红土镍矿基材料吸附及有氧降解水体污染物
王冰凝,刘守军,3,杨 颂,陈亮宇,刘兴阳,李 晋,上官炬**
红土镍矿基材料吸附及有氧降解水体污染物
王冰凝1,2,刘守军1,2,3,杨 颂2,3*,陈亮宇2,4,刘兴阳2,3,李 晋2,4,上官炬1,2**
(1.太原理工大学,煤科学与技术重点实验室,山西 太原 030024;2.山西省民用洁净燃料工程研究中心,山西 太原 030024;3.太原理工大学化学化工学院,山西 太原 030024;4.太原科瑞康洁净能源有限公司,山西 太原 030006)
以红土镍矿为研究对象,考察了原矿(HT)及改性矿(HT-FeNi)去除水体中罗丹明B(RhB)的效果.借助XRD、BET、IR等表征手段,结合吸附动力学和等温吸附模拟研究了HT吸附RhB的过程及机制.结果表明:HT的孔隙结构较为丰富,有良好的RhB吸附性能.当HT添加量为0.2g/L时,RhB去除率为39.03%,吸附量达到93.80mg/g.HT添加量增加,RhB去除效果增强,平衡吸附量减小.HT吸附RhB的过程更符合准二级动力学,包含表面扩散及颗粒内扩散两个步骤.等温吸附模型拟合发现Freundlich能够准确描述HT吸附RhB的过程.1/<0.5,表明吸附过程较易进行.HT经5次循环实验后,吸附量仍能达到39.67mg/g,表明HT有较好的循环使用性能.HT吸附RhB主要归因于Si-O吸附位点.通过气基还原制备得到改性矿(HT-FeNi).采用SEM、XRD、BET、XPS等手段对HT-FeNi进行表征分析,并考察了HT-FeNi降解RhB的效果.结果表明:HT-FeNi比表面积小(14.374m2/g),主要成分为铁镍双金属.HT-FeNi不能通过吸附作用去除RhB,而HT-FeNi/Air/pH=3体系在40min内RhB降解效率为94%.捕获活性氧物种的实验证明,HT-FeNi/Air/pH=3体系去除RhB过程中起主要作用的活性氧物种是羟基自由基(·OH).在酸性条件下,HT-FeNi通过活化O2生成·OH, Ni0诱导的Fe2+/Fe3+循环促使HT-FeNi/Air/pH=3体系生成更多的·OH.将HT-FeNi/Air/pH=3体系应用于去除水体中甲基橙(MO)和二硝基氯苯(DNCB),去除效率分别为47%、78%.
红土镍矿基材料;吸附;有氧降解;水体污染物
水体污染广泛存在,严重威胁人类健康.现有各种技术应用于去除水体污染物,如物理吸附、生物处理、高级氧化技术、膜处理技术等.其中,吸附法具有操作简便、价格低廉等特点,是处置废水的重要方法.常用的吸附剂有炭质材料[1]、金属氧化物[2]、生物材料[3]、天然矿土[4]等.炭质吸附剂及金属氧化物费用高,生物材料吸附剂制备工艺复杂,实际应用受限.天然矿土材料廉价易得、环境友好,在环境废水处置方面受到越来越多的关注.而高级氧化技术具有优异的废水处置效果,是近年水体环境修复研究的热点.铁基材料,尤其是纳米零价铁和铁基双金属[5]常用于高级氧化技术,但纳米材料制备成本高、能耗较高,严重制约其广泛应用.
红土镍矿储量丰富,价格低廉,其黏土状结构及富含铁、铝、镍和钙等活性成分的特性使其在替代污染物吸附剂和催化剂方面极具潜力. Mohapatra等[6]将改性红土镍矿应用于吸附去除水体中Cd2+, Fu等[7]以红土镍矿为原料制备了磁性复合吸附剂并考察其对水体中三甲胺的吸附性能.红土镍矿基材料除了作为水体污染物吸附剂,也可应用于类Fenton体系.Han等[8]利用红土镍矿浸出液合成Cu掺杂(Mg,Ni)(Fe,Al)2O4,通过非均相类Fenton体系高效降解有机污染物,但该体系需要添加H2O2.系统探讨红土镍矿原矿吸附作用及改性矿简单体系下有氧降解水体污染物具有重要意义.
本研究以红土镍矿为研究对象,分别探讨原矿(HT)和改性矿(HT-FeNi)去除水体中罗丹明B.结合仪器表征、动力学实验与等温吸附模拟等方法,提出HT吸附RhB机理;分析改性矿(HT-FeNi)有氧降解罗丹明B过程中体系变化,探讨有氧降解机理;考察了HT-FeNi去除甲基橙(MO)和二硝基氯苯(DNCB)等污染物的效果,旨在为水体污染物的高效去除与低品位资源的高值化利用提供参考.
1 材料与方法
1.1 材料
本实验药品除甲醇为色谱纯外,其他均为分析纯.超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和二苯碳酰二肼(C13H14N4O)购自西格玛奥德里奇(上海)贸易有限公司.其他药品均购自国药化学试剂有限公司.实验用水均为去离子水.
HT为来自印尼苏拉威西岛的低品位红土镍矿; HT-FeNi的制备方法:将40g红土镍矿和10gNa2S2O3填装到反应器内,打开水冷装置,通入保护气体N2(气速为100mL/min),当温度升至设定温度后,通入H2/N2(体积比:45/55),总气速控制为300mL/min,120min后关闭升温装置,在N2保护下将样品冷却至室温,制样至粒度为-0.2mm后,取5g样品,在2A电流强度下磁选分离并烘干.
1.2 分析方法
采用X射线衍射分析仪(XRD)、比表面积和孔隙度分析仪(BET)、红外光谱仪(IR)、扫描电镜(SEM)、X射线光电子能谱仪(XPS)对HT和HT- FeNi进行表征.
RhB、MO浓度测定均采用紫外-可见分光光度法[9-10].采用总有机碳仪(TOC)测定矿化效率.采用高效液相色谱仪(HPLC)测定DNCB浓度,配备的色谱柱为Trace1300.实验参数:紫外检测器波长254nm,柱温35℃,进样量10μL.流动相为45%甲醇和55%水,流速为1.0mL/min[11].用1,10-邻菲罗啉比色法在510nm波长下测定铁[12],镍含量测定采用丁二酮肟光度法[13].采用原子吸附光谱仪(AAS)测定体系中镍离子浓度.采用荧光分光光度法测定体系中双氧水(H2O2)和羟基自由基(·OH)的生成[14-15].利用气质联用仪(GC-MS)测定RhB降解过程中产生的中间产物.用二氯甲烷(CH2Cl2)作溶剂萃取RhB降解溶液中间产物,45℃旋蒸后再用1mL丙酮(CH3COCH3)复溶,将复溶后的液体转移至气质专用瓶进行GC-MS检测.色谱柱规格为30m×0.25mm× 0.25μm,初始柱温为80℃,维持8min后以4℃/min的速率升至280℃保持10min.载体流速为1.0mL/min,进样体积为1μL,传输线温度为280℃,离子源温度为230℃,全扫描模式,质核比扫描范围为50~1000,所用对比谱库NIST05[16].
