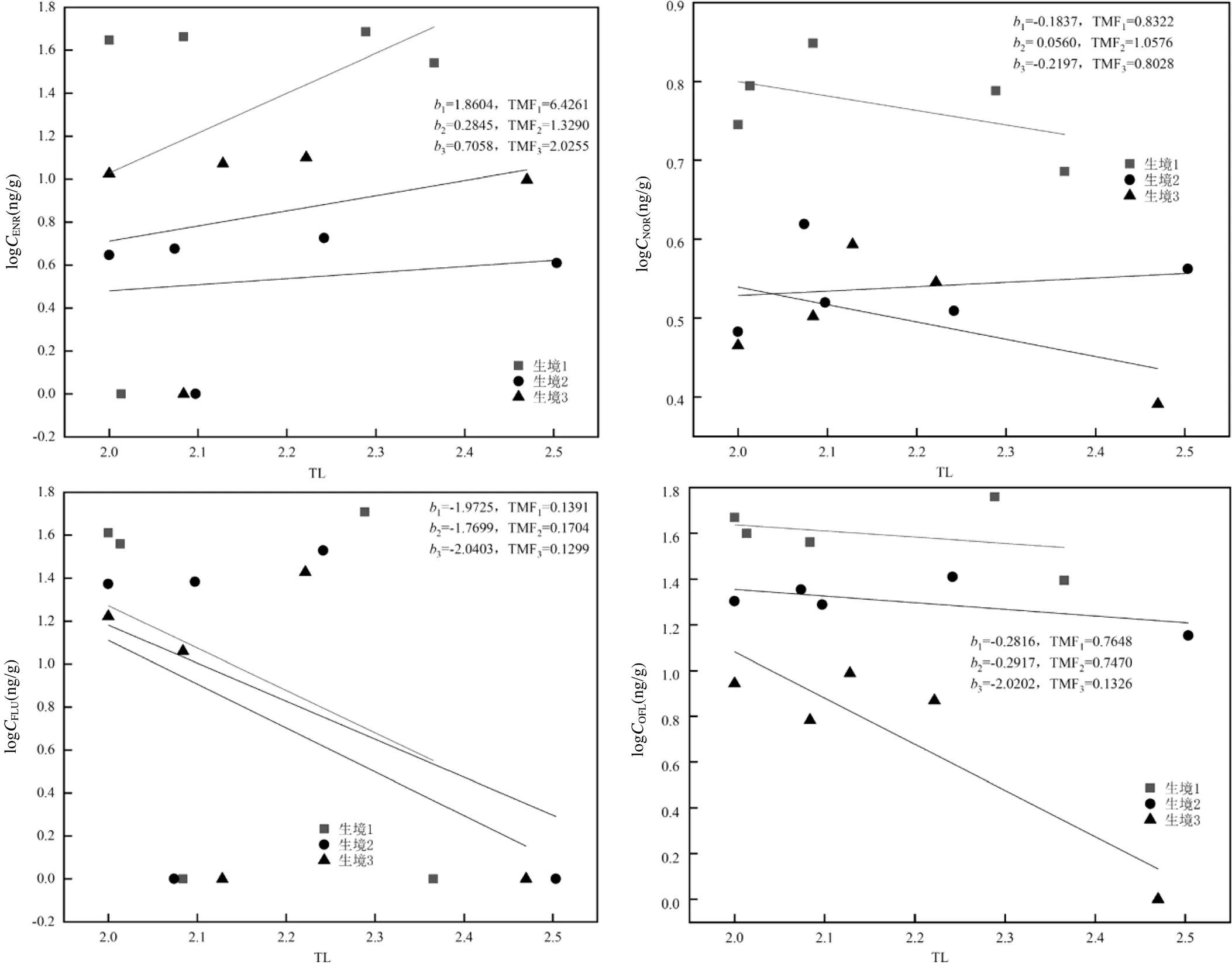
图6 3个生境中底栖动物中QNs含量对数与TL的关系
1、2和3分别为生境1、生境2和生境3中对应的公式(2)中的值;TMF1、TMF2和TMF3分别为生境1、生境2和生境3中鱼类的TMF
2.4 白洋淀QNs的BCF和TMF与环境因子的相关性

图7 环境因子与抗生素BCF和TMF相关性网络
节点根据相关性进行着色,连接表示显著(<0.05)和极显著(<0.01)相关,根据连接数加权节点大小
由图7和表3可知,部分QNs的BCF(如CIP、ENR、FLU和ORB)与WD、、SD、DO和TOCs在<0.01水平呈显著正相关,相关性系数为1.000**,其中,BCFORB和BCFPIP与其他因子相关性最高;而与COD、TP、TN、NO3--N、PO43-、沉积物总碳(TCs)、沉积物总氮(TNs)和NH3-Ns在<0.01水平呈显著负相关,相关性系数为1.000**;BCFFLE和BCFPIP与WD、、SD、DO、TOCs在<0.01水平呈显著负相关,相关性系数为1.000**;而与COD、TP、TN、NO3--N、PO43-、TNs、NH3-Ns在<0.01水平呈显著正相关,相关性系数为1.000** (表3);主成分分析结果显示(图8),和SD是底栖动物中QNs生物富集因子的主要影响因子,结果表明城市废水以及生活污水和养殖废水是底栖动物中QNs的主要来源,因此未来需要对白洋淀区域中城市废水以及生活污水和养殖废水的抗生素排放加强管理.TMFOFL与其他因子相关性最强(图7),TMFOFL与WD、、SD、DO和TOCs在<0.01水平呈显著负相关,相关性系数为1.000**;而与COD、TP、TN、NO3--N、PO43-、TCs、TNs和NH3-Ns在<0.01水平呈显著正相关,相关性系数为1.000**;TMFENR与pH值在<0.01水平呈显著正相关,相关性系数为1.000**;TMFFLU关与NO3--Ns在<0.01水平呈显著正相关,相关性系数为1.000**.

