非膜技术处理生物稳定渗滤液的效果及成本
刘婉莹,吕 凡,3,仇俊杰,黄玉龙,章 骅,3,邵立明,3,何品晶,3*
非膜技术处理生物稳定渗滤液的效果及成本
刘婉莹1,2,吕 凡1,2,3,仇俊杰1,2,黄玉龙1,2,章 骅1,2,3,邵立明1,2,3,何品晶1,2,3*
(1.同济大学固体废物处理与资源化研究所,上海 200092;2.同济大学上海污染控制与生态安全研究院,上海 200092;3.同济大学上海多源固废协同处理和能源化工程技术研究中心,上海 200092)
以经厌氧-好氧处理的生物稳定渗滤液为研究对象,分别比较了其经活性炭吸附、混凝、芬顿和电解处理后的溶解性有机碳(DOC)、COD、溶解性氮(DN)和比紫外吸光度(SUV254)的变化,及去除单位COD的成本变化.研究发现,活性炭吸附、芬顿和混凝对生物稳定渗滤液的COD、DOC和DN的去除效率均随药剂投加量的增加而提高;包含化学氧化作用的芬顿和电解技术对芳构化有机物的去除效果更好,使得SUV254减少了60%~70%,且电流密度越大,去除效率越高;活性炭吸附去除单位毫克COD的价格最高,芬顿最低;对生物稳定渗滤液而言,活性炭投加量为5g/L、芬顿试剂投加量为0.605g/L、混凝剂投加量为4.92mmol/L Fe时,性价比较高,具体还应根据原水浓度与参考标准进行选择.
吸附;混凝;芬顿;电解;非膜技术;生物稳定渗滤液;经济分析
生活垃圾卫生填埋场产生的渗滤液,含有大量以有机质[1]、氨氮[2-3]、重金属[4-5]和无机盐[6]为主的污染物[7],也是释放动物激素[8-9]、抗生素[10-11]、塑化剂[12-13]和微塑料[14]等各类新兴污染物的重要源头.根据各地降雨量、垃圾含水量的不同,我国每吨垃圾能产生70~950L渗滤液[15].
生物处理因其技术可靠、简易且经济的特点而被广泛应用于处理新鲜垃圾渗滤液.根据反应条件,可分为好氧和厌氧两大类.厌氧和好氧处理的生物稳定渗滤液一般无法达到《污水排入城镇下水道水质标准》(GB/T 31962-2015)[16]、《生活垃圾填埋场污染控制标准》(GB 16889-2008)[17]等,还需采用吸附[18-19]、混凝[20-21]、芬顿[21]、电解[22]等非膜技术,或微滤、超滤、纳滤、反渗透等[23]膜技术进行深度处理.研究表明[24],升流式厌氧污泥床(UASB)或活性污泥法组合反渗透几乎可去除渗滤液中所有的COD和氨氮.微量污染物上,研究发现纳滤能去除地表水中90%的天然有机物(NOM),以及近100%的三卤甲烷生成潜能(THMFP)[25].然而,与此同时,分子质量、分子大小(长和宽)、酸解离常数、亲疏水性和扩散系数等[26]因素均会影响纳滤和反渗透的截留率,膜污染[27]及浓缩液[28]仍是亟待解决的问题.
随着水处理工艺的发展,吸附、混凝、芬顿和电解等非膜技术展现出良好潜力,这使其替代膜技术成为可能,也为深度处理技术的比选与组合提供了更多选择.活性炭吸附能去除生物稳定渗滤液中38%~90%的COD[29],且相比于COD和溶解性有机碳(DOC),对紫外光淬灭物质的去除效果更好[30].研究发现,在利用混凝-臭氧工艺处理生物稳定渗滤液时,混凝可去除67%的COD和96%的色度[31].而芬顿不仅能去除废水中难降解的溶解性有机物(DOM)[32],提高其可生化性[33],还能去除填埋场渗滤液中的邻苯二甲酸酯和双酚A等内分泌干扰素[12].电解则能够高效去除废水中的COD、色度和各类新兴污染物[34].
考虑到不同非膜技术的优势和弊端,如何平衡去除效果与经济成本,以使其更好地替代膜技术,成为问题的关键.以往研究除了膜技术[35],多局限于对电解[36]、混凝或芬顿[37]等同类技术的比较,或对单线处理流程的经济分析[38],缺乏对多类非膜技术以及复杂流程的比较研究.而对各类非膜技术去除效率与经济成本的分析,不仅能为处理过程中污染物去除的潜力提供依据,也能为技术的选择或组合提供参考.本文以经厌氧-好氧处理的生物稳定渗滤液为研究对象,分别比较了其经活性炭吸附、混凝、芬顿和电解处理后DOC、COD、溶解性氮(DN)和比紫外吸光度(SUV254)的变化,及去除单位COD的成本变化,并以《污水排入城镇下水道水质标准》(GB/T 31962-2015)[16]等为判别标准,筛选出了较好的非膜技术,针对每种技术给出了建议的药剂投加量及电流密度,以期为生物稳定渗滤液非膜技术的比选与组合提供依据.
1 材料与方法
1.1 实验材料
生物稳定渗滤液来自5L/d处理规模的产甲烷同时反硝化反应器(SDM-AS)[39].该反应器已稳定运行180d以上,进水来自上海市某生活垃圾焚烧厂的储坑渗滤液(垃圾分类前),其性质与新鲜渗滤液相似.反应器的处理出水即为典型的生物稳定渗滤液,水质如表1所示.
1.2 装置与药剂
活性炭吸附所用试剂为粉末活性炭(PAC),将颗粒活性炭研磨、过筛(75µm)[40],取筛下物.其比表面积经BET测试(ASAP2460, Micromeritics Instrument Corporation, USA)确定为1420m2/g.取100mL渗滤液-活性炭(粉末)或水-活性炭(粉末)混合液于150mL锥形瓶中,将锥形瓶置于恒温摇床中以200r/min震荡5h,此后静置12h(恒温25℃),采用0.45µm聚醚砜针式过滤器过滤.粉末活性炭的投加量为2~10g/L[41],投加梯度为:0.5,1,2,5,10,20g/L.

表1 生物稳定渗滤液的典型水质特征
注:-为文章中未列出.
混凝所用装置为六联搅拌器(MY3000-6D,梅宇,中国),所用混凝剂为氯化铁溶液(FeCl3·6H2O, 250g/L).分别向烧杯中加入400mL生物稳定渗滤液和超纯水作为实验组和空白组.向生物稳定渗滤液中加入盐酸(质量分数15%~20%)以调节其pH(4.0± 0.2),250r/min搅拌30min去除大部分碳酸盐及碳酸氢盐;向超纯水中加入等量盐酸,同样以250r/min搅拌30min,作为空白.然后,依次向烧杯中加入混凝剂和氢氧化钠(12mol/L),启动混凝程序,采用0.45 µm聚醚砜针式过滤器过滤.混凝剂的投加梯度为:0.3,0.9,1.9,3.7,4.9,6.9,11.1,14.8,18.5mmol Fe/L.