1.3 吸附实验
1.3.1 吸附动力学实验 准确称取一定质量的HT于100mL锥形瓶中,加入50mL浓度为50mg/L的RhB溶液,在(25±1)℃下恒温震荡,间隔一定时间取样测定,利用式(1)~(2)分别计算不同时间吸附材料吸附量()和RhB去除率().
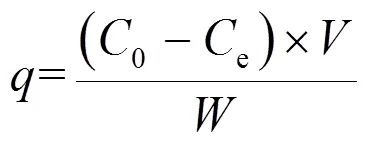

式中:为时刻HT对RhB的吸附量,mg/g;0为溶液的初始浓度,mg/L;e为吸附后溶液的浓度, mg/L;为溶液体积,L;为HT质量,g;为时刻RhB去除率.
采用准一级[17]、准二级[18]和颗粒内扩散[19]3种动力学方程拟合材料吸附RhB的动力学行为.
1.3.2 等温吸附实验 准确称取一定质量HT于100mL锥形瓶中,分别加入50mL一系列浓度(10,25,50,75,100)mg/L的RhB溶液,在温度为(25±1)℃条件下震荡24h,吸附达到平衡后取样测定.
采用Langmuir[20]、Freundlich[21]和Temkin[22]3种等温吸附方程对实验数据进行拟合.
1.3.3 循环使用性能评价实验 吸附实验完成后过滤、洗涤收集HT,于60℃恒温干燥后继续作为吸附剂使用.HT的添加量为0.4g/L.
1.4 降解实验
取50mL初始浓度为25mg/L的RhB溶液于100mL锥形瓶中,加入0.2g/L的HT-FeNi,鼓入空气/氩气(1.5L/min)开始反应.间隔10min取样,样品经0.22μm滤膜过滤后测定其浓度.使用1mol/L的NaOH和HCl溶液调节体系初始pH值.
MO溶液初始浓度为20mg/L;DNCB溶液初始浓度为10mg/L,材料加入量为1g/L,取样间隔时间为30min.其他过程与HT-FeNi降解RhB的实验相同.
2 结果与讨论
2.1 HT吸附RhB研究
2.1.1 HT理化特性分析 HT主要元素中Ni、Fe含量为1.41%、21.41%,Mg、Si、Al含量分别为8.75%、13.70%、1.85%.由图1a可见,HT主要晶相为蛇纹石Mg3Si2O5(OH)4、针铁矿FeOOH、石英SiO2.由图1b和1c可见,吸附后HT样品表示为RHT.HT及RHT均显示典型的Ⅳ型等温线,H3滞后环.HT吸附前后主要孔均为2~20nm的中孔,还有少量孔径大于50nm的大孔,说明HT和RHT均为介孔材料,具有较为丰富稳定的孔隙结构.HT比表面积为117.94m2/g,总孔容积为0.2101cm3/g,平均孔径为7.12nm,较大的比表面积和丰富的孔隙结构有利于吸附有机染料[23].而RHT比表面积和总孔容积均降低,分别为82.41m2/g和0.1855cm3/g;平均孔径增大至9.00nm.这可能是由于吸附过程中RhB堵塞及部分孔坍塌造成的.

由图1d可知, HT及RHT主要出现了蛇纹石类矿物的红外光谱特征[24].在高频区,3684cm-1为八面体层阳离子Mg所引起的面外OH伸缩震动; 3412cm-1的弱吸收峰为八面体层面内OH伸缩震动;1631cm-1为H2O的弯曲振动吸收峰.在低频区,1011cm-1为Si-O伸缩振动吸收峰,628和455cm-1分别为OH摆动带和Si-O弯曲振动峰.此外798cm-1处还出现了石英所引起的吸收峰.吸附RhB后,1011和455cm-1处的Si-O吸收峰明显减弱,这说明可能是HT表面的Si-O官能团主要参与了RhB的吸附,为RhB的吸附提供了吸附位点.
2.1.2 HT吸附RhB效果实验 在初始浓度为50mg/L的RhB溶液中分别加入0.2,0.4,0.6g/L HT,吸附效果如图2所示.随着HT添加量的增加,去除RhB的效果增强,吸附量下降.HT添加量由0.2g/L增加到0.6g/L,HT去除RhB效率由39.03%提高至75.24%;吸附量由93.80mg/g下降至60.25mg/g.HT添加量越多,其吸附容量利用越不充分.
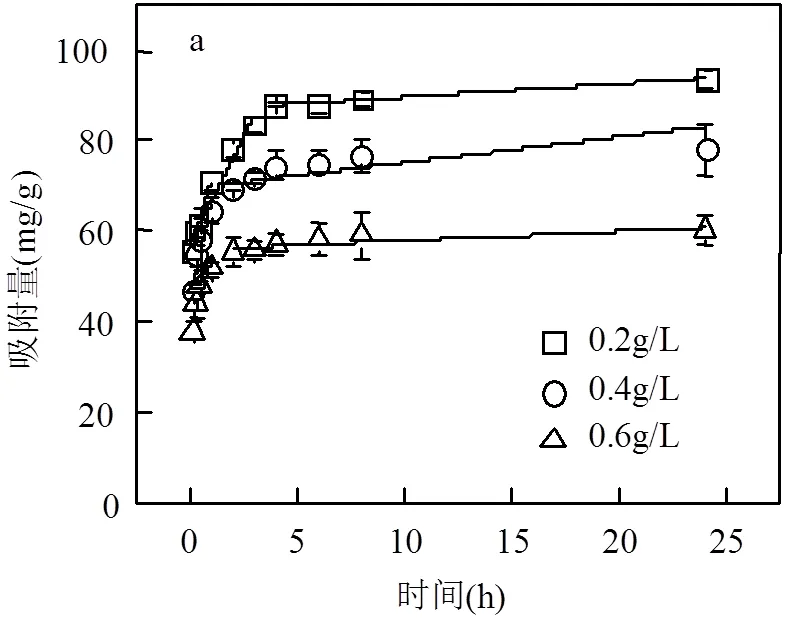
2.1.3 吸附动力学 由表1可见,准一级和准二级方程的相关系数(2)分别为0.8924~0.9639、0.9622~0.9957,均达到显著水平,1和2变化明显,而准一级动力学模型拟合的平衡吸附量e,cal与实验值e,exp差别较大;准二级动力学方程拟合所得e,cal与e,exp更为接近,拟合性更好,能够准确描述吸附过程.
