Association and prognostic significance of alpha-L-fucosidase-1 and matrix metalloproteinase 9 expression in esophageal squamous cell carcinoma
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is the predominantly diagnosed histological subtype in China, accounting for approximately 90% of all esophageal carcinomas (EC)[1]. Although more advances in early screening and multimodal therapy have been approved for use in patients with ESCC, the long-term survival rates even after curative surgery remain unsatisfactory[2-4]. This undesirable prognosis appears to be triggered mostly by aggressive tumor cell invasion and metastasis. Novel modalities for ESCC treatment that target molecular pathways are required to change the prognostic dilemma[5]. In addition, patients diagnosed with ESCC of the same stage show varied prognoses[2,3]. Hence, it is also necessary to explore the potential molecular mechanisms of ESCC as prognostic factors to distinguish those patients with a high-risk of local recurrence and/or distant metastasis[2].
The alpha-L-fucosidase-1 () gene, targeted by thetumor suppression gene, encodes a lysosomal enzyme named FUCA1[6]. Its main biological function in human cells is to degrade alpha-L-fucose-containing glycoproteins and glycolipids to inhibit cell growth and induce cell death[5-7]. Moreover, a recent study elaborated that FUCA1 could inhibit the activation and fucosylation of epidermal growth factor receptor (EGFR), thereby blocking the EGFR signaling pathway[6]. Therefore, in theory, the molecular function of FUCA1 appears to diminish the invasion capacity of tumor cells (Figure 1). The association of high FUCA1 expression in serum or tumor tissue with a favorable prognosis in triple-negative breast cancer and intrahepatic cholangiocarcinoma has been confirmed[8,9]. However, probably due to molecular mechanistic heterogeneity, some studies have reported that elevated FUCA1 content predicts worse survival outcomes in hepatocellular carcinoma and glioma[7,10]. To our knowledge, it is unknown whether FUCA1 is expressed in ESCC and whether the expression status of FUCA1 is related to the prognostic outcome of ESCC.
The matrix metalloproteinase (MMP) family, consisting of a group of zinccontaining enzymes that can degrade the extracellular matrix and destroy the basement membrane, plays a critical role in epithelial and mesenchymal tumor invasion and metastasis (Figure 1)[11,12]. Many previous studies and meta-analyses have proven that overexpression of MMP family proteins in ESCC is associated with an unfavorable survival[11-13]. Notably, further studies discovered that FUCA1 could downregulate MMP-9 expression and activity, thereby diminishing the invasive ability of intrahepatic cholangiocarcinoma and breast cancer[9,14]. However, no relationship between FUCA1 expression and MMP-9 expression in ESCC has been reported.

Therefore, the main aim of this study was to evaluate the prognostic significance of FUCA1 and MMP-9 expression in ESCC and investigate the correlation of FUCA1 expression with MMP-9 expression.
MATERIALS AND METHODS
The Cancer Genome Atlas data acquisition
The gene expression profiles in tumor tissues, clinical information, survival times, and outcomes of patients diagnosed with ESCC without distant metastasis were obtained from the public The Cancer Genome Atlas (TCGA) database (https://tcga-data.nci.nih.gov/tcga/). The skewed data ofandexpression profiles were log-transformed to reduce skewness. Then, on the basis of the best cutoff value determined by using X-tile 3.6.3 software (Copyright Yale University 2003), the logtransformed gene expression levels were divided into two groups (high/low).
Certainly, a man who takes in the Intelligencer may live merrily and be buried contentedly9, and by the end of his life will have such a capital stock of paper that he can lie on a soft bed of it, unless he prefers wood shavings for his resting-place
Detection of FUCA1 and MMP-9 in ESCC tissues was carried out by immunohistochemistry (IHC), as described in related studies[6,8]. Specifically, the paraffin slices were incubated with a rabbit anti-human FUCA1 polyclonal antibody (dilution, 1:400;Abcam, Cambridge, United Kingdom) and a rabbit anti-human MMP-9 monoclonal antibody (dilution, 1:300; D603H, Cell Signaling Technology, Danvers, MA, United States). The expression of FUCA1 and MMP-9 was independently evaluated by two pathologists (Zhang MQ and Huang WT) who all engaged in pathological diagnosis over 5 years. If there was inconsistent interpretation, reevaluation under a doublehead microscope was performed to obtain a consistently reliable result.
Patient selection
The clinical, pathological, and follow-up information of all patients who underwent curative esophagectomy for EC from January 1, 2014 to December 31, 2014 at the Sun Yat-Sen University Cancer Center (SYSUCC) was retrospectively collected from the Hospital Information System. All tumor-node-metastasis (TNM) staging was reclassified according to the 8edition of the American Joint Committee on Cancer Staging Manual. Subsequently, only patients who met the following criteria were retained:(1)Diagnosed with thoracic ESCC; (2) Underwent complete removal (R0); (3) No induction therapy; (4) No death occurred within 30 d after operation; (5) No other primary neoplasm; (6) Had adequate paraffin-embedded specimens for immunohistochemical staining; and (7) Had complete follow-up information. A total of 119 consecutive patients were enrolled in this study (Figure 2).