利用同一个六联搅拌器(MY3000-6D,梅宇,中国)进行芬顿实验,芬顿试剂包括硫酸亚铁(FeSO4·7H2O, 250g/L)和过氧化氢(质量分数30%).向生物稳定渗滤液中加入适量硫酸(质量分数30%~40%)以调节pH(4.0±0.2),减少碳酸盐和碳酸氢盐对羟基自由基的捕捉效应[21];向等量超纯水中加入等量硫酸,作为空白.通过预实验确定硫酸亚铁与过氧化氢的较优比例为2.5(数据未列出),在此比例下,分2次投加芬顿试剂,并改变其投加量如表2所示.
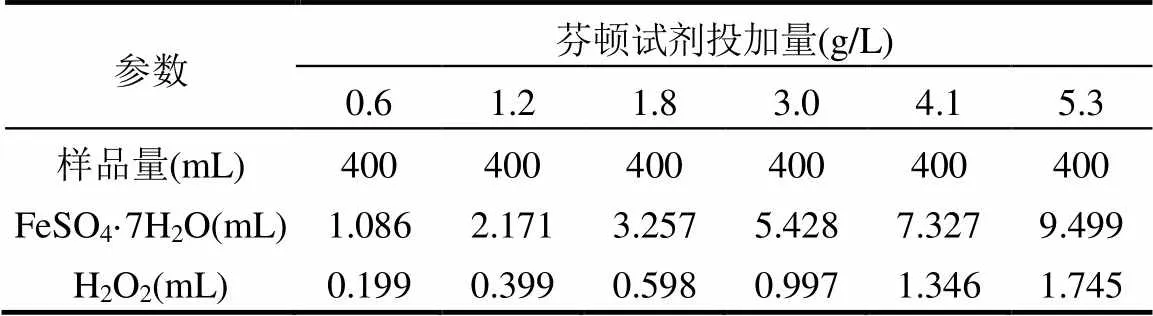
表2 芬顿试剂投加量
电解所用阳极和阴极分别为Ti/PbO2和不锈钢,极板间距为10mm,阴极板在两侧,阳极板在中间,电流密度分别为2.5,5,10A/dm2,处理的生物稳定渗滤液体积为2.4L.首先,向生物稳定渗滤液中加入适量硝酸(质量分数15%~20%),将其pH调节至(4.0±0.2),快速搅拌,以去除大部分碳酸根离子和碳酸氢根离子;然后,定时取样,并采用0.45µm聚醚砜针式过滤器过滤.由于盐酸和硫酸均会产生相关自由基[46],本实验最终采用硝酸调节样品pH值.所有实验均设置3组平行.
1.3 分析方法
DOC和DN的测试由总有机碳分析仪(TOC- VCPH,岛津,日本)和总氮分析仪(TNM-l,岛津,日本)完成,因生物稳定渗滤液及其处理后出水均经0.45µm聚醚砜滤膜过滤,故测试所得总有机碳(TOC)及总氮(TN)即为DOC和DN;COD的测试由分光光度计(DRB200,哈希,美国)完成;氯离子浓度由瓶口滴定器(Titrette 50mL,普兰德,德国)测定;SUV254等于紫外吸光度(ultraviolet absorbance at 254nm,UV254)比DOC,其值代表了生物稳定渗滤液及其处理后出水的芳构化程度[47],UV254的测试采用紫外分光光度计(UV-1800,岛津,日本).
1.4 数据处理
本文中各类非膜技术对污染物的去除率公式如下式(1);去除污染物的成本计算如下式(2),单位为元/mg COD.


2 结果与分析
2.1 活性炭吸附
如图1所示,粉末活性炭对DOC、COD和DN的吸附去除率均随投加量增加而提高,且其对DOC的去除率最高.就DOC而言,当粉末活性炭的投加量为5g/L时,去除率接近80%;其后,随着粉末活性炭投加量增加,去除率的增加明显趋缓;当投加量为20g/L时,去除率最高达89.1%.因经历了好氧硝化过程,生物稳定渗滤液中的氨氮浓度较低,亚硝酸盐、硝酸盐和有机氮构成了DN的主要部分,粉末活性炭对此部分氮去除率最高仅为11.5%(投加量20g/L).
粉末活性炭不能降解污染物,而主要通过空间位阻、范德华力和亲疏水性去除污染物[48],其吸附容量随分子尺寸的减少而增加[49].根据不同活性炭投加量下DOC和COD的浓度,可由吸附容量(表3),计算得到DOC和COD的Fruendlich吸附等温式.DOC的吸附等温式中,e, DOC为本文获得的PAC对DOC的吸附容量(见表3),mg DOC/g PAC;DOC为与吸附比表面积、温度有关的系数,本文经计算为0.1052;DOC为与温度有关的常数,本文经计算为0.6567;DOC为吸附平衡时DOC浓度,mg/L.
COD的吸附等温式中,e, COD为本文研究获得的PAC对COD的吸附容量(表3),mg COD/g PAC;COD为与吸附比表面积、温度有关的系数,本文经计算为9.616×10-13;COD为与温度有关的常数,本文经计算为0.1808;COD为吸附平衡时COD浓度,mg/L.