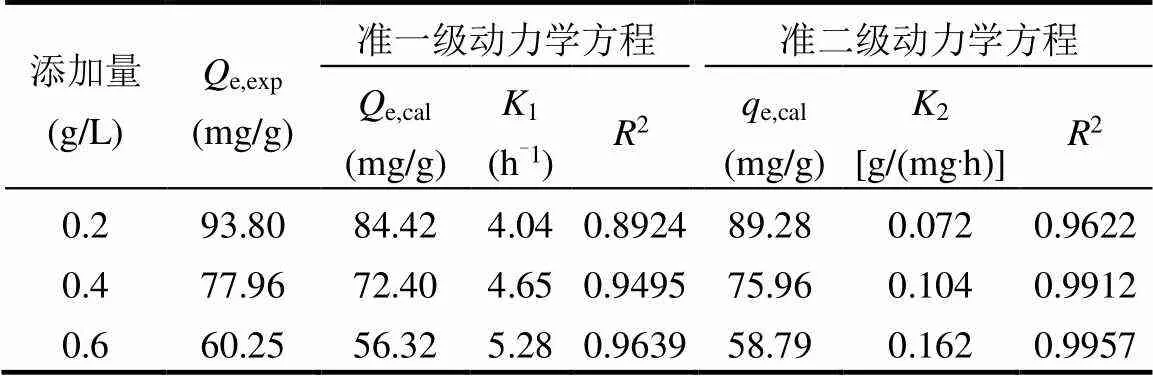
表1 HT吸附RhB的动力学参数
注:e,exp为实验所得平衡吸附量,mg/g;e,cal为准一级动力学方程拟合计算所得平衡吸附量,mg/g;e,cal为准二级动力学方程拟合计算所得平衡吸附量,mg/g;1,2分别为速率常数,h-1,g/(mg·h).
为了进一步研究溶液中RhB在HT上的扩散机理,利用颗粒内扩散模型对数据进行拟合.由图2a可知,HT吸附RhB包括2个阶段:第一阶段吸附速率较快,相应拟合直线截距不为零,说明除颗粒内扩散外,表面扩散也影响吸附过程;第二阶段为吸附材料颗粒的孔内扩散,速率较低,说明颗粒内扩散为影响吸附速度的控制步骤[17].
2.1.4 等温吸附模拟 由图3、表2可知,Temkin方程拟合相关系数较低,不适用于描述RhB吸附过程;Langmuir和Freundlich方程拟合相关系数高,都能较好地描述HT吸附RhB的过程, Freundlich吸附模型拟合结果更好.表明HT吸附RhB过程以多分子层吸附为主,同时伴有单分子层吸附. Freundlich模型中,反映吸附位点能量分布特征,值越大,表征吸附强度越大;F反映吸附能力的强弱,F值越大,表征吸附能力越大[25-26].一般认为, 1/>2,吸附过程困难;0.1<1/<0.5,吸附较为容易[27].HT吸附RhB,1/为0.2094~0.2406,吸附过程较为容易.当HT投加量为0.4g/L时,1/最小,吸附强度最大.
2.1.5 循环使用性能评价 如图4所示, HT经5次循环实验后,吸附量仍能达到39.67mg/g,去除效率仍可保持35%,表明HT具有较好的循环使用性能.

图3 RhB吸附等温线的非线性拟合
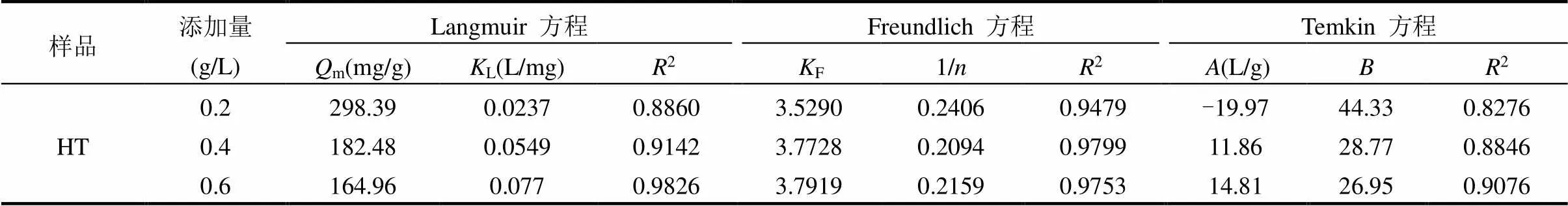
表2 HT吸附RhB等温方程参数
注:m为饱和吸附量,mg/g;L为Langmuir平衡常数;F为Freundlich平衡常数;为无量纲常数.
