Tailoring the optical and magnetic properties of La-BaM hexaferrites by Ni substitution
Hafiz T.Ali M.Ramzan M Imran Arshad Nicola A.Morley M.Hassan Abbas Mohammad YusufAtta Ur Rehman Khalid Mahmood Adnan Ali Nasir Amin and M.Ajaz-un-Nabi
1Department of Mechanical Engineering,College of Engineering,Taif University,Taif 21944,Saudi Arabia2Department of Physics,Government College University,Faisalabad,Pakistan3Department of Materials Science and Engineering,University of Sheffield,Sir Robert Hadfield Building,Mapping St.,Sheffield,S1 3JD,UK4Department of Clinical Pharmacy,College of Pharmacy,Taif University,Taif 21944,Saudi Arabia
We investigate the impact of Ni insertion on the structural, optical, and magnetic properties of Ba0.8La0.2Fe12−xNixO19 hexaferrites (Ni substituted La-BaM hexaferrites). Samples were prepared using the conventional co-precipitation method and sintered at 1000 °C for 4 hours to assist the crystallization process. An analysis of the structure of the samples was carried out using an x-ray diffraction (XRD) spectrometer. The M-type hexagonal structure of all the samples was confirmed using XRD spectra. The lattice parameters a and c were found to be in the ranges of 5.8925±0.001 nm—5.8952±0.001 nm and 23.2123±0.001 nm—23.2219±0.001 nm,respectively. The M-type hexagonal nature of the prepared samples was also indicated by the presence of corresponding FT-IR bands and Raman modes in the FT-IR and Raman spectra, respectively. EDX results confirmed the successful synthesis of the samples according to the required stoichiometric ratio. A UV-vis spectrometer was used to record the absorption spectra of the prepared samples in the wavelength range of 200 nm—1100 nm. The optical energy bandgap of the samples was found to be in the range of 1.21 eV—3.39 eV. The M—H loops of the samples were measured at room temperature at an applied magnetic field range of 0 kOe—60 kOe. A high saturation magnetization of 99.92 emu/g was recorded in the sample with x=0 at a microwave operating frequency of 22.2 GHz. This high value of saturation magnetization is due to the substitution of La3+ ions at the spin-up (12k, 2a, and 2b) sites. The Ni substitution is proven to be a potential candidate for the tuning of the optical and magnetic parameters of M-type hexaferrites. Therefore, we suggest that the prepared samples are suitable for use in magneto-optic applications.
Keywords: M-type hexaferrite,nanostructured materials,magnetic properties,optical properties
1. Introduction
In recent years, hexagonal magnetic nanoparticles have attracted great scientific interest due to their potential for use in various technological applications such as permanent magnets, microwave absorbers, highdensity magnetic media,magneto-optic recording media, and stealth technology.[1—4]Hexagonal ferrites are divided into five sub-categories based on their crystal structure and chemical formula: M-type(AFe12O19), W-type (AFe16O27), X-type (AFe28O46), Y-type(AFe12O22), and Z-type (AFe24O41), whereAis the divalent cation such as Ba2+, Sr2+, Pb2+, Ca2+.[5—7]Among these, M-type hexaferrites have gained more attention due to their distinguished properties, such as their very high magnetic anisotropy,higher coercivity,higher corrosion resistance,and high thermal and chemical stability.[8—11]Barium M-type(BaM)hexaferrites are ferrimagnetic materials with the chemical formula BaFe12O19and the space groupP63/mmc. They consist of tetrahedral (4f1), octahedral (12k, 4f2, and 2a),and hexahedral (2b) sites. Fe3+ions at the 4f1and 4f2sites(eight ions per unit cell) exhibit a spin-down character and Fe3+ions at the 12k, 2a, and 2bsites (sixteen ions in a unit cell)exhibit a spin-up character.[12,13]The substitution of various magnetic and non-magnetic ions at these sites can modify the structural, magnetic, and other properties of BaM ferrites. Some examples of substitution at different sites of the hexaferrite structure are divalent ion (A2+) substitution,[14,15]trivalent ion(A3+)substitution,[16,17]and tetravalent ion(A4+)substitution.[14,18]To achieve the best hexaferrite properties,it is very important to optimize the quantity and type of the substituent cations. Various reports have been published on the topic of modifying the microstructure, electrical, dielectric, optical, and magnetic properties of BaM hexaferrite by replacing the Fe3+and Ba3+cations with divalent and trivalent cations. Recently, Cerneaet al.[9]reported the magnetic properties of Ni2+substituted BaFe12O19hexaferrite.They observed a decreasing trend in the coercivity, retentivity, and maximum energy product with an increase in Ni2+.Iqbalet al.[19]studied the effect of Pr—Ni substitution in the crystal lattice of BaSr hexaferrite. They reported an increase in the electrical resistivity of the prepared samples with an increase in the Ni content. Beheraet al.[20]also investigated the effect of Ni2+incorporation in the lattice matrix ofBaFe12O19synthesized by the sol—gel method. They found a reduction in the saturation magnetization from 68.16 emu/g to 58.99 emu/g when the Ni2+content (x) increased from 0 to 0.5, and an increase in the ferrimagnetic transition temperature (TC) was observed. Guneret al.