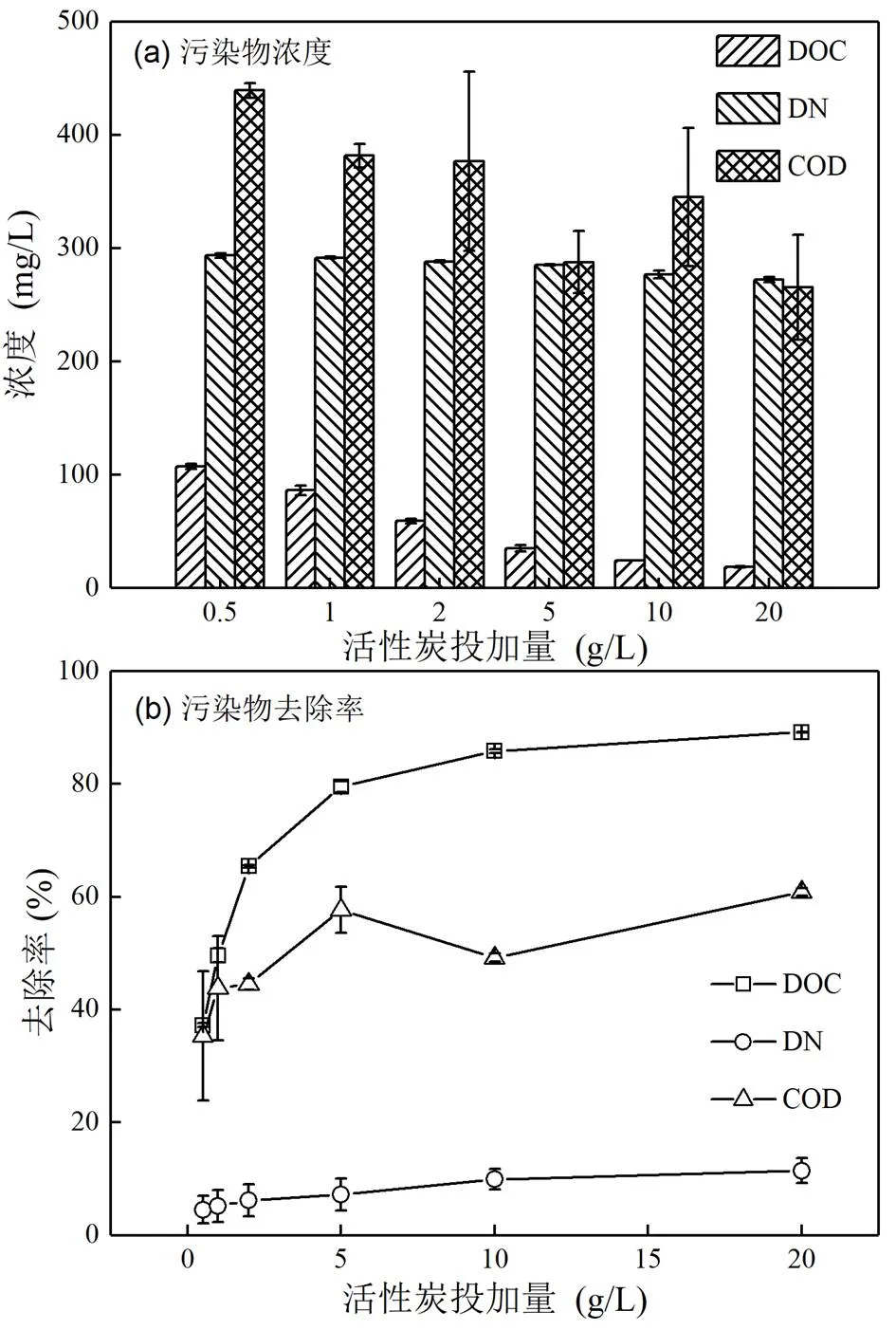
图1 不同粉末活性炭投加量下的污染物去除效果
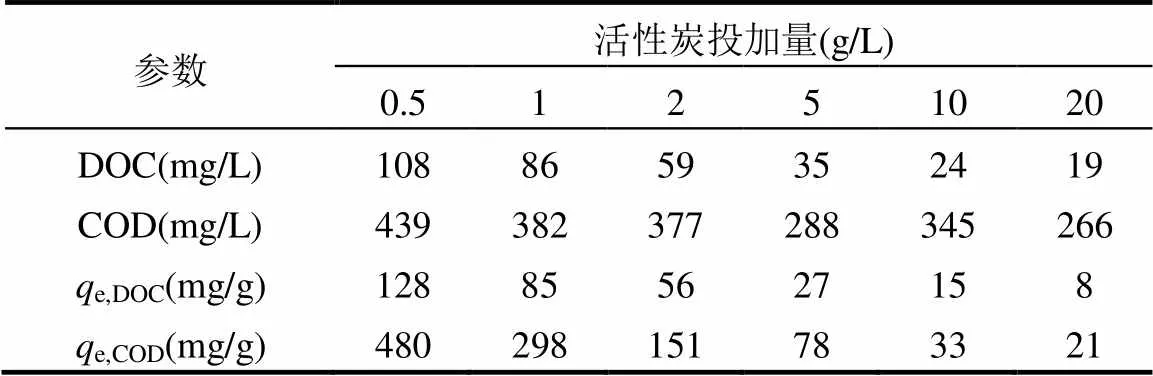
表3 不同粉末活性炭投加量下的DOC和COD吸附容量
注:e,DOC为本文获得的PAC对DOC的吸附容量,mg DOC/g PAC;e,COD为本文获得的PAC对COD的吸附容量,mg COD/g PAC.
2.2 混凝
因铁盐对于天然有机物的去除效果优于铝盐[50],本实验采用三氯化铁作为混凝剂.由图2可知,随着混凝剂投加量的增加,DOC和COD的去除率先快速提高,后逐渐变缓.当FeCl3的投加量大于14.77mmol/L时,DOC的去除率从72.4%下降至62.4%,COD的去除率从62.3%下降至61.6%,这可能与混凝机理的变化有关.此外,DN的去除率变化不明显,在混凝剂投加量最大时(FeCl3=18.46mmol/ L),DN去除率仍仅为6.2%.
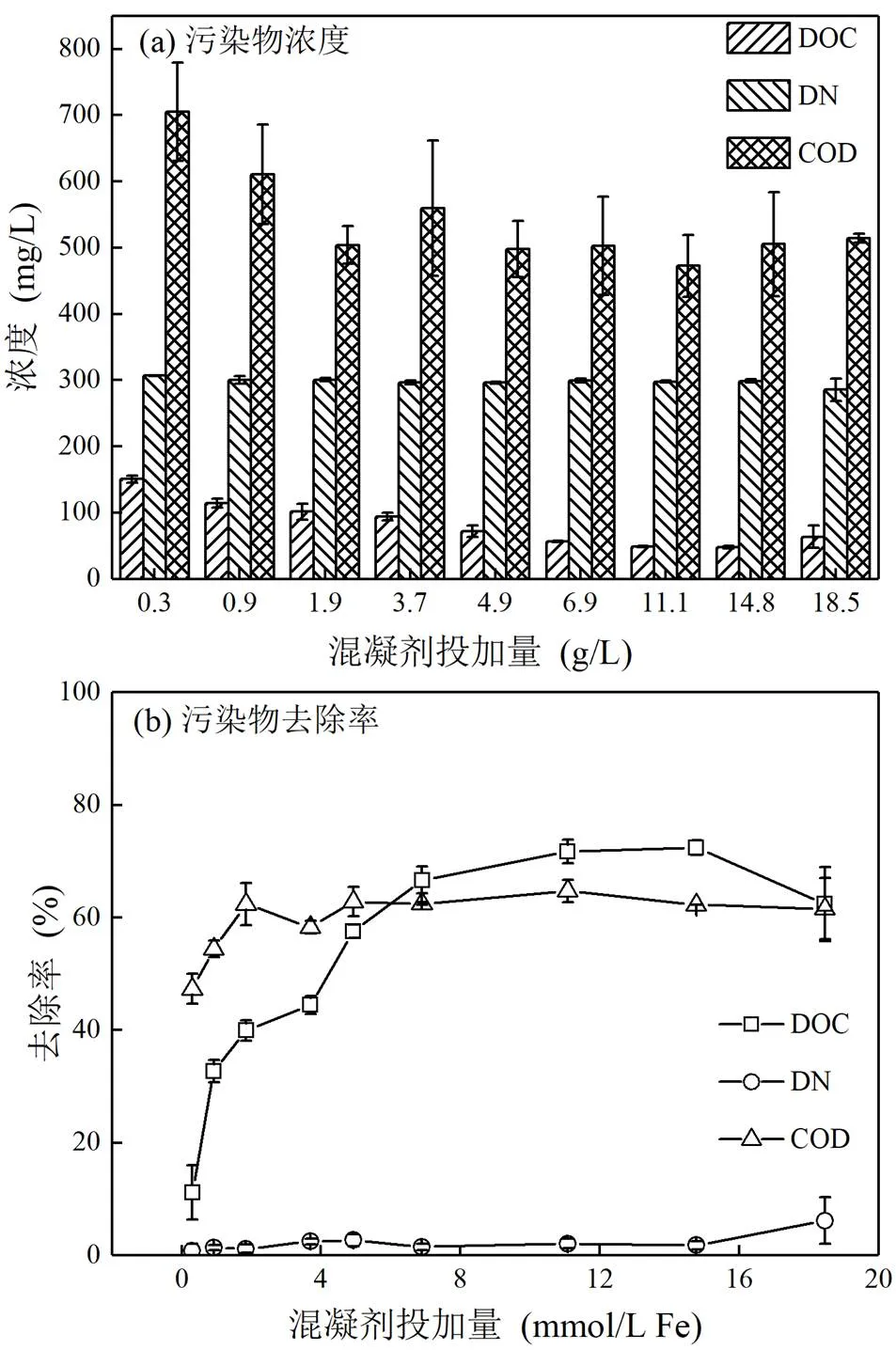
图2 不同混凝剂投加量下污染物去除效果
2.3 芬顿
芬顿包括氧化和混凝2个过程,氧化过程中,有机物被部分降解;混凝过程中,有机物从水相转移至铁泥中.由图3可知,随着投加量增加,DOC、DN和COD的去除率逐渐提高.其中,DOC的去除率从58.7%提高至81.0%,增幅与混凝及活性炭吸附相比较小.COD的去除率从42.9%提高至58.0%,范围与多数文献一致[21,29,51],变化幅度也较小,这可能与过氧化氢及硫酸亚铁的比例固定有关[21,52];此外,当2种药剂的比例不同时,氧化和混凝对COD去除的贡献不相同[53],对有机物的处理效果也具有选择性[54].DN的去除率从4.8%提高至11%.仅0.605g/L的投加量即可去除58.7%的DOC和42.9%的COD;且芬顿与混凝及活性炭吸附类似,对DN的去除率较低.

图3 不同芬顿试剂投加量下的污染物去除效果
2.4 电解