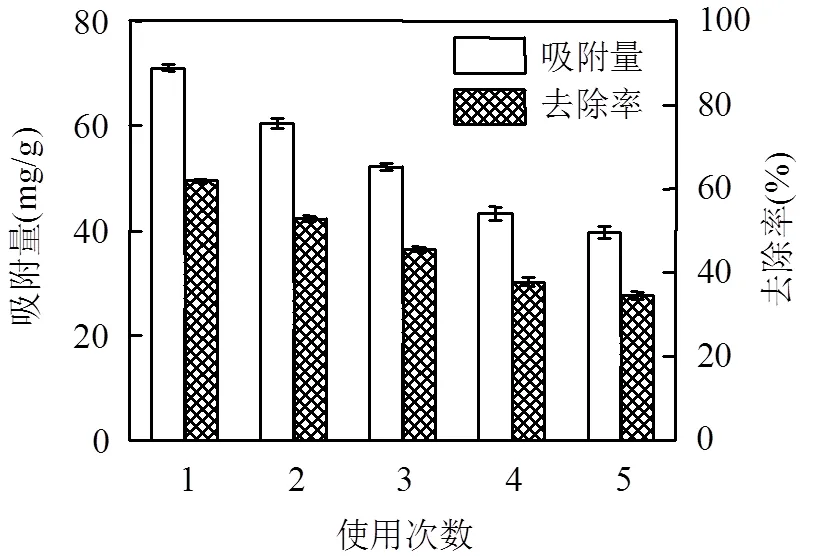
图4 HT吸附RhB循环使用效果
2.2 HT-FeNi降解RhB研究
2.2.1 HT-FeNi理化特性分析 由图5a~e可知, HT-FeNi是由大小在10~100μm左右的粒子组成,其表面除了均匀分布的铁和镍,还含有氧元素,这可能是由于制备过程中表面零价铁和零价镍氧化生成了铁氧化物和镍氧化物.图5f表明,HT-FeNi主要结晶相为铁镍合金、Ca2Mg0.7Fe0.6Si1.7O7和镁橄榄石.测得HT-FeNi的主要成分为零价铁和零价镍,分别占85.68%和13.23%.结合XRD分析,HT-FeNi的主要组成为铁镍双金属.图5g表明,在最大相对压力下, HT-FeNi的吸附容量为21.51cm3/g.其孔隙体积为0.033cm3/g,比表面积为14.374m2/g.HT-FeNi的比表面积远小于HT.由图5h~j可知,HT-FeNi表面存在碳、铁、镍、氧等元素.表面碳可能是样品制备过程暴露于大气中导致的[28]. 711.1和724.6eV左右的峰对应Fe2O3中Fe(III)的2p3/2和2p1/2结合能.FeO中Fe(II)的2p3/2和2p1/2结合能分别为709.5和722.8eV左右.Ni(II)2p的XPS光谱在855.0和860.9eV处拟合出2个峰,这是Ni(II)在NiO中的Ni2p1/2和Ni2p3/2的特征.XPS光谱图未检测到零价铁和零价镍的信号峰,这是由于HT-FeNi表面覆盖了较厚的铁氧化物和镍氧化物,这与SEM-EDS结果一致.
2.2.2 HT-FeNi降解RhB效果实验 如图6所示, HT-FeNi无法通过吸附作用去除RhB,这可能是其比表面积过小导致的.HT-FeNi主要成分为铁镍合金,但其较厚的氧化层会阻碍降解反应进行,故调节体系pH值至酸性,考察HT-FeNi去除RhB的效果.当调节体系初始pH值为3时,空气气氛下HT-FeNi去除RhB能力较强,40min内去除效率达94%.去除过程遵循准一级动力学,去除速率常数达到0.0703min-1.为了进一步探究氧气在HT-FeNi去除RhB过程中的作用,同时进行了氩气气氛下HT-FeNi去除RhB效果实验.结果表明,氩气气氛下HT-FeNi在40min内去除效率为12%.由此可知,在空气气氛下,HT-FeNi高效去除RhB是氧化和还原共同作用的结果,而氧气在HT-FeNi去除RhB的过程中起着至关重要的作用.氩气气氛下,HT-FeNi去除RhB可能归因于吸附在还原剂表面的原子氢直接还原RhB.零价铁可以将H+原位还原为气态H2(式3).镍是加氢催化剂,吸附在HT-FeNi表面的H2进一步离解为活性H原子(式4)[29].
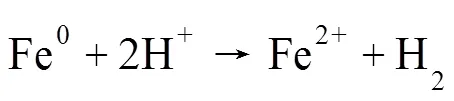
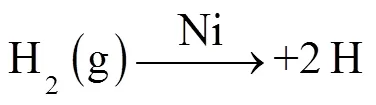
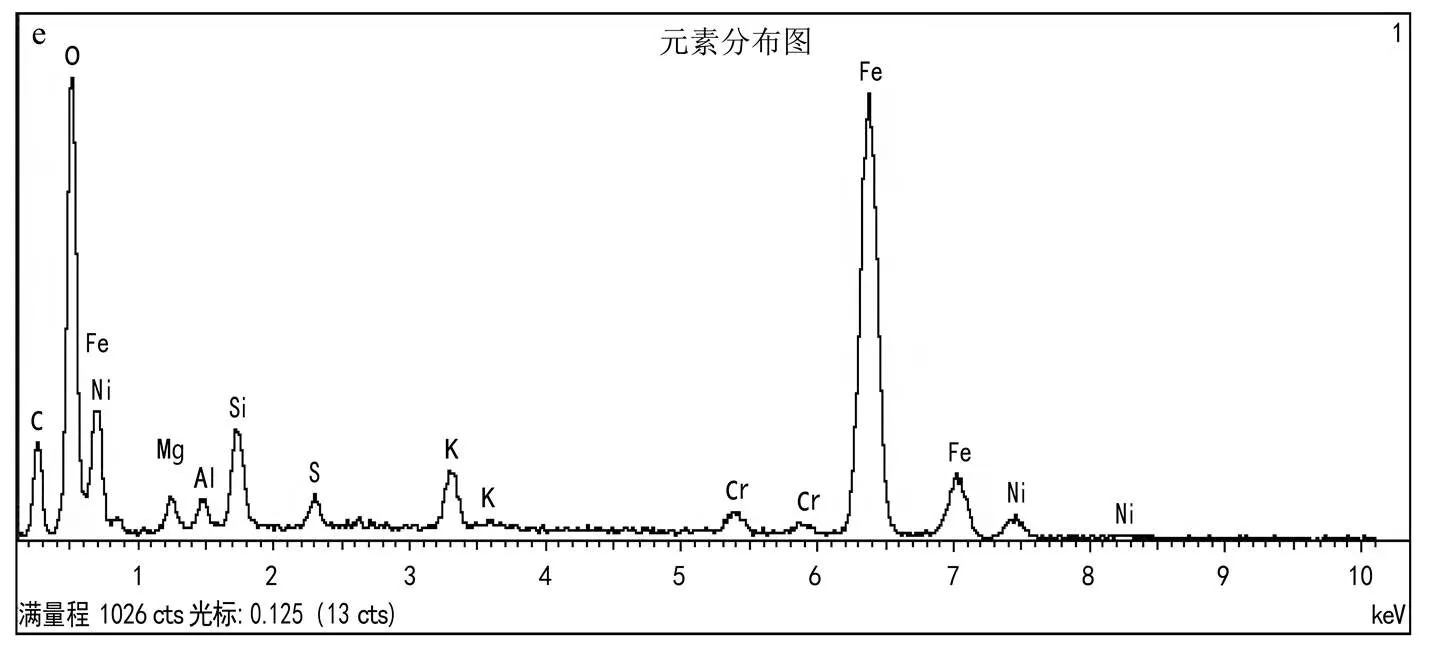