表3 环境因子与QNs的BCF/TMF的Spearman相关性
注:*表示<0.05水平显著相关;**表示<0.01水平显著相关.
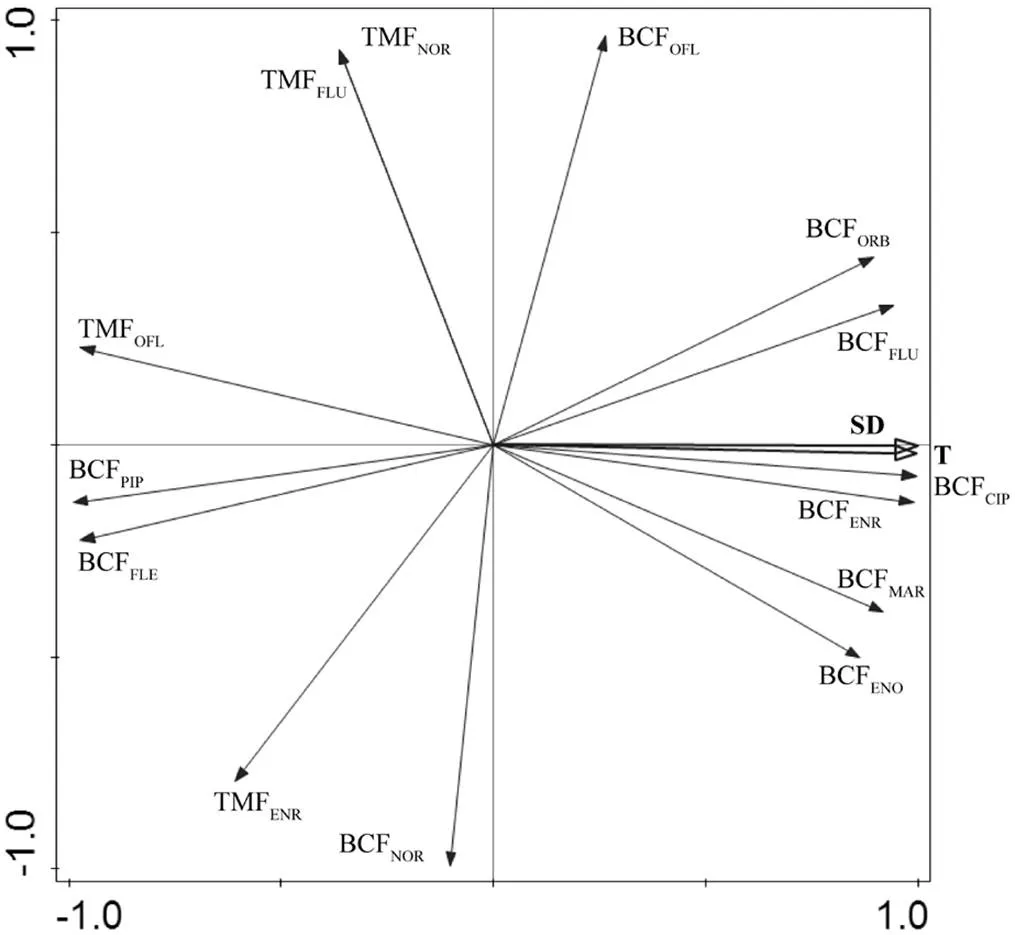
图8 环境因子与QNs的BCF和TMF间的PCA分析
2.5 白洋淀QNs的BCF和TMF与底栖动物生物量的相关性

图9 底栖动物生物量与抗生素BCF和TMF相关性网络
节点根据相关性进行着色,连接表示显著(<0.05)和极显著(<0.01)相关,根据连接数加权节点大小
由图9和表4可知,BCF和TMF与底栖动物生物量存在相关性(相关性为-1.000**~1.000**).结果表明,底栖动物生物量与TMF呈显著相关, TMFOFL与其他物质相关性最高,其中中国圆田螺和中华圆田螺的生物量与TMFOFL在<0.01水平呈显著负相关,相关性系数为1.000**;克氏螯虾和中华绒毛蟹的生物量与TMFOFL在<0.01水平呈显著正相关,相关性系数为1.000**;梨形环棱螺的生物量与TMFNOR在<0.01水平