主要考察电流密度对电解效果的影响.采用恒电流模式,由于电解过程中生物稳定渗滤液的体积变化较小,近似认为极水比(电极面积与处理水量之比)不变.由图4可知,随着电流密度增大,电解对COD和DOC的去除率明显提高.180min后,电流密度分别为2.5,5,10A/dm2的电解对COD和DOC的去除率分别达到79.8%、94.7%、92.5%和23.0%、37.5%、47.4%.电解结束时,COD和DOC的浓度分别为151,41,59mg/L和130,116,91mg/L.

图4 不同电流密度下电解处理污染物的去除效果
与活性炭吸附及混凝不同,电解可以降解有机物,其作用分为完全矿化和部分氧化[46].完全矿化时,有机物可被彻底降解为CO2;部分氧化时,有机物的氧化态变高,但仍留存于样品中[46,55].如图4(c)和(d)所示,DOC的去除率低于50%,表明电解只完全矿化了不到50%的有机物;而COD的去除率超过90%,表明几乎所有的有机物都被部分氧化成小分子,但在后续阶段,只有一部分被完全矿化成CO2.
由于生物稳定渗滤液的氨氮浓度较低(2~ 21mg/L),而电解对氮的去除主要体现在氨氮上[45],本文不讨论电解对氮的去除效果.因此,为避免引入氯离子产生氯的自由基,或引入硫酸根离子产生过硫酸基[46],实验采用稀硝酸调节溶液pH值,以阻止碳酸盐、碳酸氢盐捕获羟基自由基[21].电解体系引入了硝酸根离子,贡献了DN.
3 讨论
3.1 不同深度处理技术出水的SUV254
其中电解2.5A/dm2、电解5A/dm2和电解10A/dm2分别表示电流密度为2.5,5,10A/dm2的处理效果.对于活性炭吸附和混凝,生物稳定渗滤液的SUV254分别从3.79L/(mg×m)下降至2.02,2.49L/ (mg×m),而后有所上升;这表明出水中芳构化有机物的比例先下降,其后随着DOC下降有所上升[47].芬顿处理后,生物稳定渗滤液的SUV254从3.79L/ (mg×m)下降至0.59L/(mg×m),表明亲水性小分子有机物的比例增加,这与芬顿的氧化作用相关[56].
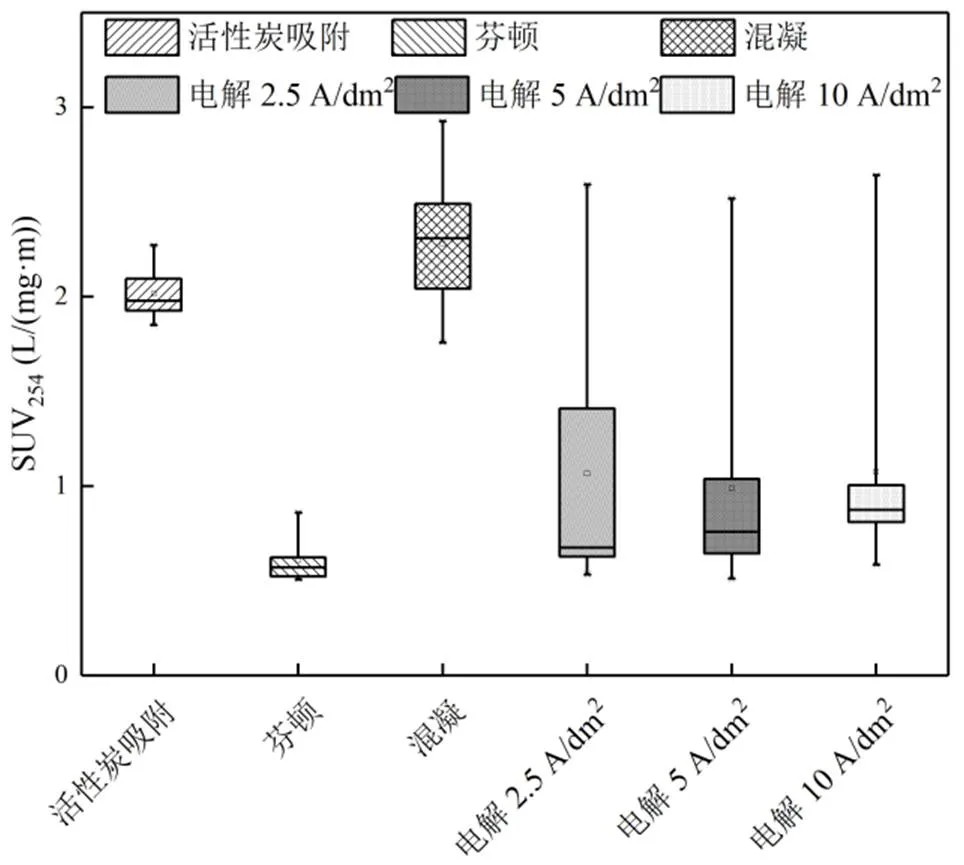
图5 PAC、混凝、Fenton和电解处理出水的SUV254