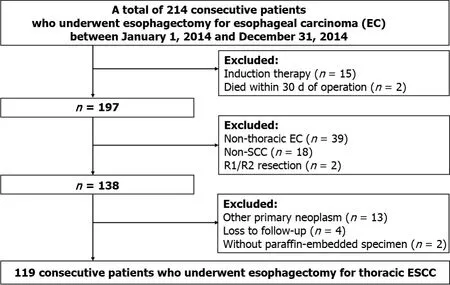
Written informed consent was obtained from all patients themselves during preoperative conversations. This retrospective study was approved by the Research Ethics Committee at the Sun Yat-Sen University Cancer Center (No. 308–2015–012).
Immunohistochemical staining and interpretation
When this was over the slaves escorted him to the outer gate, and took leave of him with every mark of esteem73 and politeness, to which it is to be feared he responded but indifferently, since the gate was no sooner opened than he took to his heels, and fled away with all his might, his one idea being to put as much space as possible between himself and the dreary74 place into which he had ventured so rashly, just to consult a tedious Oracle who after all had told him nothing
The staining intensity was graded on a four-step scale:Negative, weak, moderate,and strong, scored as 0, 1, 2, and 3, respectively. The percentage of the chromogenic reaction, counted in five random fields per section using 100 × magnification, was grouped into < 25%, 25%-50%, 50%-75%, and ≥ 75% (scored 1, 2, 3, and 4, respectively).The case was interpreted as high expression of FUCA1 and MMP-9 if moderate to strong cytoplasmic staining was observed in ≥ 25% of ESCC cells with reference to previous studies[13,15]. In addition, the total immunoreaction score was calculated according to the staining intensity (scores 0-3) multiplied by the percentage of stained ESCC cells (scores 1-4) to generate a total score from 0 to 12[13,15].
Statistical analysis
In 2015, Tzu-Chun Cheng and his colleagues reported that upregulation of FUCA1 expression in triple-negative breast cancer (adenocarcinoma) conferred a favorable OS by degrading cell surface glycoproteins and glycolipids to inhibit cell growth and induce cell death[8]. Subsequently, the association of higher FUCA expression (≥ 20.85 U/L) with a better prognosis in patients with intrahepatic cholangiocarcinoma(adenocarcinoma) was also confirmed. Further mechanistic investigations revealed that FUCA could diminish the invasive ability of tumor cells by downregulating MMP-9 expression[9]. In addition, another recent study on thyroid cancer demonstrated that FUCA1 expression was higher in normal thyroids and papillary thyroid carcinomas than in poorly differentiated, metastatic, and anaplastic thyroid carcinoma;in other words, lower FUCA1 expression was related to a worse prognosis of thyroid cancer (adenocarcinoma)[15]. Similarly, Otero-Estévez[16] reported that the expression and activity of FUCA1 in colorectal cancer (adenocarcinoma) showed a gradual decrease from early to advanced stage, and patients with a low FUCA1 level were significantly associated with a higher tumoral recurrence rate. However, there were controversial reports concerning the relationship between FUCA1 expression and prognostic survival in non-adenocarcinoma malignancies (, hepatocellular carcinoma, glioma, and ESCC)[5,7,10,17]. Two early studies reported that a high preoperative serum FUCA level (> 35/μL) was significantly associated with a worse recurrence-free survival and OS in patients with hepatocellular carcinoma following hepatectomy[7,17]. Recently, another study also found that FUCA overexpression had a negative effect on the prognosis of glioma[10]. The prognostic roles of FUCA1 overexpression in hepatocellular carcinoma and glioma were in agreement with our findings in ESCC. Through further mechanistic studies, they elaborated the following novel mechanisms. First, the lack of FUCA1 protein could promote the development of numerous acidic vacuoles that participate in the autophagic cell death process. Second,FUCA1 overexpression induced tumor-associated macrophage recruitment by upregulating chemokines 2/5 expression, but this pathway could be inhibited by introducingsilenced RNA[10]. Moreover, correlation analyses showed that FUCA1 overexpression was associated with higher proportions of local invasion, higher pathological grade, and lymphatic metastasis[7,10,17]. The above evidence from mechanistic studies and retrospective cohort studies all illustrated that FUCA1 may have the ability to promote tumor cell invasion and metastasis among patients with nonadenocarcinoma[7,10,17]. Therefore, FUCA1 may be used not only as a prognostic biomarker but also as a novel therapeutic target for hepatocellular carcinoma, glioma,and ESCC. In addition, on the basis of the above studies, it became apparent that the molecular mechanisms of FUCA1 were quite different in adenocarcinoma and nonadenocarcinoma malignancies, and further studies are warranted to elucidate the potential molecular pathways in ESCC.