呈显著负相关,相关性系数为1.000**;对于生物富集系数而言, BCFPIP与其他物质相关性最强,中国圆田螺和中华圆田螺的生物量与BCFCIP、BCFENR、BCFFLU、BCFORB在<0.01水平呈显著正相关,相关性系数为1.000**,与BCFPIP和BCFFLE在<0.01水平呈显著负相关,相关性系数为1.000**;克氏螯虾和中华绒毛蟹的生物量BCFCIP、BCFENR、BCFFLU、BCFORB在<0.01水平呈显著负相关,相关性系数为1.000**,与BCFPIP和BCFFLE在<0.01水平呈显著正相关,相关性系数为1.000**;中华米虾的生物量与BCFENO、BCFMAR在<0.01水平呈显著负相关,相关性系数为1.000**.这可能与高浓度抗生素对底栖动物胁迫有关[40].
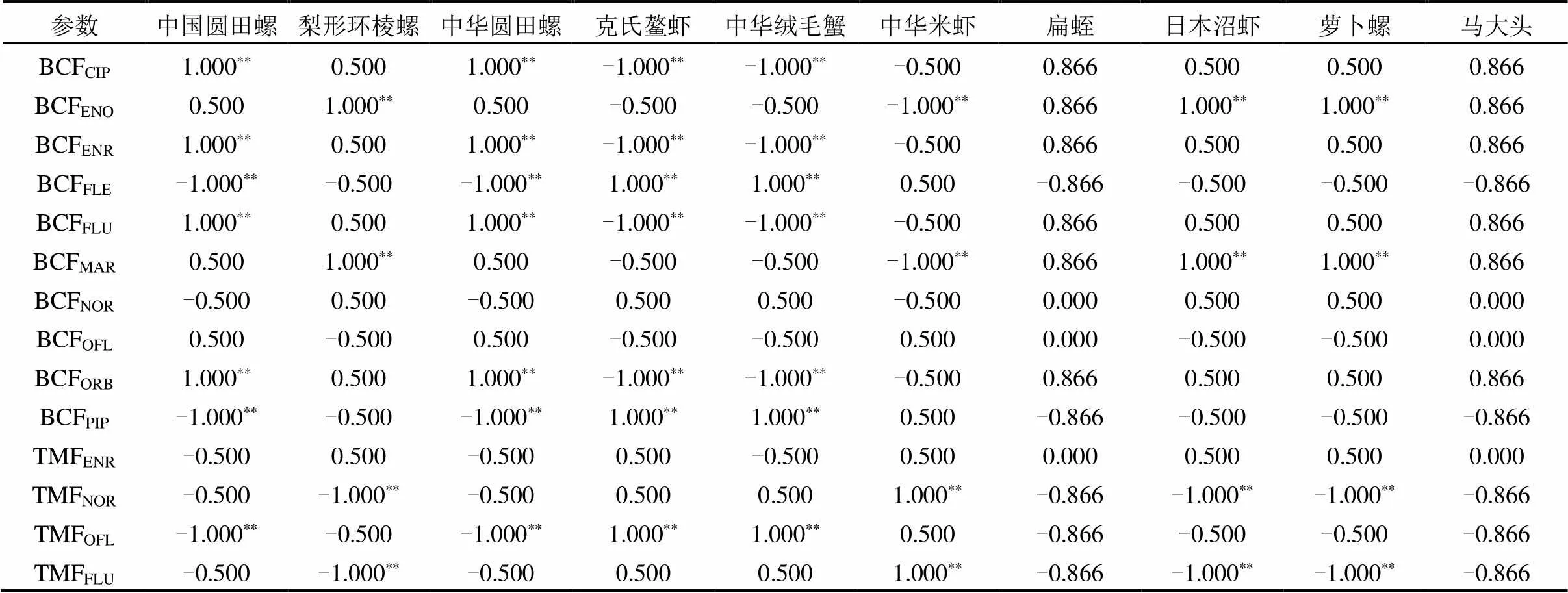
表4 底栖动物生物量与QNs的BCF/TMF的Spearman相关性
注:*表示<0.05水平显著相关;**表示<0.01水平显著相关.