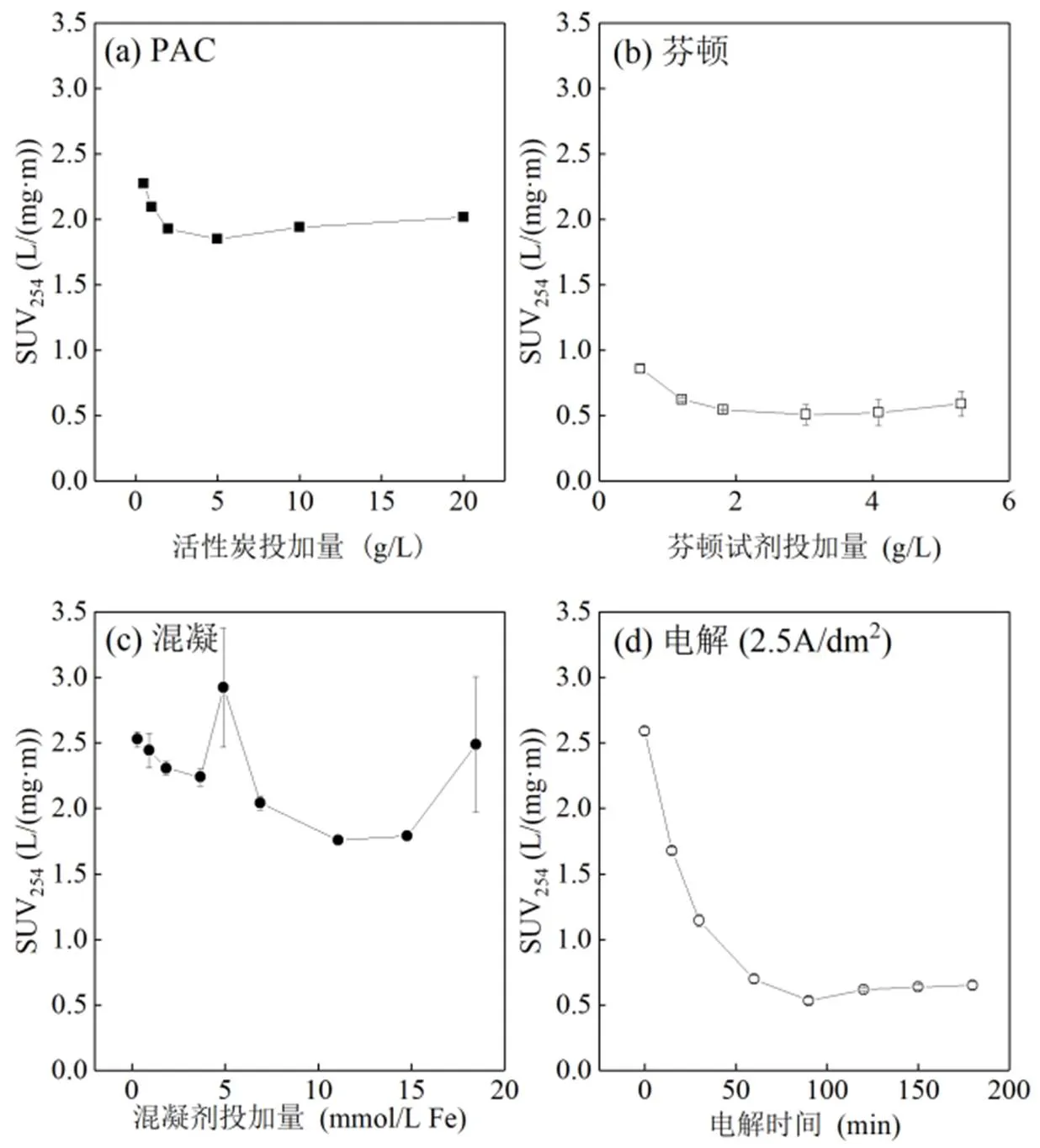
经3种(2.5,5和10A/dm2)电流密度电解后,与芬顿处理后的出水相似, 3种电流密度下,出水SUV254均从2.5L/(mg×m)左右快速下降至0.5L/(mg×m),再缓慢上升至0.75~1.0L/(mg×m).电流密度越大,SUV254下降至最低值所需的时间越短,即氧化速率越快(图6).
上述结果表明,均存在氧化反应的芬顿和电解,出水SUV254的下降幅度较大,为60%~70%;只存在相际迁移的活性炭吸附和混凝,出水SUV254的下降幅度较小,为40%~50%.
3.2 不同技术的最佳去除率及成本分析
根据不同渗滤液处理设施的规模与工艺,考虑到设备折旧、人工及污泥处理等费用的复杂性,本文主要讨论药剂及部分电耗成本的直接运行成本;并根据各种药剂的市场价格及电费标准.由图7比较可得,活性炭吸附去除单位毫克COD的价格最高,且与10A/m2的电解数量级相当,均比其他技术高出一个数量级.由于药剂选择等原因,芬顿去除单位毫克COD的价格比混凝更低.此外,对于活性炭吸附、芬顿和混凝而言,去除单位COD的价格与药剂投加量基本呈正相关;而对于2.5,5和10A/m2的电解而言,去除单位COD的价格与电解时间呈凸函数关系.
图7(a)中,当活性炭投加量分别为2,5,10g/L时,DOC和COD的去除率分别为65.4%、79.5%、85.8%和44.6%、57.7%、49.2%,DOC和COD的浓度分别为59,35,24mg/L,377,288,345mg/L,价格分别为3.1×10-5,5.9×10-5和1.4×10-4元/mg COD;其中,DOC的去除率先增加了14.1%、后增速放缓,COD的去除率先增加后降低,COD的浓度在活性炭投加量为5g/L时低于《污水排入城镇下水道水质标准》(GB/T 31962-2015)C级标准300mg/L[16],去除单位毫克COD的价格则先增加了2.8×10-5元、后增速提高.上述结果表明,对于生物稳定渗滤液,活性炭投加量为5g/L时的性价比最高,此时DOC和COD的去除率分别接近80%和60%.