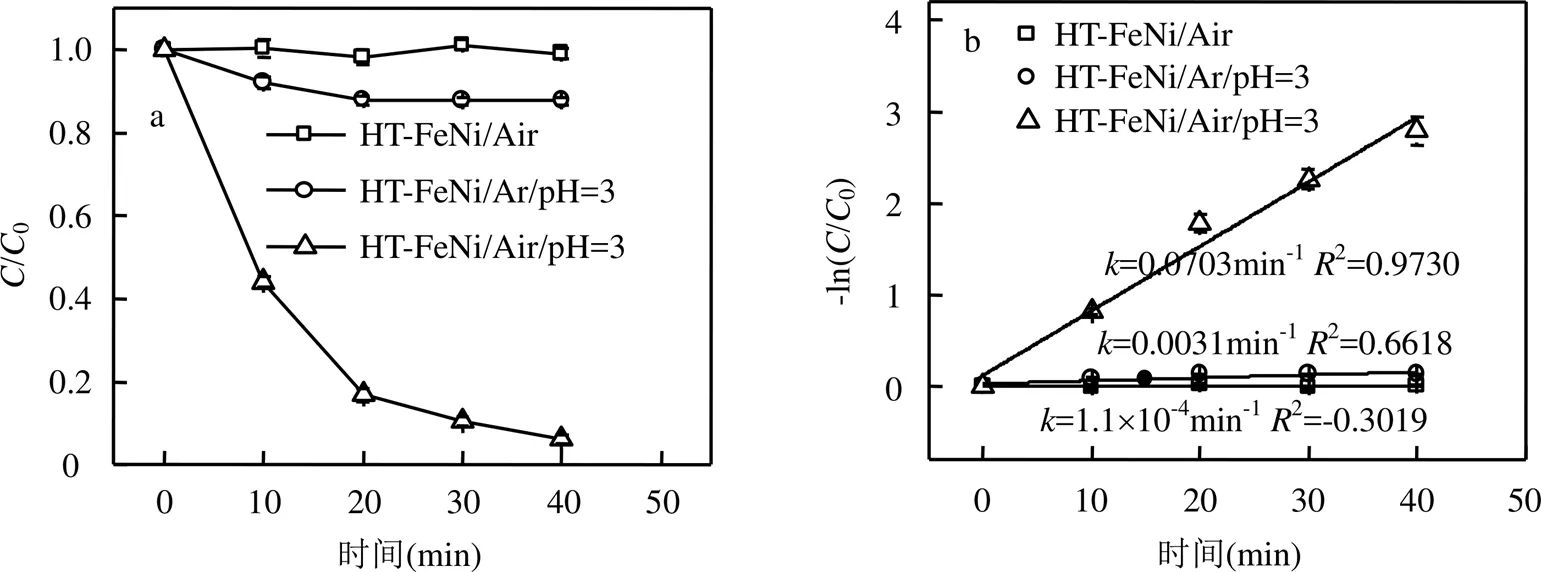
图6 HT-FeNi降解RhB效率
2.2.3 HT-FeNi降解RhB机理研究 (1)活性氧物种捕获实验:如图7所示,向体系中加入3种活性氧物种捕获剂后,降解效率均有降低.加入TBA捕获·OH后,降解效率由94%降至18%;加入CAT捕获H2O2后,降解效率降低至38%;加入SOD捕获·O2-后,降解效率降至71%.由此可知,HT-FeNi去除RhB过程中起主要作用的是·OH.

图7 加入ROS捕获剂后HT-FeNi/Air/pH=3体系降解RhB效率
(2)活性氧物种及金属离子测定:采用荧光探针法检测HT-FeNi/Air/pH=3体系中·OH和H2O2的生成情况(图8a),结果表明HT-FeNi/Air/pH=3体系降解RhB过程中生成了·OH和H2O2,验证了活性氧物种捕获实验的可靠性.由图8b可见,随着反应时间的延长,Fe2+、Fe3+和Ni2+浓度均呈增大趋势.HT- FeNi/Air/pH=3体系反应40min后,镍的溶出量为2.37mg/L,远小于体系中铁的溶出量,这是由于HT- FeNi中铁含量远大于镍含量,且在酸性环境中铁更容易失电子.HT- FeNi/Air/pH=3体系反应40min后,水相体系中Fe2+含量大于Fe3+,分别为11.55和8.85mg/L.体系可通过多个途径释放Fe2+.Fe0将电子转移到H+生成H2和Fe2+[式(3)];也可将转移电子到O2生成H2O2和Fe2+(式5),从而通过Fenton反应(式6)生成.OH降解污染物.Ni2+可能是Ni和O2反应的产物,O2被一次还原为·O2-[式(7)][30].Ni2+/Ni0的标准氧化还原电位(-0.257,298K)高于Fe2+/Fe0的标准氧化还原电位(-0.447,298K),Fe0可以将Ni2+转化为Ni0.Ni0又可参与反应将Fe3+还原为Fe2+,形成Fe2+/Fe3+循环[29][式(8)],使水相Fe2+含量持续高于Fe3+,有效提高了铁元素利用率,生成更多·OH氧化降解污染物.

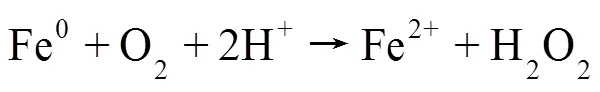
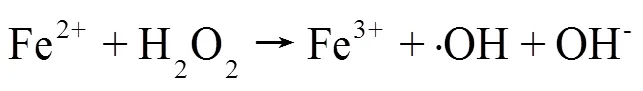
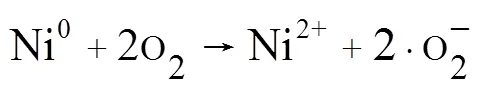
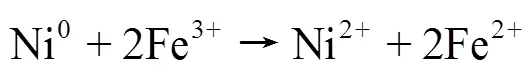
(3)HT-FeNi/Air/pH=3体系降解RhB机理:利用GC-MS检测了HT-FeNi/Air/pH=3体系降解RhB的中间产物,检测到的中间产物主要有4-羟基-4-甲基-2戊酮、2-羟基-2-苯基乙酸等(表3).图9为RhB吸收光谱图,RhB在544nm处的最大吸收峰减少并蓝移,且吸收峰减小的速度明显快于蓝移的速度,推测RhB降解的第一步反应为脱乙基和发色团裂解的同时发生,且发色团裂解为主要反应,脱乙基为次要反应.中间产物4-羟基-4-甲基-2戊酮等的生成证明第二步反应可能为苯环开环.