3 讨论
3.1 白洋淀与国内其它湖泊沉积物以及底栖动物中QNs含量比较
将氧氟沙星、环丙沙星、以及恩诺沙星与典型河流沉积物中含量进行对比.结果发现,白洋淀沉积物中氧氟沙星(10.91ng/g)的平均含量高于黄浦江(4.100ng/g)[41]、中国渤海(1.500ng/g)[42]和胶州湾(2.700ng/g)[43],稍高于中国太湖(9.900ng/g)[44];但是其最高含量可达92.83ng/g,远高于黄浦江(12.40ng/g)和太湖(28.40ng/g)沉积物中氧氟沙星含量;环丙沙星(6.870ng/g)平均含量高于中国渤海(4.400ng/g)和胶州湾(1.410ng/g),远高于大亚湾(0.1500ng/g)和海陵岛(0.4500ng/g)[45],稍低于太湖(9.800ng/g),但远低于北意大利波湖(26.15ng/g)[46];恩诺沙星(4.260ng/g)平均含量远高于胶州湾(0.1800ng/g),稍高于渤海(2.000ng/g)和黄浦江(3.200ng/g),最高含量(5.530ng/ g)稍低于渤海(5.700ng/g)和黄浦江(8.900ng/g),高于胶州湾(1.970ng/g)和长江口(4.800ng/g).比较发现白洋淀沉积物中喹诺酮类抗生素污染处于较高水平,应引起重视,加强白洋淀入淀水质、生活污水集中处理以及对畜禽养殖业的管控.
底栖动物中FLU浓度为11.50~51.10ng/g、OFL浓度为3.700~46.60ng/g和NOR浓度为2.460~ 10.50ng/g.与之前白洋淀的研究相比,本研究中底栖动物的QNs浓度低于Li等[22]的研究结果(17.80~ 167.0ng/g),也低于辽河流域[25]底栖动物中QNs的研究结果(770.1ng/g).这可能一方面是因为白洋淀水环境中抗生素浓度降低,进而底栖动物吸收到体内的浓度降低[25-26,28];另一方面可能是由于近几年白洋淀水环境质量好转,底栖动物生物量显著增加[47-49],进而产生了生物量稀释效应.
3.2 QNs的生物累积特征及其与环境因子的相关性
白洋淀3个生境中底栖动物中10种QNs的BCF为219.6L/kg(BCFMAR,生境2)~1754L/kg (BCFENR,生境3),表明QNs在底栖动物的生物富集相对较高[38].BCFENR、BCFNOR、BCFOFL、BCFCIP、BCFENO、BCFFLU、BCFORB、BCFFLE、BCFPIP、BCFMAR的平均值分别为1639,1193,1111,838.5,622.0,523.3, 508.8,467.6,395.3和240.8L/kg,低于白洋淀之前底栖动物QNs的研究(219.0~1990L/kg(BCFCIP)、1720~ 3120L/kg(BCFENR)、246.0~2000L/kg(BCFFLE)、267.0~1400L/kg(BCFOFL))[22];明显低于之前底栖动物体内QNs的研究(4.200~462.4L/kg(BCFNOR)、0.5800~394.0L/kg(BCFCIP)、52.10~2542L/kg (BCFENR)、67.30~3040L/kg(BCFOFL)和4.560~ 587.0L/kg(BCFENO));本研究中的TMFOFL(0.1326~ 0.7648)高于之前的白洋淀[22](0.3800~0.5200)和太湖[23]研究中的值(0.4500~0.5200);环境因子对抗生素的归趋和残留起着重要的作用[50-51],这种现象可能是环境因子在不同地区的时空差异所导致[23,52].
TMFs/BCFs与理化因子的相关性网络和spearman秩相关分析结果显示,大多数理化因子都与TMFs/BCFs显著相关,主要影响因子为和SD;白洋淀之前的研究结果显示,TOC、和SD是影响底栖动物的春季主要影响因子,水体温度是底栖生物生长发育的重要因素,适宜的温度对于底栖动物的生存发展具有促进作用[53-54],而温度及pH值的变化也会改变抗生素在沉积物中的吸附条件,从而解吸到水体中被底栖动物吸收[55-57];也有研究表明,抗生素在水体中存在状态也受环境因子的影响(pH值、、盐度、SD),环境因子的改变会使抗生素降解为离子态存在于水体中[53,58-60],降低水体中抗生素的浓度以及水生生物吸收到体内的抗生素,进而影响抗生素在水生生物体内的生物富集与营养传递.
4 结论
4.1 白洋淀优势底栖动物中QNs存在空间差异,且不同底栖动物中QNs的浓度也存在差异.