图7(b)中,当芬顿试剂的投加量分别为0.605,4.086g/L时,DOC和COD的去除率分别为58.7%、79.4%和42.9%、60.4%,DOC和COD的浓度分别为70,35mg/L和383,265mg/L,其中,COD的浓度分别低于《污水排入城镇下水道水质标准》(GB/T 31962-2015)A、B级和C级标准[16],去除单位毫克COD的价格分别为1.8×10-6和1.1×10-5元/mg COD,比臭氧协同双氧水氧化渗滤液的成本更低[57].而随着芬顿试剂投加量的增加,去除单位毫克COD的价格直线上升,但与其他技术相比增幅较小;如前所述,DOC、COD和DN去除率的增幅较小,但前两者的去除率仍分别大于58%和40%.由此可见,芬顿具有较大的价格优势,可推广于实际应用,但同时也应考虑处理流程的成本及操作难度.
如图7(c),当混凝剂的投加量分别为1.85,3.69, 4.92,6.90mmol/L Fe时,DOC和COD的去除率分别为39.9%、44.5%、57.6%、66.7%和62.4%、58.3%、62.8%、62.5%,DOC和COD的浓度分别为102,94, 72,57mg/L和504,559,498,503mg/L,价格分别为2.910-6,6.1×10-6,7.6×10-6和1.1×10-5元/mg COD.其中,DOC的去除率在混凝剂的投加量分别为3.69和4.92mmol/L Fe之间时变化最大,COD的去除率维持在60%左右,COD的浓度在混凝剂投加量为4.92mmol/L Fe时低于《污水排入城镇下水道水质标准》(GB/T 31962-2015)A、B级标准500mg/L[16],去除单位毫克COD的价格基本呈直线变化.由此可见,对于生物稳定渗滤液,混凝剂投加量为4.92mmol/L Fe时性价比最高,具体情况还应视原水浓度与达标要求而定.
在图7(d)、(e)和(f)中,电解技术去除单位毫克COD的价格均在15min时增速变缓,这可能与作用自由基的变化有关;而分别在120,90,90min时增速变快,这与DOC去除率的变化趋势相反.根据焦耳定律,恒电流电解时,消耗的电能与电阻和时间呈正相关,由此可见,去除的DOC影响了电解体系的电阻,这可能与渗滤液中的腐殖酸相关.此外,由于凸函数的特性,与芬顿和混凝相比,电解较难在低于1.0×10-5元/mg COD时获得较好的去除效果.
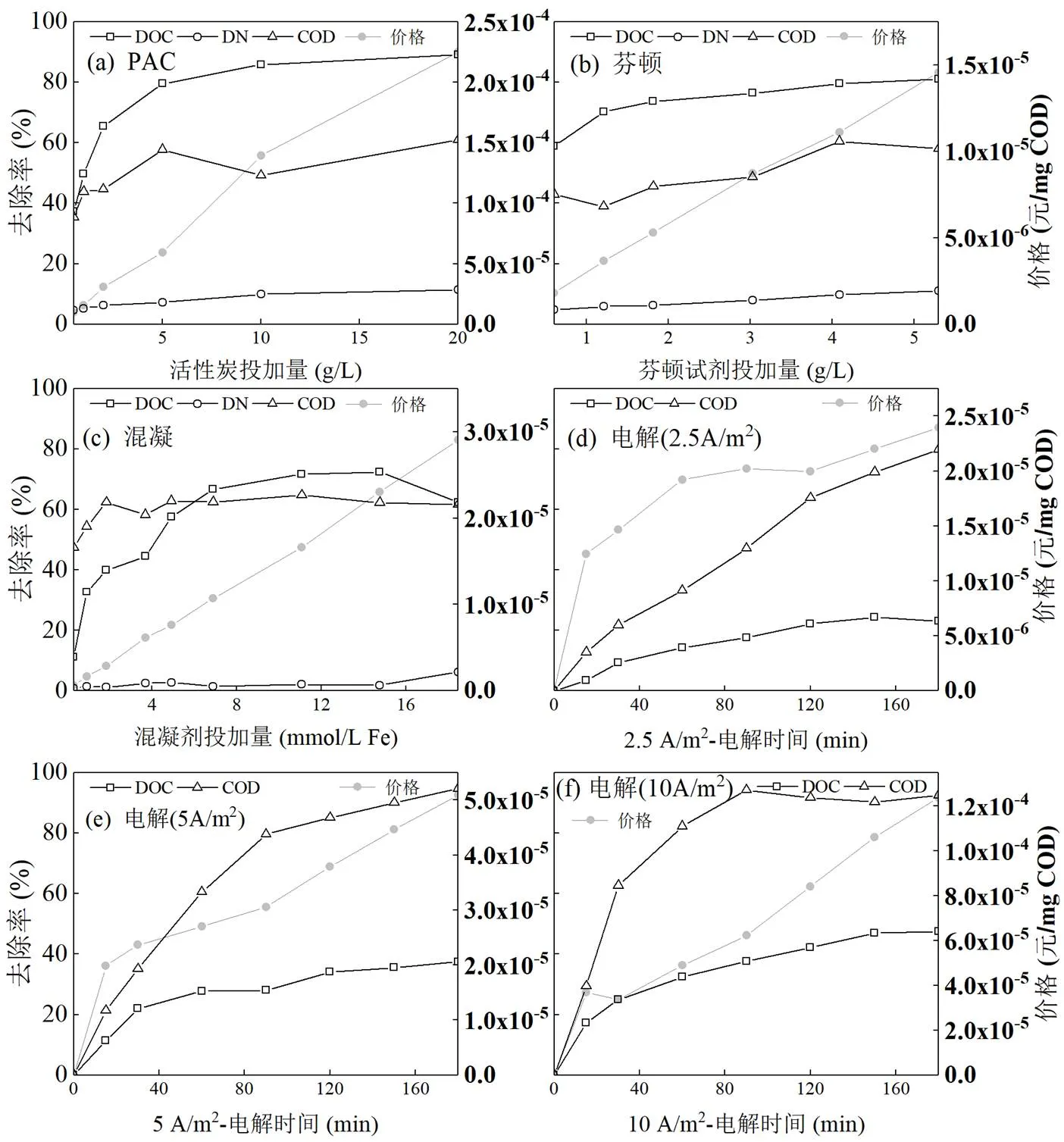
图7 不同技术去除单位毫克COD的价格随药剂量或电解时间的变化
4 结论
4.1 活性炭吸附、芬顿和混凝对生物稳定渗滤液的COD、DOC和DN的去除效率均随药剂投加量的增加而提高,对DN的去除率均低于15%.
4.2 SUV254的结果表明,包含化学氧化的芬顿和电解对芳构化有机物的去除效果更好,SUV254减少了60%~70%,且电流密度越大,去除效率越高;只存在相际迁移的活性炭吸附和混凝效果较差,SUV254减少了40%~50%.
4.3 在活性炭吸附、芬顿、混凝和电解(电流密度分别为2.5,5,10A/m2)4种技术中,活性炭吸附去除单位毫克COD的价格最高,芬顿最低.对生物稳定渗滤液而言,活性炭投加量为1g/136mgCOD、芬顿试剂投加量为1g/1372mgCOD、混凝剂投加量为1mmol Fe/169mgCOD时,性价比较高,具体还应根据原水浓度与参考标准进行选择.