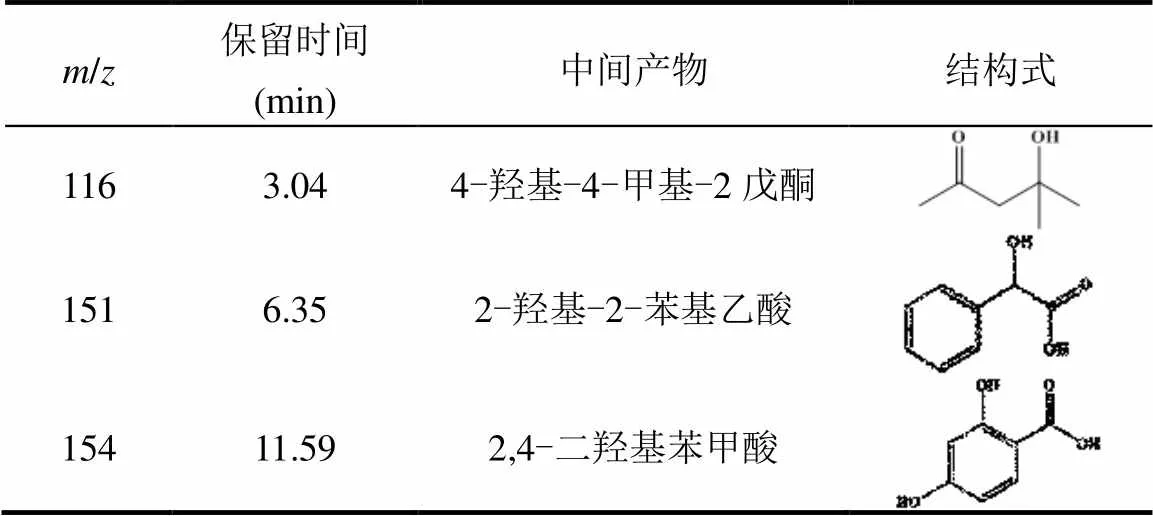
表3 HT-FeNi/Air/pH=3体系降解RhB的中间产物
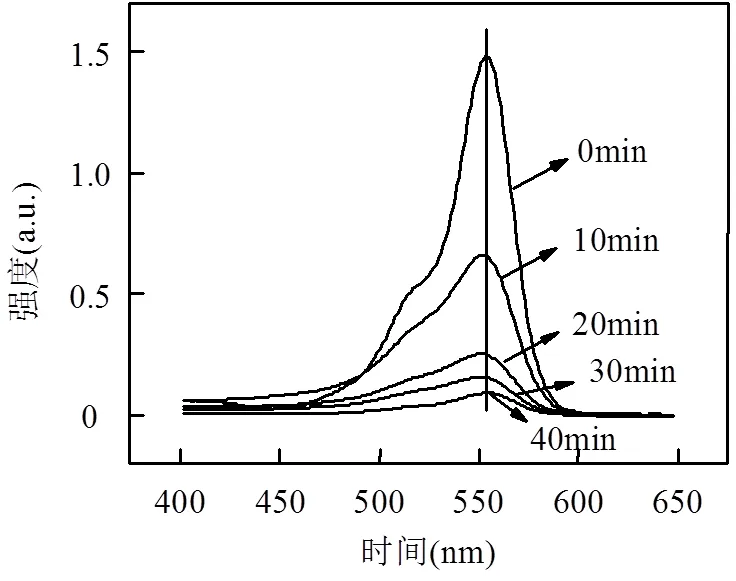
图9 RhB吸收光谱图
HT-FeNi/Air/pH=3体系降解RhB的机理:1)主要路径是Fe0通过活化分子氧生成·OH氧化降解RhB,在该过程中,HT-FeNi中的Ni0促进Fe2+/Fe3+循环,使得体系生成更多的·OH;2)Ni0催化生成的活泼H原子可通过还原作用降解小部分RhB.可能的降解机理如图10所示.
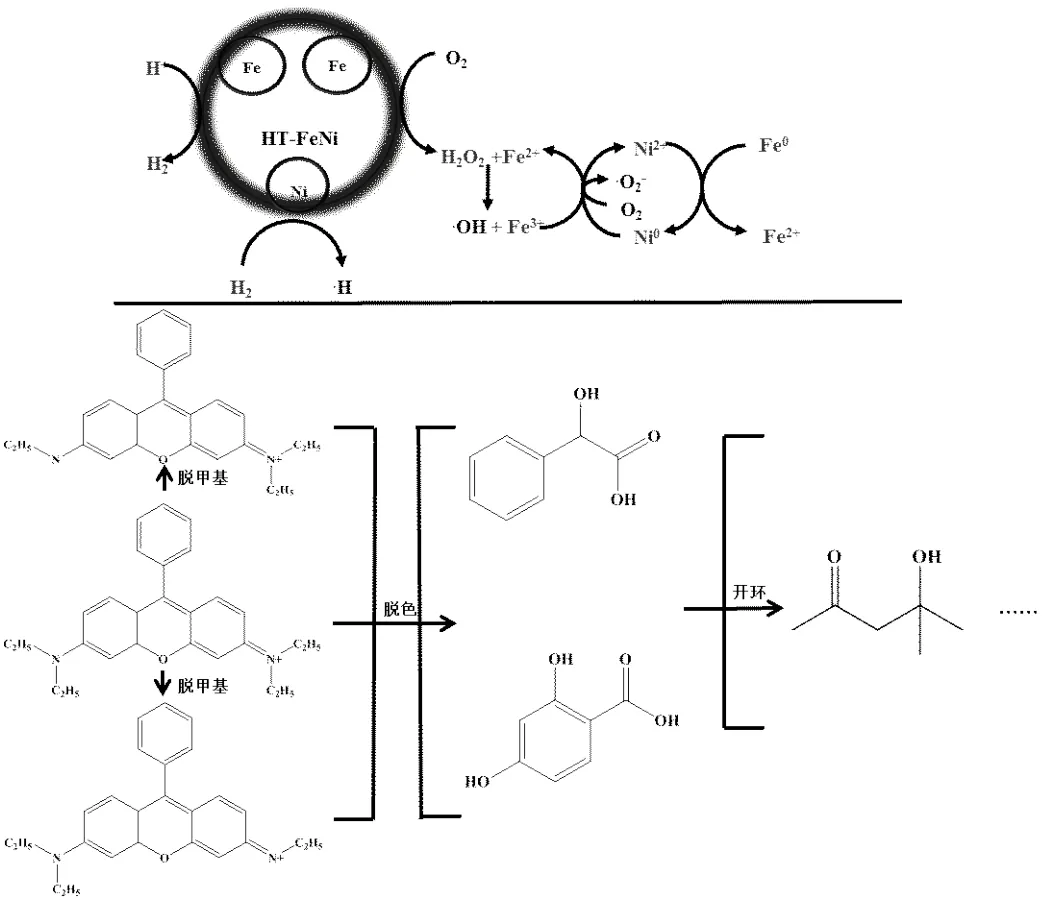
图10 HT-FeNi/Air/pH=3降解RhB可能的机理
2.2.4 HT-FeNi/Air/pH=3体系应用 ·OH氧化电位为2.8V,具有极强的氧化性.其能够有效去除水体环境难降解污染物如偶氮染料、氯代化合物、硝基苯等.将HT-FeNi/Air/pH=3体系应用于去除水体环境中MO和DNCB,效果如图11所示.在40min内该体系去除MO、DNCB 2种污染物的效率分别达到47%和78%.结果表明,HT-FeNi/Air/ pH=3体系可有效去除多种污染物,在水体环境修复中有广泛的应用潜力.