4.2 底栖动物对QNs的生物富集系数在生境3最高,其次为生境2和生境1;其中,ENR和NOR呈营养放大,FLU和OFL呈营养稀释.
4.3 多数水体和沉积物理化参数与QNs在底栖动物中的生物富集和传递行为显著相关,尤其是SD和.
4.4 白洋淀底栖动物生物量与QNs的BCF和TMF呈显著负相关,会产生生物量稀释效应.
[1] Zhang Q Q, Ying G G, Pan C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015,49(11):6772-6782.
[2] Zhou L J, Ying G G, Zhao J L, et al. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China [J]. Environmental Pollution, 2011,159(7):1877-1885.
[3] Carvalho I T, Santos L. Antibiotics in the aquatic environments: A review of the European scenario [J]. Environment International, 2016,94:736-757.
[4] Yang S F, Lin C F, Lin Y C, et al. Sorption and biodegradation of sulfonamide antibiotics by activated sludge: Experimental assessment using batch data obtained under aerobic conditions [J]. Water Research, 2011,45(11):3389-3397.
[5] Zhang L L, Qin S, Shen L N, et al. Bioaccumulation, trophic transfer, and human health risk of quinolones antibiotics in the benthic food web from a macrophyte-dominated shallow lake, North China [J]. Science of the Total Environment, 2020,712:136557.
[6] 刘家尊.氟喹诺酮类药物的临床用药及不良反应分析[J]. 中国实用医药, 2019,14(7):125-126.
Liu J Z. Analysis of clinical medication and adverse reactions of fluoroquinolones[J]. Chinese Practical Medicine, 2019,14(7):125-126.
[7] Rossi R, Saluti G, Moretti S, et al. Multiclass methods for the analysis of antibiotic residues in milk by liquid chromatography coupled to mass spectrometry: A review [J]. Food Additives & Contaminants Part A, 2017,35(2):241-257.
[8] Shen L N, Zhang L L, Qin S, et al. The correlation between quinolone antibiotics and microbial community structure and diversity in Baiyangdian Lake [J]. Acta Scientiae Circumstantiae, 2020,40(2):574- 584.
[9] 申立娜,张璐璐,秦 珊,等.白洋淀喹诺酮类抗生素与微生物群落结构和多样性相关性研究[J]. 环境科学学报, 2020,40(2):574-584.
Shen L N, Zhang L L, Qin S, et al. The correlation between quinolone antibiotics and microbial community structure and diversity in Baiyangdian Lake [J]. Journal of Environmental Science, 2020,40(2): 574-584.
[10] Wang Z, Han M, Li E, et al. Distribution of antibiotic resistance genes in an agriculturally disturbed lake in China: Their links with microbial communities, antibiotics, and water quality [J]. Journal of Hazardous Materials, 2020,393:122426.
[11] Zhou L J, Wang W X, Lv Y J, et al. Tissue concentrations, trophic transfer and human risks of antibiotics in freshwater food web in Lake Taihu, China [J]. Ecotoxicology and Environmental Safety, 2020,197: 110626.
[12] Cheng D M, Liu X H, Zhao S N, et al. Influence of the natural colloids on the multi-phase distributions of antibiotics in the surface water from the largest lake in North China [J]. Science of the Total Environment, 2017,578:649-659.
[13] 孙秋根,王智源,董建玮,等.太湖流域河网4种典型抗生素的时空分布和风险评价[J]. 环境科学学报, 2018,38(11):4400-4410.
Sun Q G, Wang Z Y, Dong J W, et al. Spatial-temporal distribution and risk evaluation of four typical antibiotics in river networks of Taihu Lake Basin [J]. Journal of Environmental Science, 2018,38(11):4400- 4410.
[14] 张国栋.南四湖流域典型抗生素时空分布及迁移规律研究[D]. 济南:山东师范大学, 2019:21-26.
Zhang G D. Spatial and temporal distribution and migration of typical antibiotics in nansihu lake basin [D]. JiNan: Shandong Normal University, 2019:21-26.
[15] 刘 昔,王 智,王学雷,等.我国典型区域地表水环境中抗生素污染现状及其生态风险评价[J]. 环境科学, 2019,40(5):2094-2100.
Liu X, Wang Z, Wang X L, et al. Status of antibiotic contamination and ecological risks assessment of several typical Chinese surface- water environments [J]. Environmental Science, 2019,40(5):2094- 2100.
[16] Yao L L, Wang Y X, Tong L, et al. Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, central China [J]. Ecotoxicology and Environmental Safety, 2017,135:236-242.
[17] 陈卫平,彭程伟,杨 阳,等.北京市地下水中典型抗生素分布特征与潜在风险[J]. 环境科学, 2017,38(12):5074-5080.