[1] He P J, Xue J F, Shao L M, et al. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill [J]. Water Research, 2006,40(7): 1465-1473.
[2] He P J, Shao L M, Guo H D, et al. Nitrogen removal from recycled landfill leachate by ex situ nitrification and in situ denitrification [J]. Waste Management, 2006,26(8):838-845.
[3] He P J, Shao L M, Guo H D, et al. Nitrogen removal from landfill leachate using single or combined processes [J]. Environmental Technology, 2005,26(4):373-380.
[4] He P J, Xiao Z, Shao L M, et al. In situ distributions and characteristics of heavy metals in full-scale landfill layers [J]. Journal of Hazardous Materials, 2006,137(3):1385-1394.
[5] Qu M, He P J, Shao L M, et al. Heavy metals mobility in full-scale bioreactor landfill: Initial stage [J]. Chemosphere, 2008,70(5):769- 777.
[6] Xia Y, He P J, Pu H X, et al. Inhibitory effect of high calcium concentration on municipal solid waste leachate treatment by the activated sludge process [J]. Waste Management & Research, 2017, 35(5):508-514.
[7] 何品晶.固体废物处理与资源化技术 [M]. 北京:高等教育出版社, 2011: 399-401.
He P J. Treatment and recycling technologies of solid waste [M]. Beijing: Higher education press, 2011:399-401.
[8] Zhang H, Chang C H, Lu F, et al. Estrogenic activity of fractionate landfill leacahte [J]. Science of the Total Environment, 2009,407(2): 879-886.
[9] Zhang H, Chang C H, Lu F, et al. Fluorescent characteristics of estrogenic compounds in landfill leachate [J]. Environmental Technology, 2009,30(9):953-961.
[10] He P J, Huang J H, Yu Z F, et al. Antibiotic resistance contamination in four Italian municipal solid waste landfills sites spanning 34years [J]. Chemosphere, 2021,266:129182.
[11] Yu Z F, He P J, Shao L M, et al. Co-occurrence of mobile genetic elements and antibiotic resistance genes in municipal solid waste landfill leachates: A preliminary insight into the role of landfill age [J]. Water Research, 2016,106:583-592.
[12] He P J, Zheng Z, Zhang H, et al. PAEs and BPA removal in landfill leachate with Fenton process and its relationship with leachate DOM composition [J]. Science of the Total Environment, 2009,407(17): 4928-4933.
[13] Zheng Z, He P J, Shao L M, et al. Phthalic acid esters in dissolved fractions of landfill leachates [J]. Water Research, 2007,41(20):4696- 4702.
[14] He P J, Chen L Y, Shao L M, et al. Municipal solid waste (MSW) landfill: A source of microplastics?-Evidence of microplastics in landfill leachate [J]. Water Research, 2019,159:38-45.
[15] Yang N, Damgaard A, Kjeldsen P, et al. Quantification of regional leachate variance from municipal solid waste landfills in China [J]. Waste Management, 2015,46:362-372.
[16] GB/T31962-2015 污水排入城镇下水道标准 [S].
[17] GB/T31962-2015 Wastewater quality standards for discharge to municipal sewers [S].
[18] GB 16889-2008 生活垃圾填埋场污染控制标准[S].
[19] GB 16889-2008 Standard for pollution control on the landfill site of municipal solid waste [S].
[20] Xiang Y J, Xu Z Y, Wei Y Y, et al. Carbon-based materials as adsorbent for antibiotics removal: mechanisms and influencing factors [J]. Journal of Environmental Management, 2019,237:128-138.
[21] Talebi A, Razali Y S, Ismail N, et al. Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process [J]. Science of the Total Environment, 2020,707: 134533.
[22] Tatsi A A, Zouboulis A I, Matis K A, et al. Coagulation– flocculation pretreatment of sanitary landfill leachates [J]. Chemosphere, 2003, 53(7):737-744.
[23] Deng Y, Englehardt J D. Treatment of landfill leachate by the Fenton process [J]. Water Research, 2006,40(20):3683-3694.
[24] Sarkka H, Bhatnagar A, Sillanpaa M. Recent developments of electro-oxidation in water treatment - a review [J]. Journal of Electroanalytical Chemistry, 2015,754:46-56.
[25] Zhang B L, Shan C, Hao Z N, et al. Transformation of dissolved organic matter during full-scale treatment of integrated chemical wastewater: molecular composition correlated with spectral indexes and acute toxicity [J]. Water Research, 2019,157:472-482.
[26] Kurniawan T A, Lo W H, Chan G Y S. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate [J]. Journal of Hazardous Materials, 2006,129(1-3):80-100.
[27] De La Rubia A, Rodriguez M, Leon V M, et al. Removal of natural organic matter and THM formation potential by ultra- and nanofiltration of surface water [J]. Water Research, 2008,42(3):714- 722.
[28] Bellona C, Drewes J E, Xu P, et al. Factors affecting the rejection of organic solutes during NF/RO treatment - a literature review [J]. Water Research, 2004,38(12):2795-2809.
[29] Tang C Y, Chong t H, Fane A G. Colloidal interactions and fouling of NF and RO membranes: A review [J]. Advances in Colloid and Interface Science, 2011,164(1/2):126-143.
[30] Van Der Bruggen B, Lejon L, Vandecasteele C. Reuse, treatment, and discharge of the concentrate of pressure-driven membrane processes [J]. Environmental Science & Technology, 2003,37(17):3733-3738.
[31] Renou S, Givaudan J G, Poulain S, et al. Landfill leachate treatment: review and opportunity [J]. Journal of Hazardous Materials, 2008, 150(3): 468-493.
[32] Deng Y, Jung C I, Zhao R Z, et al. Adsorption of UV-quenching substances (UVQS) from landfill leachate with activated carbon [J]. Chemical Engineering Journal, 2018,350:739-746.
[33] Monje-Ramirez I, Orta De Velasquez M T. Removal and transformation of recalcitrant organic matter from stabilized saline landfill leachates by coagulation-ozonation coupling processes [J]. Water Research, 2004,38(9):2358-2366.
[34] Sun G X, Zhang Y, Gao Y X, et al. Removal of hard COD from biological effluent of coking wastewater using synchronized oxidation-adsorption technology: performance, mechanism, and full- scale application [J]. Water Research, 2020,173:115517.
[35] De Morais J L, Zamora P P. Use of advanced oxidation processes to improve the biodegradability of mature landfill leachates [J]. Journal of Hazardous Materials, 2005,123(1-3):181-186.
[36] Oturan N, Van Hullebusch E D, Zhang H, et al. Occurrence and Removal of Organic Micropollutants in Landfill Leachates Treated by Electrochemical Advanced Oxidation Processes [J]. Environmental Science & Technology, 2015,49(20):12187-12196.
[37] Ribera-Pi J, Badia-Fabregat M, Espi J, et al. Decreasing environmental impact of landfill leachate treatment by MBR, RO and EDR hybrid treatment [J]. Environmental Technology, 2020:1-15.