Of the 119 patients from the SYSUCC, 21 (17.6%) were women, and the mean(standard deviation, SD) age of all patients was 59.0 (8.9) years. The majority of patients were smokers at the time of diagnosis (80, 67.2%) and had a history of alcohol use (74, 62.2%). The middle third of the thoracic esophagus was the most common site of ESCC (69, 58.0%), followed by the lower third (36, 30.3%). Upon pathological examination of resected specimens, the majority of tumors were limited to location between the mucosa and adventitia (pT1, 6.7%; pT2, 16.8%; pT3, 52.1%) under the microscope and had regional lymph node metastasis (pN1, 35.3%; pN2, 10.9%; pN3,8.4%). The constituent ratios of pathological TNM stages I, II, and III were 5.0%, 39.5%,and 55.5%, respectively. In addition, 67.2% of all patients (80/119) received postoperative adjuvant chemotherapy. Among these 80 patients, 69 (86.3%) received a docetaxel plus nedaplatin/carboplatin regimen, and the remaining 11 were given a paclitaxel plus nedaplatin/carboplatin regimen.
RESULTS
Patient characteristics
At last he spoke9 to the queen: Dear wife, this man has done me a great service, and has, besides, behaved like a gentleman in not allowing me to send back the money
Association of FUCA1 and MMP-9 expression with clinicopathological features
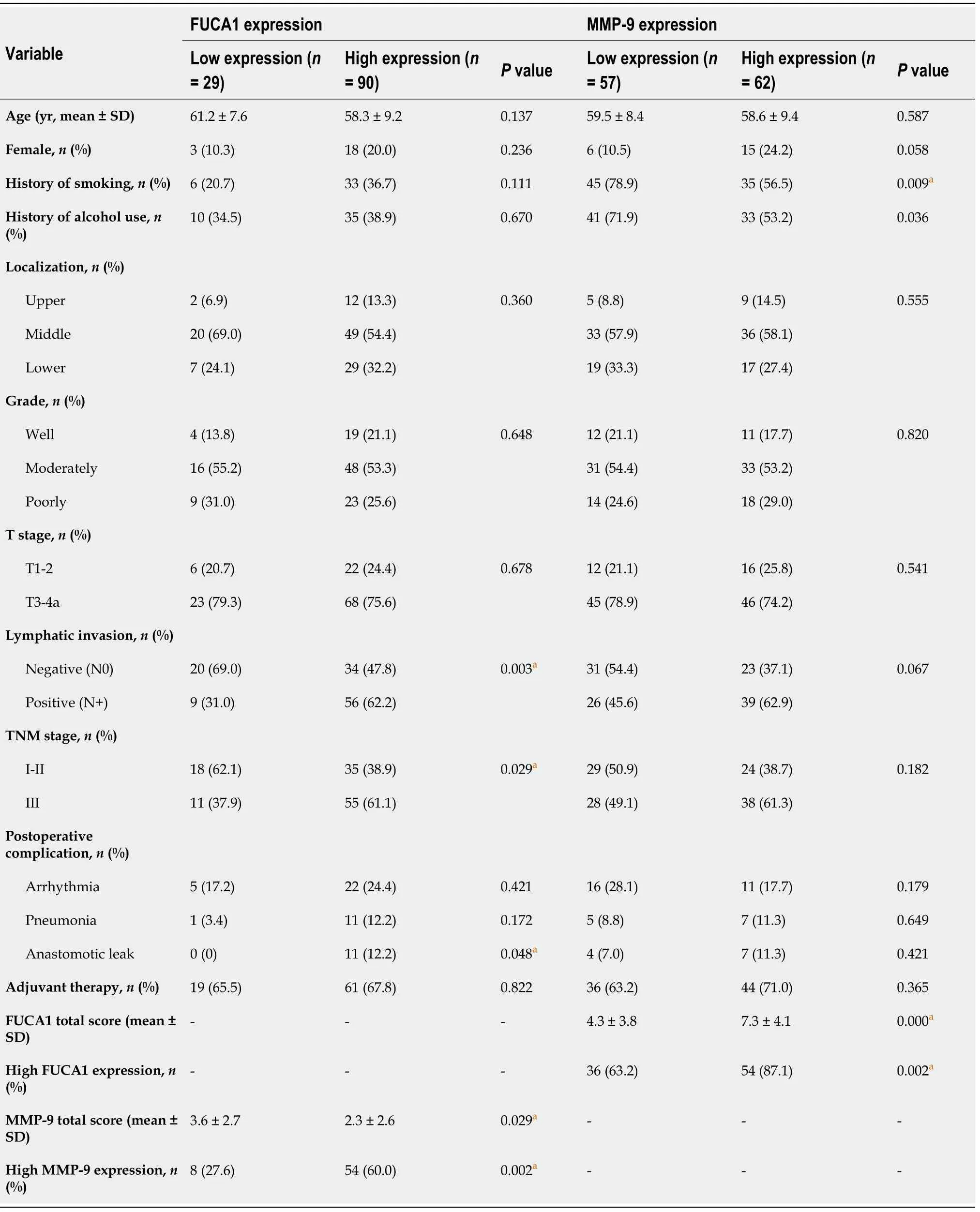
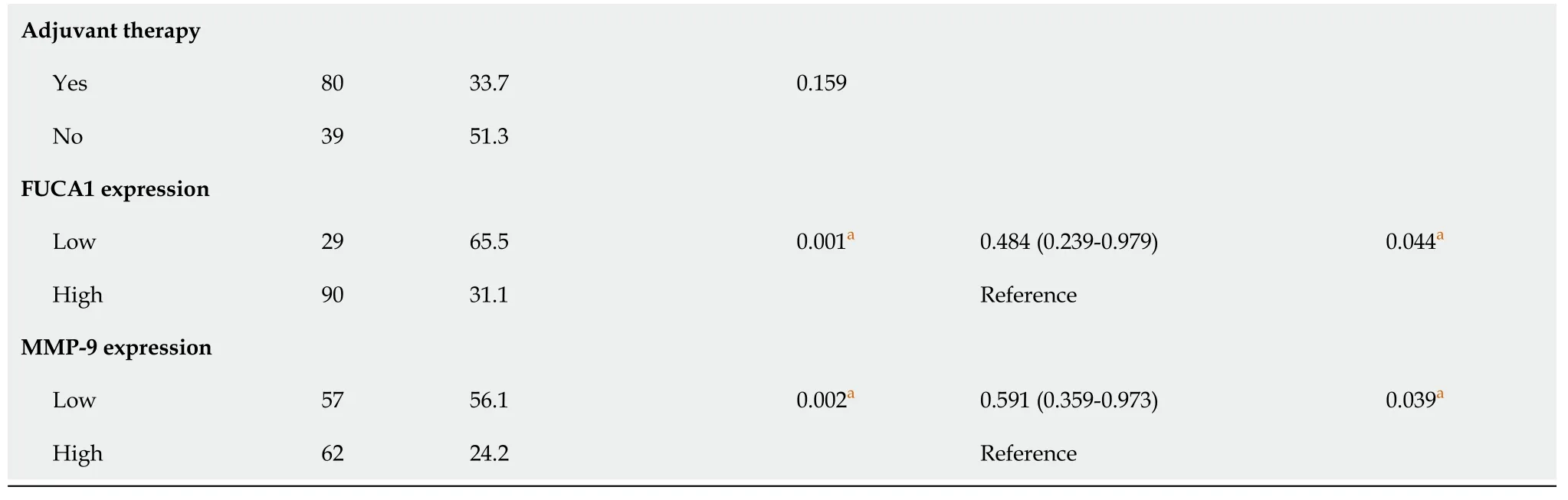
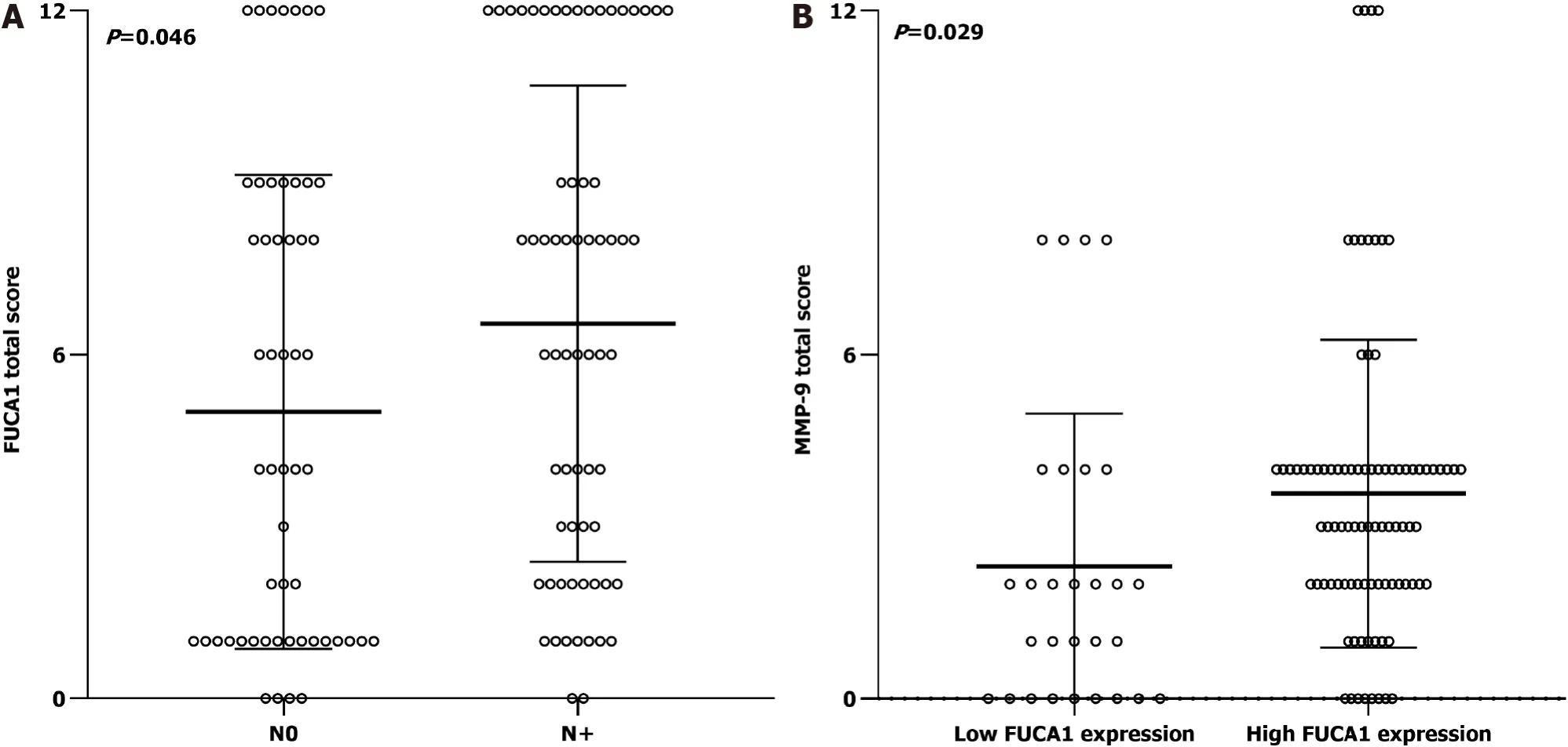
The clinicopathologic features of patients from the SYSUCC and TCGA databases according to the expression status of FUCA1 and MMP-9 are summarized in Table 1 and Supplementary Table 1, respectively. High expression of FUCA1 and MMP-9 in ESCC tumor cells was observed in 90 (75.6%, with a median total score of 6) and 62 patients (52.1%, with a median total score of 3), respectively. The IHC staining for FUCA1 and MMP-9 is shown in Figure 3. High FUCA1 expression was more frequent in patients with positive regional lymph node metastasis (= 0.003, Figure 4A) and advanced stage tumors (= 0.029). In the high MMP-9 expression group, patients showed a lower proportion of smoking history (56.5%78.9%,= 0.009) and alcohol use (53.2%71.9%,= 0.036). The FUCA1 expression status and total score were positively related to the MMP-9 expression status (= 0.002) and total score (= 0.258,= 0.005, Figure 4B), respectively. However, in TCGA data analysis, we only found that male patients (= 0.035) and patients without lymph node metastasis (= 0.050)had a relatively higher proportion of low MMP-9 gene expression (Supplementary Table 1).
My Dad looked at me for the longest time, and his eyes started to tear up. I had never seen him cry. He turned and looked out the windshield. You re right, he said. You are a big boy....a man. I won t kiss you anymore.
Survival outcomes and prognostic analysis
Although our present study clarified the prognostic significance of FUCA1 expression and the correlation of FUCA1 and MMP-9 expression in ESCC for the first time, several limitations cannot be ignored when extrapolating these results. First, this retrospective study was carried out in a single-center, small sample size cohort, which inevitably caused selection bias. Moreover, external validation in other patient cohorts was also lacking. Second, due to intratumoral heterogeneity, the paraffin section used for IHC staining may not represent the entire tumor mass. To obtain the most representative staining, all paraffin slices used in this study included ESCC tissues and corresponding