图11 HT-FeNi/Air/pH=3体系降解MO、DNCB效率
3 结论
3.1 HT孔隙结构较为丰富,有良好的RhB吸附性能,HT吸附RhB主要归因于Si-O吸附位点.随着吸附材料添加量的增加,RhB去除效果增强,平衡吸附量减小.准二级动力学模型能较好地描述HT吸附RhB的过程,吸附过程包含表面扩散及颗粒内扩散两个步骤.Langmuir和Freundlich方程均能较好地描述吸附过程,Freundlich吸附模型拟合效果更好.HT对RhB的吸附过程以多分子层吸附为主,同时伴有单分子层吸附,吸附过程较易进行.
3.2 改性红土镍矿HT-FeNi主要成分为铁镍双金属.HT-FeNi/Air/pH=3体系能有效降解RhB,起主要作用的活性氧物种是·OH.Ni0诱导的Fe2+/Fe3+循环促使HT-FeNi/Air/pH=3体系生成更多的·OH.HT- FeNi/Air/pH=3体系还可高效去除MO及DNCB,表明HT-FeNi在水体环境修复方面有广泛的应用潜力.
[1] 马志强,胥思勤,姬江浩,等.改性水稻生物炭对水体中Sb(Ⅲ)的吸附 [J]. 中国环境科学, 2021,41(6):2706-2716.
Ma Z Q, Xu S Q, Ji J H, et al. Adsorption of Sb(Ⅲ) in water by modified rice straw biochar [J]. China Environmental Science, 2021, 41(6):2706-2716.
[2] 宋 歌,张文静,毕 贞,等.多因素对ANAMMOX菌利用零价铁还原硝酸盐过程影响 [J]. 中国环境科学, 2019,39(11):4666-4672.
Song G, Zhang W J, Bi Z, et al. Effects of multiple factors on the process of ANAMMOX bacteria strengthening nitrate reduction by zero-valent iron [J]. China Environmental Science, 2019,39(11): 4666-4672.
[3] 郝凯越,李远威,宗永臣,等.高原生境下A2O工艺对污水处理的微生物机制 [J]. 中国环境科学, 2021,41(5):2240-2251.
Hao K Y, Li Y W, Zong Y C, et al. Microbial mechanism of A2O process for wastewater treatment in plateau habitat [J]. China Environmental Science, 2021,41(5):2240-2251.
[4] Ngulube T, Gumbo J R, Masindi V, et al. An update on synthetic dyes adsorption onto clay based minerals: A state-of-art review [J]. Journal of Environmental Management, 2017,191:35-57.
[5] Hug S J, Leupin O. Iron-Catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction [J]. Environmental Science & Technology,2003, 37(12):2734-2742.
[6] Mohapatra M, Anand S. Cd(II) adsorption on high iron containing lateritic ore of Orissa [J]. Indian Journal of Environmental Protection, 2007,27(6):509-515.
[7] Fu L, Liu Y, Hao S, et al. Preparation of magnetic composite adsorbents from laterite nickel ore for organic amine removal [J]. Arabian Journal of Chemistry, 2021,14(2):102933.
[8] Han X, Gou L, Tang S, et al. Enhanced heterogeneous Fenton-like degradation of refractory organic contaminants over Cu doped (Mg,Ni)(Fe,Al)2O4synthesized from laterite nickel ore [J]. Journal of Environmental Management, 2021,283:111941.
[9] Zhao J C, Wu T X, Oikawa K Q, et al. Photoassisted degradation of dye pollutants. 3. degradation of the cationic dye rhodamine B in aqueous anionic surfactant/TiO2dispersions under visible light irradiation: evidence for the need of substrate adsorption on TiO2particles [J]. Environmental Science & Technology, 1998,32(16): 2394-2400.
[10] Wang P Y, Yang L P, Li J, et al. Zn/ZnO heterostructure for the application of MO degradation and NO removal [J]. Catalysis Letters, 2020,150(7):1985-1992.
[11] Shen, J Y, Zhou Z Y, Ou C J, et al. Reductive transformation and detoxification mechanism of 2,4-dinitrochlorobenzene in combined zero valent iron and anaerobic-aerobic process [J]. Joumal of Environmental Sciences, 2012,24(11):1900-1907..
[12] Handler R M, Beard B L, Johnson C M, et al. Atom exchange between aqueous Fe(II) and goethite- an Fe isotope tracer study [J]. Environmental Science & Technology, 2009,43(4):1102-1107.
[13] GB/T 15555.10-1995 固体废物镍的测定丁二酮肟分光光度法 [S].
GB/T 15555.10-1995 Solid waste - determination of nickel - diedione oxime spectrophotometric method [S].
[14] Walling C. Intermediates in the reactions of Fenton type reagents [J]. Accounts of Chemical Research, 1998,31(4):155-157.
[15] Khlyustova A, Sirotkin N. Plasma-assisted oxidation of benzoic acid [J]. Frontiers of Chemical Science and Engineering, 2019,14(4):513- 521.
[16] Shi J G, Ai Z H, Zhang L Z, et al. Fe@Fe2O3core-shell nanowires enhanced Fenton oxidation by accelerating the Fe(III)/Fe(II) cycles [J]. Water Research, 2014,59(1):145-153.