Chen W P, Peng C W, Yang Y, et al. Distribution characteristics and risk analysis of antibiotic in the groundwater in Beijing [J]. Environmental Science, 2017,38(12):5074-5080.
[18] Zhang R J, Zhang G, Tang J H, et al. Levels, spatial distribution and sources of selected antibiotics in the East River (Dongjiang), South China [J]. Aquatic Ecosystem Health & Management, 2012,15(2): 210-218.
[19] 廖 杰,魏晓琴,肖燕琴,等.莲花水库水体中抗生素污染特征及生态风险评价[J]. 环境科学, 2020,41(9):205-211.
Liao J, Wei X Q, Xiao Y Q, et al. Pollution characteristics and risk assessment of antibiotics in Lianhua Reservoir [J]. Environmental Science, 2020,41(9):205-211.
[20] Liu K, Yin X F, Zhang D L, et al. Distribution, sources, and ecological risk assessment of quinotone antibiotics in the surface sediments from Jiaozhou Bay wetland, China [J]. Marine Pollution Bulletin, 2018, 129(2):859-865.
[21] Liu S S, Zhao H X, Lehmler H J, et al. Antibiotic pollution in marine food webs in Laizhou Bay, North China: Tropho dynamics and human exposure implication [J]. Environmental Science & Technology, 2017, 51(4):2392-2400.
[22] Li W H, Shi Y L, Gao L H, et al. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China [J]. Chemosphere, 2012,89:1307-1315.
[23] Xie Z X, Lu G H, Yan Z H, et al. Bioaccumulation and trophic transfer of pharmaceuticals in food webs from a large freshwater lake [J]. Environmental Pollution, 2017,222:356-366.
[24] Han Q F, Zhao S, Zhang X R, et al. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China [J]. Environment International, 2020,138:105551.
[25] Bai Y , Meng W , Xu J , et al. Occurrence, distribution and bioaccumulation of antibiotics in the Liao River Basin in China [J]. Environmental Science Processes & Impacts, 2014,16(3):586-593.
[26] Qi W, Cgpab C, Yhwab C, et al. Antibiotics in a subtropical food web from the Beibu Gulf, South China: Occurrence, bioaccumulation and trophic transfer [J]. Science of The Total Environment, 2020,751: 141718.
[27] Chen H, Shan L, Xu X R, et al. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure [J]. Marine Pollution Bulletin, 2015,90(1/2):181-187.
[28] Cheng D M, Liu X H, Wang L, et al. Seasonal variation and sediment-water exchange of antibiotics in a shallower large lake in North China [J]. Science of the Total Environment, 2014,476-477: 266-275.
[29] 申立娜,张璐璐,秦 珊,等.白洋淀喹诺酮类抗生素污染特征及其与环境因子相关性研究[J]. 环境科学学报, 2019,39(11):3888-3897.
Shen L N, Zhang L L, Qin S, et al. The occurrence and distribution of quinolones (QNs) and correlation analysis between QNs and physical-chemical parameters in Baiyangdian Lake, North China [J]. Journal of Environmental Science, 2019,39(11):3888-3897.
[30] Cheng D M, Liu X H, Zhao S N, et al. Influence of the natural colloids on the multi-phase distributions of antibiotics in the surface water from the largest lake in North China [J]. Science of the Total Environment, 2017,578:649-659.
[31] Zhang L L, Shen L N, Qin S, et al. Quinolones antibiotics in the Baiyangdian Lake, China: Occurrence, distribution, predicted no-effect concentrations (PNECs) and ecological risks by three methods [J]. Environmental Pollution, 2020,256:113458.
[32] Zhang L L, Qin S, Shen L N, et al. Bioaccumulation, trophic transfer, and human health risk of quinolones antibiotics in the benthic food web from a macrophyte-dominated shallow lake, North China [J]. Science of the Total Environment, 2020,712:136557.
[33] 刘 珂.胶州湾典型海岸带沉积物中喹诺酮抗生素时空分布特征及风险评价[D]. 青岛:青岛大学, 2018.
Liu K. Spatial, temporal distribution and risk assessment of quinotine antibiotics in the surface sediments of coastal zone, Jiaozhou Bay [D]. Qingdao: Qingdao University, 2018.
[34] 郭宁宁,李 希,周训军,等.亚热带丘陵区人工湿地底栖动物群落特征研究[J]. 中国环境科学, 2021,41(2):930-940.
Guo N N, Li X, Zhou X J, et al. Characteristics of zoobenthos community in constructed wetlands in subtropical hilly area [J]. China Environmental Science, 2021,41(2):930-940.
[35] Arnot J A, Gobas F A. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms [J]. Environmental Reviews, 2006,14(4):257-297.
[36] Post, D M. Using stable isotopes to estimate trophic position: models, methods, and assumptions [J]. Ecology, 2002,83(3):703-718.