normal esophageal tissues without necrotic tissue. Third, the antibodies selected in this study were not completely the same as those used in previous studies[12,13,15,18]. Undeniably, different antibodies may vary the IHC staining results.Fourth, the consensus cutoff values for FUCA1 and MMP-9 staining interpretation had not been determined; thus, there may be variation in the statistical results in which different cutoff values were used. In this study, the threshold value was selected for interpreting high and low FUCA1/MMP-9 expression with reference to previous large cohort studies[12,13,15,18].
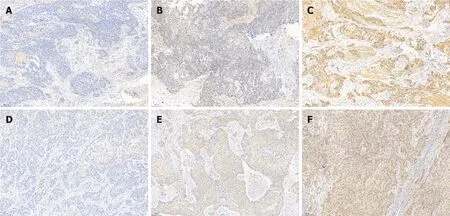
Many studies observed MMP-9 overexpression detected by IHC staining in ESCC(34.8%-90.0%), and the related mechanistic studies elaborated that MMP-9 could participate in the development and progression of ESCC mainly by degrading type IV collagen to promote tumor cell metastasis and resulted in poor prognosis, which was completely consistent with our present study[11]. However, few studies have focused on the association between FUCA1 and MMP-9 expression. Shuang[9] reported that AFU (another abbreviation form for FUCA1) significantly downregulated the expression of MMP-9 in intrahepatic cholangiocarcinoma (adenocarcinoma).Another cytological experiment carried out by Yuan[14] also demonstrated that AFU could significantly reduce MMP-9 activity and expression in human breast cancer cell lines (adenocarcinoma). Conversely, our study found that FUCA1 staining was positively associated with MMP-9 staining in ESCC, which was first reported in squamous cell carcinoma. The detailed molecular mechanisms need to be explored in the future to obtain more effective therapeutic targets in ESCC.