[17] Unlu N, Ersoz M. Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions [J]. Journal of Hazardous Materials, 2006,136(2):272-280.
[18] Cerne M, Palcic I, Paskovic I, et al. The effect of stabilization on the utilization of municipal sewage sludge as a soil amendment [J]. Waste Management, 2019,94:27-38.
[19] Zhang L, Wu W T, Liu J Y, et al. Removal of phosphate from water using raw and activated laterite: batch and column studies [J]. Desalination and Water Treatment, 2013,52(4-6):775-783.
[20] Langmuir I. The constitution and fundamental properties of solids and liquids [J]. Journal of the American Chemical Society, 1916,38(11): 2221-2295.
[21] Freundlich H M. Ueber die adsorption in loesungen. Zeitschrift fur physikalische chemie-international [J]. Journal of Research in Physical Chemistry & Chemical Physics, 1906,57:385-470.
[22] Ghasemi Z A, Seif A B, Ahmadi T S, et al. Thermodynamic and kinetic studies for the adsorption of Hg (II) by nano-TiO2from aqueous solution [J]. Advanced Powder Technology, 2012,23(2):148-156.
[23] Adeyi A A, Fasina F D, Giwa A. Kinetics and isotherm studies of liquid phase adsorption of cationic dye onto chitosan synthesized from crab shells [J]. International Journal of Engineering Research in Africa, 2018,4840(74):112-126.
[24] 杨梦力,付 伟,王葆华,等.硅酸盐型红土镍矿石的红外光谱研究:印尼与中国不同产地矿石样品的对比[J]. 光谱学与光谱分析, 2015, 35(3):631-634.
Yang M L, Fu W, Wang B H, et al. Infrared spectroscopic study of silicate laterite nickel ore: Comparison of ore samples from different origins in Indonesia and China [J]. Spectroscopy and Spectral Analysis, 2015,35(3):631-634.
[25] Malandrino M, Abollino O, Giacomino A, et al. Adsorption of heavy metals on vermiculite: influence of pH and organic ligands [J]. Journal of Colloid and Interface Science, 2006,299(2):537-546.
[26] 高淑玲,杨翠玲,罗鑫圣,等.坡缕石黏土污泥对水相中亚甲基蓝吸附研究[J]. 中国环境科学, 2014,34(1):78-84.
Gao S L, Yang C L, Luo X S, et al. Study on methylene-blue adsorption from water phase by palygorskite clay sludge [J]. China Environmental Science, 2014,34(1):78-84.
[27] Ahamad K U, Singh R, Baruah I. Equilibrium and kinetics modeling of fluoride adsorption onto activated alumina, alum and brick powder [J]. Groundwater for Sustainable Development, 2018,(7):452-458.
[28] Li X Q, Zhang W X. Sequestration of metal cations with zerovalent iron nanoparticles-a study with high resolution X-ray photoelectron spectroscopy (HR-XPS) [J]. Journal of Physical Chemistry C, 2017, 111(19):6939-6946.
[29] Liu W, Ai Z H, Zhang L Z. Design of a neutral three-dimensional electro-fenton system with foam nickel as particle electrodes for wastewater treatment [J], Journal of Hazardous Materials, 2012,243: 257-264.
[30] Shen W J, Mu Y, Wang B N, et al. Enhanced aerobic degradation of 4-chlorophenol with iron-nickel nanoparticles [J]. Applied Surface Science, 2017,393:316-324.
Adsorption and aerobic degradation of water pollutants by laterite nickel ore-based materials.
WANG Bing-ning1,2, LIU Shou-jun1,2,3, YANG Song2,3*, CHEN Liang-yu2,4, LIU Xing-yang2,3, LI Jin2,4, SHANGGUAN Ju1,2**
(1.Key Laboratory of Coal Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China;2.Shanxi Civil Clean Fuel Engineering Research Center, Taiyuan 030024, China;3.College of Chemistry and Engineering, Taiyuan University of Technology, Taiyuan 030024, China;4.Taiyuan Green Coke Energy Co., Ltd, Taiyuan 030006, China)., 2022,42(2):736~744
Taking laterite nickel ore as the research object, the removal efficiencies of rhodamine B(RhB) with the raw ore (HT) and the modified ore (HT-FeNi) were investigated systematically. The adsorption mechanism of RhB was studied by X-ray diffraction, specific surface area analyser, infrared spectrum, and the kinetic and isothermal adsorption characteristic analyses. The results show that the HT possesses a relatively rich pore structure and a good adsorption performance on RhB. When added 0.2g/Lof HT, a RhB removal efficiency of 39.03% and a adsorption capacity of 93.80mg/g were reached. The RhB removal efficiency was enhanced, while the equilibrium adsorption capacity was decreased with the increasing dosage of HT. The adsorption kinetics and isotherms of RhB were well fitted by pseudo-second-order kinetic and Freundlich equation, respectively. The adsorption process might include two steps: surface diffusion and intra-particle diffusion. The1/was less than 0.5, which indicated that the adsorption process occurred more easily. The adsorption capacity of 39.67mg/g was still remained after 5cycles of experiments, indicating a good recycling performance of HT. The adsorption of RhB by HT was mainly attributed to the adsorption site of Si-O. The modified ore (HT-FeNi) was prepared by gas reduction of raw ore (HT). SEM、XRD、BET and XPS were used to characterize HT-FeNi and the degradation efficiency of RhB with this material was investigated. The specific surface area of HT-FeNi was smaller (14.374m2/g),and its main component was Fe-Ni bimetallic. HT-FeNi could not remove RhB by adsorption. However, the degradation efficiency of RhB could reach 94% in HT-FeNi/Air/pH=3system within 40min. The reactive oxygen species capture experiments indicated that the hydroxyl radical (·OH) played a major role for the RhB degradation. HT-FeNi could activate O2under acidic condition and ·OH formed. The Fe2+/Fe3+cycle induced by Ni0promoted the production of ·OH. Then HT-FeNi/Air/pH=3system was applied to the removal of MO and DNCB, the removal efficiencies were 47% and 78%, respectively.
laterite nickel ore-based materials;adsorption;aerobic degradation;water pollutants
X753
A
1000-6923(2022)02-0736-09
王冰凝(1989-),女,山西运城人,工程师,太原理工大学博士研究生,主要从事环境功能材料制备及应用研究.发表论文5篇.
2021-06-24
国家自然科学基金资助项目(21878210);山西省专利推广实施资助计划项目(20200719)
* 责任作者, 讲师, yangsong@tyut.edu.cn; ** 教授, shangguanju@tyut.edu.cn