[37] Gujarathi N P, Han B, Linden J C, et al. Phytoremediation of Oxytetracycline from Wastewater [C]. The 2005Annual Meeting, 2005.
[38] Iglesias C, Meerhoff M, Johansson L S, et al. Stable isotope analysis confirms substantial differences between subtropical and temperate shallow lake food webs [J]. Hydrobiologia, 2017,784(1):111-123.
[39] Ponsard V S. Sources of Variation in Consumer-Diet15N Enrichment: A Meta-Analysis [J]. Oecologia, 2003,136(2):169-182.
[40] 周品成,刘希强,康兴生,等.4种水生植物对兽用抗生素去除效果比较[J]. 华南农业大学学报, 2019,40(6):67-73.
Zhou P C, Liu X Q, Kang X S, et al. Removal effects of four aquatic plants on veterinary antibiotics [J]. Journal of South China Agricultural University, 2019,40(6):67-73.
[41] 申立娜,张璐璐,秦 珊,等.白洋淀喹诺酮类抗生素污染特征及其与环境因子相关性研究[J]. 环境科学学报, 2019,39(11):3888-3897.
Shen L N, Zhang L L, Qin S, et al. The occurrence and distribution of quinolones (QNs) and correlation analysis between QNs and physical-chemical parameters in Baiyangdian Lake, North China [J]. Acta Scientiae Circumstantiae, 2019,39(11):3888-3897.
[42] Chen K, Zhou J L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China [J]. Chemosphere, 2013,95:604- 612.
[43] Liu X H, Zhang H B, Li L Z, et al. Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries [J]. Marine Pollution Bulletin, 2016,109(1): 597-602.
[44] 刘 珂,张道来,刘 娜,等.胶州湾海岸带表层沉积物中典型喹诺酮类抗生素污染特征研究[J]. 海洋环境科学, 2017,36(5):655-661.
Liu K, Zhang D L, Liu N, et al. Investigation on the typical quinolone antibiotics in the surface sediments of Jiaozhou bay, China [J]. Marine Environmental Science, 2017,36(5):655-661.
[45] Xu J, Zhang Y, Zhou C B, et al. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China [J]. Science of the Total Environment, 2014,497-498: 267-273.
[46] He X T, Wang Z H, Nie X P, et al. Residues of fluoroquinolones in marine aquaculture environment of the Pearl River Delta, South China [J]. Environmental Geochemistry and Health, 2012,34(3):323-335.
[47] Calamarid D, Zuccato E, Castiglioni S, et al. Strategic survey of therapeutic drugs in the rivers Po and Lambro in North-ern Italy [J]. Environmental Science & Technology, 2003,37(7):1241-1248.
[48] Cai Y J, Gong Z J, Qin B Q. Benthic macroinvertebrate community structure in Lake Taihu, China: Effects of trophic status, wind-induced disturbance and habitat complexity [J]. Journal of Great Lakes Research, 2012,38(1):39-48.
[49] Cai Y J, Gong Z J, Xie P. Community structure and spatiotemporal patterns of macrozoobenthos in Lake Chaohu (China) [J]. Aquatic Biology, 2012,17(1):35-46.
[50] 佟霁坤.近十年白洋淀水质特征及营养状态分析[D]. 保定:河北大学, 2020.
Dong J K. Analysis of water quality characteristics and nutrient status in Baiyang Lake in recent ten years [D]. Baoding :Hebei University, 2020.
[51] Wenk J, Von Gunten U, Canonica S. Effect of dissolved organic matter on the transformation of contaminants induced by excited triplet states and the hydroxyl radical [J]. Environmental Science & Technology, 2011,45(4):7945.
[52] Petrie B, Barden R, Barbara K H. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring [J]. Water Research, 2015,72:3-27.
[53] Du B, Haddad S P, Luek A, et al. Bioaccumulation of human pharmaceuticals in fish across habitats of a tidally influenced urban bayou [J]. Environmental Toxicology and Chemistry, 2016,35(4):966- 974.
[54] 陈泽豪,杨 文,王 颖,等.白洋淀大型底栖动物群落结构及其与环境因子关系[J]. 生态学杂志, 2021,40(7):2175-2185.
Chen Z H, Yang W, Wang Y, et al. Community structure of macrozoobenthos and its relationship with environmental factors in Baiyangdian Lake [J]. Journal of Ecology, 2021,40(7):2175-2185.
[55] 李光锦.赣江干流底栖动物群落结构及其与环境因子的关系[D]. 南昌:南昌工程学院, 2019.
Li G J. The characteristics of zoobenthos community and their relationships to environment factors in Mainstem of Gnjiang River [D]. Nanchang :Nanchang Institute of Technology, 2019.