DISCUSSION
In this study, we detected FUCA1 protein overexpression in most ESCC tissues by IHC for the first time and found that high FUCA1 expression was positively associated with high MMP-9 expression, regional lymph node metastasis (pN+), and advanced TNM stage (III). Additionally, multivariable survival analysis showed that the FUCA1 and MMP-9 expression status could independently predict the postoperative survival of patients with resected ESCC.
All statistical analyses were performed using SPSS 24.0 software (IBM, Chicago, IL,United States), and a two-sidedvalue less than 0.05 was defined as a statistically significant difference. Student’stest andtest were used to compare the differences in continuous variables and categorical variables, respectively, between the high expression group and the low expression group. Overall survival (OS) months were counted from the date of ESCC diagnosis to the date of death or the last follow-up(December 31, 2020). The Kaplan-Meier method was applied to identify the potential prognostic variables using the log-rank test. Subsequently, all of the above statistically significant variables were retained in the Cox proportional hazards model. In addition,scatter plots and survival curves were drawn using GraphPad Prism 8.0 software (San Diego, CA, United States).
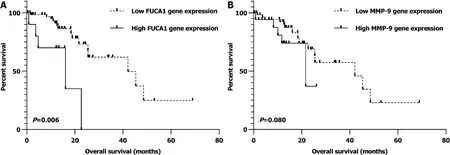
Similarly, after univariate and multivariate analyses, we found that nonmetastatic ESCC patients (I-III) with lowgene expression in the TCGA database were also significantly related to a better OS (= 0.006; Supplementary Table 2 and Figure 6A).However, the relationship of low MMP-9 expression with favorable prognosis was observed as a trend but with no statistical significance (= 0.080; Supplementary Table 2 and Figure 6B).
One evening the Lion said to the King: So you think you have got twelve huntsmen, do you? Yes, certainly, said the King, they _are_ twelve huntsmen
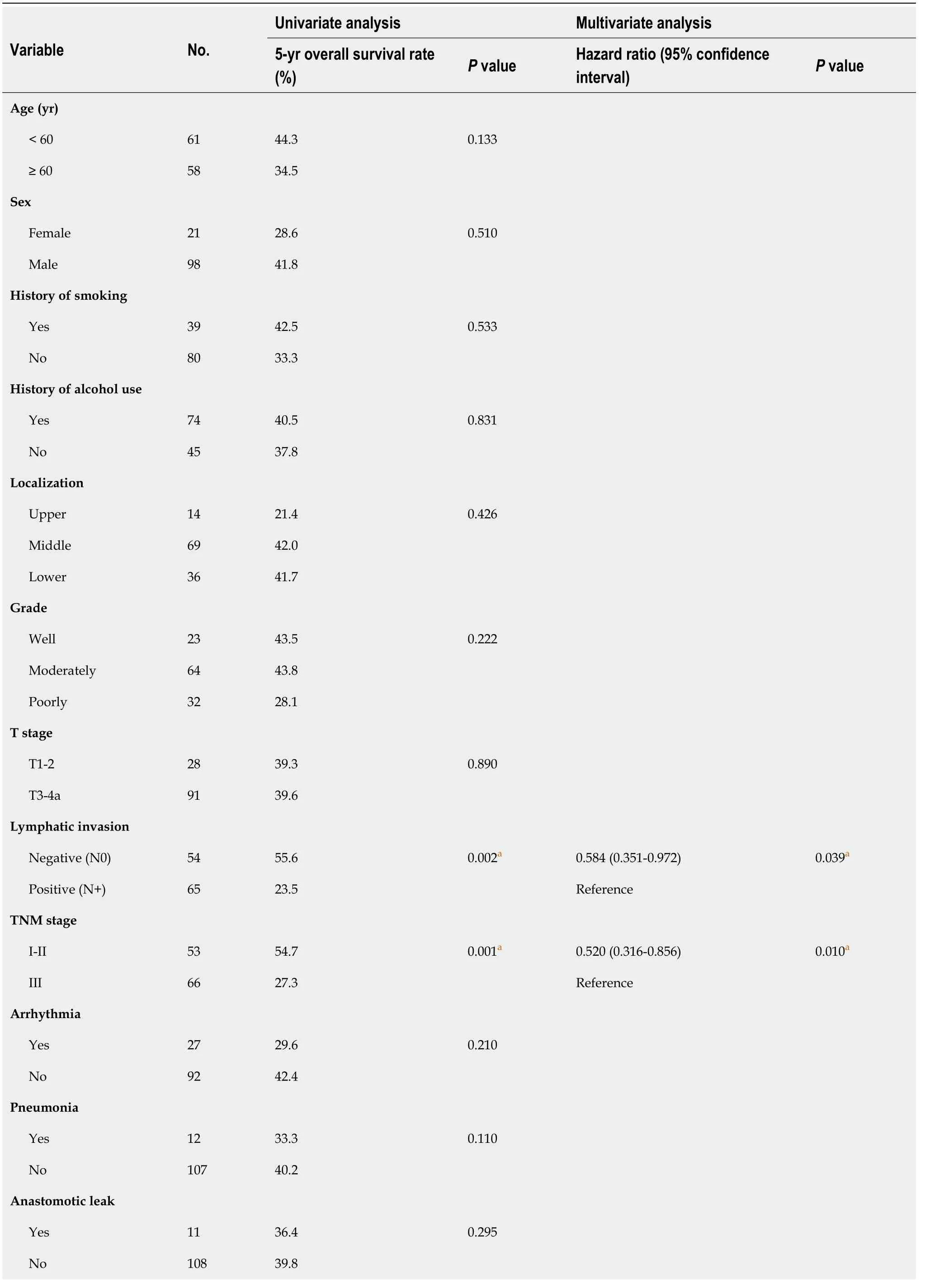
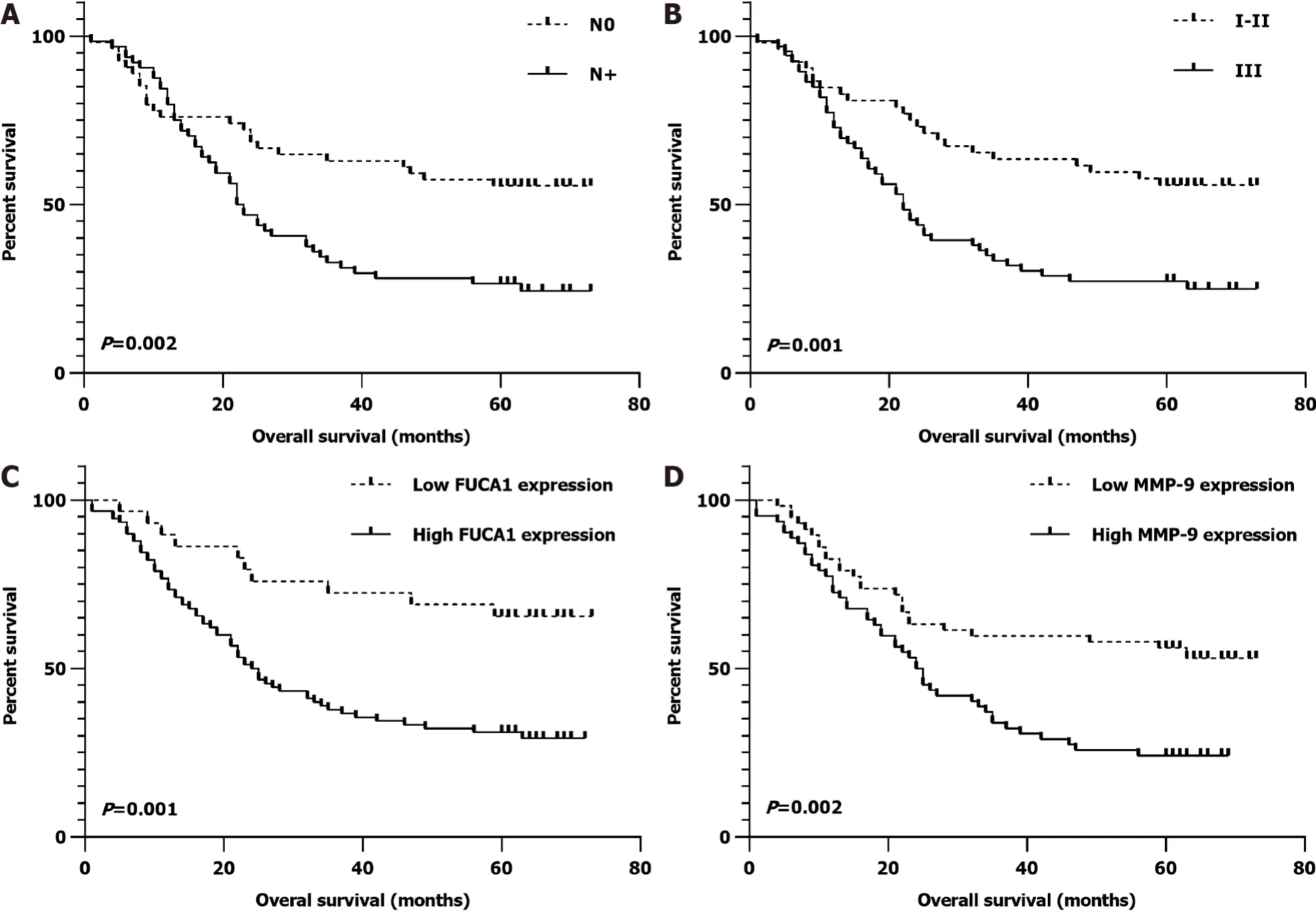
In the SYSUCC cohort, the median OS time was 32.2 mo (range, 1-73 mo) and the 5-year OS rate was 39.5%. In univariate survival analysis, no regional lymph node metastasis (N0,= 0.002), earlier TNM stage (I-II,= 0.001), low FUCA1 expression (= 0.001), and low MMP-9 expression (= 0.002) were significantly associated with a favorable OS (Table 2). After further adjustment in the Cox proportional hazards model, the above four variables still had strong prognostic value for OS (Table 2 and Figure 5).