[38] Mandal P, Dubey B K, Gupta A K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope [J]. Waste Management, 2017,69:250-273.
[39] Amor C, De Torres-Socias E, Peres J A, et al. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes [J]. Journal of Hazardous Materials, 2015,286: 261-268.
[40] Di Maria F, Sisani F. A life cycle assessment of conventional technologies for landfill leachate treatment [J]. Environmental Technology & Innovation, 2017,8:411-422.
[41] Qiu J J, Lu F, Zhang H, et al. Persistence of native and bio-derived molecules of dissolved organic matters during simultaneous denitrification and methanogenesis for fresh waste leachate [J]. Water Research, 2020,175:115705.
[42] Welander U, Henrysson T. Physical and chemical treatment of a nitrified leachate from a municipal landfill [J]. Environmental Technology, 1998,19(6):591-599.
[43] Foo K Y, Hameed B H. An overview of landfill leachate treatment via activated carbon adsorption process [J]. Journal of Hazardous Materials, 2009, 171(1-3):54-60.
[44] Zhao J S, Ouyang F, Yang Y X, et al. Degradation of recalcitrant organics in nanofiltration concentrate from biologically pretreated landfill leachate by ultraviolet-Fenton method [J]. Separation and Purification Technology, 2020,235:116076.
[45] Qiu J J, Lu F, Zhang H, et al. UPLC Orbitrap MS/MS-based fingerprints of dissolved organic matter in waste leachate driven by waste age [J]. Journal of Hazardous Materials, 2020,383:121205.
[46] Zheng Z, Zhang H, He P J, et al. Co-removal of phthalic acid esters with dissolved organic matter from landfill leachate by coagulation and flocculation process [J]. Chemosphere, 2009,75(2): 180-186.
[47] Fernandes A, Santos D, Pacheco M J, et al. Nitrogen and organic load removal from sanitary landfill leachates by anodic oxidation at Ti/Pt/PbO2, Ti/Pt/SnO2-Sb2O4and Si/BDD [J]. Applied Catalysis B: Environmental, 2014,148:288-294.
[48] Panizza M, Cerisola G. Direct and mediated anodic oxidation of organic pollutants [J]. Chemical Reviews, 2009,109(12):6541-6569.
[49] Weishaar J L, Aiken G R, Bergamaschi B A, et al. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon [J]. Environmental Science & Technology, 2003,37(20):4702-4708.
[50] Phungsai P, Kurisu F, Kasuga I, et al. Changes in dissolved organic matter composition and disinfection byproduct precursors in advanced drinking water treatment processes [J]. Environmental Science & Technology, 2018,52 (6):3392-3401.
[51] Velten S, Knappe D R U, Traber J, et al. Characterization of natural organic matter adsorption in granular activated carbon adsorbers [J]. Water Research, 2011,45(13):3951-3959.
[52] Matilainen A, Vepsalainen M, Sillanpaa M. Natural organic matter removal by coagulation during drinking water treatment: a review [J]. Advances in Colloid and Interface Science, 2010,159(2):189-197.
[53] 王 杰,马溪平,唐凤德,等.微波催化氧化法预处理垃圾渗滤液的研究 [J]. 中国环境科学, 2011,31(7):1166-1170.
Wang J, Ma X P, Tang F D, et al. Study on pretreatment of landfill leachate by microwave catalytic oxidation [J]. China Environmental Science, 2011,31(7):1166-1170.
[54] Umar M, Aziz H A, Yusoff M S. Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate [J]. Waste Management, 2010,30(11):2113-2121.
[55] Deng Y. Physical and oxidative removal of organics during Fenton treatment of mature municipal landfill leachate [J]. Journal of Hazardous Materials, 2007,146(1/2):334-340.
[56] He P J, Liu W Y, Qiu J J, et al. Improvement criteria for different advanced technologies towards bio-stabilized leachate based on molecular subcategories of DOM [J]. Journal of Hazardous Materials, 2021,414: 125463.
[57] Moreira F C, Boaventura R A R, BRILLAS E, et al. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters [J]. Applied Catalysis B: Environmental, 2017,202:217-261.
[58] 陈炜鸣,张爱平,李 民,等.O3/H2O2降解垃圾渗滤液浓缩液的氧化特性及光谱解析 [J]. 中国环境科学, 2017,37(6):2160-2172.
Chen W M, Zhang A P, Li M, et al. Oxidation characteristics and spectral analysis of concentrated leachate degraded by O3/H2O2[J]. China Environmental Science, 2017,37(6):2160-2172.
Cost-benefit analysis of different non-membrane based technologies for the treatment of bio-stabilized leachate.
LIU Wan-ying1,2, LÜ Fan1,2,3, QIU Jun-jie1,2, HUANG Yü-long1,2, ZHANG Hua1,2,3, SHAO Li-ming1,2,3, HE Pin-jing1,2,3*
(1.Institute of Waste Treatment and Reclamation, Tongji University, Shanghai 200092, China;2.Shanghai Institute of Pollution Control and Ecological Security, Tongji University, Shanghai 200092, China;3.Shanghai Multi-source Solid Waste Collaborative Treatment and Energy Engineering Technology Research Center, Tongji University, Shanghai 200092, China)., 2022,42(2):644~653
In this paper, four non-membrane based techniques, including activated carbon adsorption, coagulation, Fenton and electrolysis, were applied to treat the anaerobic-aerobic stabilized leachate. The respective water quality parameters, such as dissolved organic carbon (DOC), chemical oxygen demand (COD), dissolved nitrogen (DN) and specific ultraviolet absorbance at 254nm (SUV254), were determined for comparison. Cost change curve of removing unit COD was also depicted. When activated carbon adsorption, Fenton and coagulation were employed, the removal efficiencies of COD, DOC and DN increased with enhancing the dosages of agents. Fenton and electrolysis as chemical oxidation techniques shows superior performance on the removal of aromatized organics, resulting in 60%~70% reduction of SUV254. In addition, the removal efficiency linearly increased with the current density. For every unit of COD removal, the cost for applying activated carbon adsorption is highest among all the tested techniques while the expense of Fenton will be minimal. To make these techniques economical, it is recommended that 5g/L of activated carbon, 0.605g/L of Fenton reagent and 4.92mmol/L Fe of coagulant were used for each process. When applied in practical application, it is worth noting that the actual dosage need be optimized on basis of the properties of on-site leachate and the local discharge standards.
Adsorption;Coagulation;Fenton;Electrolysis;Non-membrane based technology;Bio-stabilized leachate;Cost-benefit analysis
X703.5
A
1000-6923(2022)02-0644-10
刘婉莹(1997-),女,湖北潜江人,硕士,主要从事固体废物处理与资源化利用的相关研究.发表论文3篇.
2021-07-12
国家重点研发计划项目(2018YFD1100600);国家自然科学基金资助项目(22076145)
* 责任作者, 教授, solidwaste@tongji.edu.cn