[56] 李 娟,王沛芳,纪靓靓,等.抗生素在沉积物中的吸附机制及其影响因素[J]. 四川环境, 2013,32(2):87-88,94-95,93.
Li J, Wang P F, Ji L L, et al. Mechanisms and Influencing Factors for Sorption of Antibiotics in Sediments [J]. Sichuan Environment. 2013,32(2):87-88,94-95,93.
[57] 孔慧敏,杨四福,迟宝明,等.喹诺酮类抗生素在水环境中降解和吸附影响因素[J]. 科学技术与工程, 2021,21(3):1196-1201.
Kong H M, Yang S F, Chi B M, et al. Factors influencing the degradation and absorption of Quinolone antibiotics in aqueous environment [J]. Science Technology and Engineering, 2021,21(3): 1196-1201.
[58] Dorival G N, Zafra G A, Navalon A, et al. Removal and degradation characteristics of quinolone antibiotics in laboratory- scale activated sludge reactors under aerobic, nitrifying and anoxic conditions [J]. Journal of Environmental Management, 2013,120:75-83.
[59] Peng K, Qin B Q, Cai Y J, et al. Water column nutrient concentrations are related to excretion by benthic invertebrates in Lake Taihu, China [J]. Environmental Pollution, 2020,261:114161.
[60] Yang Y F, Yi Y J, Zhou Y, et al. Spatio-temporal variations of benthic macroinvertebrates and the driving environmental variables in a shallow lake-Science direct [J]. Ecological Indicators, 2019,110: 105948.
The bioaccumulation characteristics of quinolones (QNs) in macrobenthos-A case study of Baiyangdian Lake.
FU Yu1, JU Ze-jia1, CHEN Hui1, ZHAO Xin-yu1, SONG Yuan-meng1, WANG Guang-zhi1, ZHANG Lu-lu1,2*, CUI Jian-sheng1,2
(1.Academy of Environmental Science, Hebei University of Science and Technology, Shijiazhuang 050000, China;2.Biotechnology laboratory for pollution control in Hebei Province, Shijiazhuang 050000, China)., 2022,42(2):878~888
The concentrations of quinolones (QNs) in water and macrobenthos samples were detected by high performance liquid chromatography tandem mass spectrometry (HPLC-MS). Correlation analysis was conducted between the characteristics of bioaccumulation for QNs in macrobenthos and environmental parameters in Baiyangdian Lake. The results showed that: The concentrations of ΣQNs in water varied from 0.7380 to 2004ng/L, the average concentrations of FLU (168.0ng/L) were the highest and the detection frequency of ofloxacin (OFL) and norfloxcin (NOR) in macrobenthos was the highest (Freq=100%), followed by flumequine (FLU) and enrofloxa (ENR) (Freq³50%), while the detection rate of other QNs was less than 40% (Freq£40%), While in the macrobenthos, the concentrations of ΣQNs varied from 23.70ng/g to 289.4ng/g, thereinto, the average concentrations of FLU(29.43ng/g) and CIP(40.38ng/g) were the highest; The bioaccumulation factors (BCF) of QNs in macrobenthos were in the range of 219.6 (BCFMAR)~1754 (BCFENR) L/kg, which indicated that the bioaccumulation of QNs was high in macrobenthos; The trophic magnification factors (TMF) of OFL, ENR, and NOR varied from 0.1299 (TMFFLU) to 6.426 (TMFENR), indicating NOR and ENR appeared trophic magnification, while FLU and OFL appeared trophic dilution; Through correlation analysis, the results showed that the BCF of ENR、CIP、FLU and ORB were significant positively correlated with water depth (WD), temperature (), secchi depth (SD), dissolved oxygen (DO), and total organic carbon (TOCs), while significant negatively correlated with chemical oxygen demand (COD), total phosphorus (TP), total nitrogen (TN), nitrate nitrogen (NO3--N), PO43-, total carbon (TCs), total phosphorus (TNs), and NH3-Ns; TMFOFLwas significantly positive correlation to COD, TP, TN, NO3-N, PO43-, TCs, TNs and NH3-Ns, while significant negatively correlated with WD,, SD, DO and TOCs, indicating that domestic sewage and aquaculture wastewater contributed the most to QNS.
Baiyangdian Lake;quinolones;dominant macrobenthos;bioaccumulation;spatial distribution;environmental parameters
X171.5
A
1000-6923(2022)02-0878-11
付 雨(1995-),女,河北唐山人,河北科技大学硕士研究生,主要从事湖泊生态学相关研究.发表论文1篇.
2021-06-28
河北省自然科学基金资助项目(D2019208152);河北省高等学校科学技术研究重点项目(ZD2021046)
* 责任作者, 副教授, zhanglulu19850703@163.com