I sat down in a child-sized chair to catch my breath, hardly aware of what was happening, when she came to me with outstretched hands, bearing a small white box, unwrapped and slightly soiled, as though it had been held many times by unwashed, childish hands
Two can play at that game, I responded. I will wear my gray suit, my Borsalino imported straw hat and a new silk tie. We will be dressed to the nines. This town will never be the same. Almost like our first date.
CONCLUSION
FUCA1 cooperation with MMP-9 may have a major role in affecting the ESCC invasion and metastatic capability and serve as a valuable prognostic biomarker in ESCC.
ARTICLE HIGHLIGHTS
Research background
Fundamental studies discovered that alpha-L-fucosidase-1 (FUCA1) could downregulate matrix metalloproteinase 9 (MMP-9) expression and activity, thereby diminishing the invasive ability of intrahepatic cholangiocarcinoma and triplenegative breast cancer; thus, high FUCA1 expression and low MMP-9 expression in serum or tumor tissue are associated with a favorable prognosis. However, likely due to molecular mechanistic heterogeneity, some studies have reported that elevated FUCA1 content predicts worse survival outcomes in hepatocellular carcinoma and glioma.
Research motivation
To explore the prognostic significance of FUCA1 and MMP-9 expression in esophageal squamous cell carcinoma (ESCC) and investigate the correlation of FUCA1 expression with MMP-9 expression.
Research objectives
A total of 119 consecutive patients who underwent esophagectomy for ESCC between January 1, 2014 and December 31, 2014 at the Sun Yat-Sen University Cancer Center(SYSUCC) were enrolled in the final analysis. In addition, the FUCA1 and MMP-9 gene expression profiles of 76 patients diagnosed with ESCC without distant metastasis were obtained from the public The Cancer Genome Atlas (TCGA) database.
Research methods
Student’s t test and χ2 test were used to compare the differences in continuous variables and categorical variables, respectively. The Kaplan-Meier method was applied to identify the potential prognostic variables using the log-rank test.Subsequently, all of the above statistically significant variables were retained in the Cox proportional hazards model.
Research results
In the SYSUCC cohort, the FUCA1 expression status (high/low) and total IHC score were positively related to the MMP-9 expression status (high/low, P = 0.002) and total IHC score (r = 0.258, P = 0.005). Moreover, after further adjusting in the Cox propor tional hazards model, low FUCA1 expression (= 0.001) and low MMP-9 expression (= 0.002) still showed significant associations with a favorable overall survival (OS).Similarly, after univariate and multivariate analysis, we found that nonmetastatic ESCC patients (I-III) with lowgene expression in the TCGA database also had a significantly better OS (= 0.006). However, the relationship of lowexpression with a favorable prognosis was observed as a trend but with no statistical significance (= 0.080).
Research conclusions
FUCA1 cooperation with MMP-9 may have a major role in affecting ESCC invasion and metastasis capability and serve as a valuable prognostic biomarker in ESCC.
Research perspectives
The present study offers a future research direction in which FUCA1 cooperation with MMP-9 may be a potential regulator in ESCC progression, which needs to be explored in fundamental studies.
 World Journal of Gastrointestinal Oncology2022年2期
World Journal of Gastrointestinal Oncology2022年2期
- World Journal of Gastrointestinal Oncology的其它文章
- Anal human papilloma viral infection and squamous cell carcinoma:Need objective biomarkers for risk assessment and surveillance guidelines
- Gastric epithelial histology and precancerous conditions
- Small bowel adenocarcinoma:An overview
- Microbiome and colorectal carcinogenesis:Linked mechanisms and racial differences
- Frankincense myrrh attenuates hepatocellular carcinoma by regulating tumor blood vessel development through multiple epidermal growth factor receptor-mediated signaling pathways
- Relation between skeletal muscle volume and prognosis in rectal cancer patients undergoing neoadjuvant therapy