[21]reported the synthesis of Bi and La doped BaM hexaferrite by the sol-gel autocombustion method. They studied the dependence of the magnetic properties of BaFe12O19on the concentrations of various dopants such as La, Bi, and Y. They reported the saturation magnetization and coercivity of the prepared samples in the range of 53.69 emu/g—67.42 emu/g and 3.812×105A/m—2.177×105A/m, respectively. Some other reports describe attempts to modify the structural, electrical, optical, or magnetic properties of BaM by doping with Ni2+, La3+, and the co-doping of these cations.[19,22—25]However,a detailed study exploring the impact of Ni2+doping on the structural, optical, and magnetic properties of La-BaM hexaferrites is still needed. High coercivity, moderate saturation magnetization,and low cost are all advantages of these materials. The objective of this work is to provide a detailed study of the impact of Ni2+substitution in La-BaM hexaferrites. A series of samples with the chemical formula Ba0.8La0.2Fe12−xNixO19(Ni-doped BL) hexaferrites was prepared via the co-precipitation route.The co-precipitation method was chosen due to its simplicity,low cost, normal temperature, reduced duration, and the homogeneity of prepared nanoparticles.[26,27]The prepared samples were investigated using different techniques including xray diffraction (XRD), energy dispersive x-ray (EDX) spectroscopy,Fourier transform infrared(FTIR)spectroscopy,Raman spectroscopy,UV-vis spectroscopy,and vibrating sample magnetometry(VSM).
2. Experimental details
2.1. Synthesis
In this study, Ba0.8La0.2Fe12−xNixO19(x=0,0.1,0.2,0.3,0.4,0.5) ferrites were prepared by the coprecipitation method. The starting materials BaCl2·2H2O,Ni(NO3)2·6H2O,Fe(NO3)3·9H2O,and La(NO3)3·6H2O were purchased from Aldrich and used as received. The required amounts of these chemicals were used to prepare a homogeneous solution in deionized water according to the required stoichiometric ratios. The solution was stirred magnetically on a hotplate at a temperature 70°C until the formation of precipitates occurred. The pH of the solution was maintained at 11 during the precipitation process by adding NaOH dropwise. The samples were placed in a water bath for 12 hours at 90°C to complete the digestion process. The precipitates were laid down at the bottom of the beakers. These precipitates were collected and cleaned with DI water and ethanol to remove impurities. The drying of the washed precipitates was accomplished by placing them in the oven for 15 hours at 110°C. The dried precipitates were then ground using a mortar and pestle to obtain fine powder samples. The samples were sintered at 1000°C in a muffle furnace for four hours to assist the crystallization process. The cooling was done at a very slow rate(~1.4°C)to avoid the development of pores.
2.2. Characterization
A phase and microstructure analysis of the sintered samples was carried out using an x-ray diffractometer (D8 Advance,Bruker),with CuKαas the x-ray source. The diffraction patterns were recorded in the 2θrange of 20°—60°. An FT-IR spectrometer (Spectrum 2, PerkinElmer) was used to obtain the infrared spectra. An EDX spectrometer was used to determine the elemental composition of the prepared samples.The Raman spectra were recorded in the wavenumber range of 150 cm−1—800 cm−1using a Raman spectrometer.The optical energy bandgap values were determined using a UV-Vis spectrometer(double beam,Lambda 25,PerkinElmer). The magnetic character of the sintered samples was studied by recordingM—Hloops at room temperature using a vibrating sample magnetometer(Lakeshore-7407).
3. Results and discussion
3.1. Phase analysis
The XRD spectra of the synthesized Ni-doped La-BaM hexaferrites, as shown in Fig. 1, were recorded at 300 K.A comparison of the XRD patterns revealed that all the labeled peaks were well-matched with the JCPDS data (card#00-051-1879) for BaM hexaferrites, and that no extra peaks were observed. This confirms the formation of a single-phase magneto-plumbite structure with the space groupP63/mmcto a very high accuracy and without any defects or impurity phase. The various crystal structure parameters including the lattice constants (a&c),[28]crystallite size, unit cell volume,[8]x-ray density,[26]and dislocation density[29,30]are calculated utilizing the indexed XRD patterns; the calculated parameters are presented in Table 1. From Fig.2,it can be observed that the lattice constantaincreased with an increased Ni2+ion content, andcalso increased with the addition of doping except atx=0.3. It can also be seen in Fig. 2 that the unit cell volume (V) was enhanced by the substitution of dopant ions. This increase is attributed to the difference in the ionic radii of Ni2+(0.69)and Fe3+(0.64).[20]The average crystallite size of the prepared nanoparticles was found to be in the range of 25.2±0.1 nm—28.5±0.1 nm and showed a decreasing trend with an increase in the Ni2+ion concentration. The calculated x-ray density of the samples was found to lie in the range of 5.144 g/cm3—5.310 g/cm3. The dislocation density of a material is inversely proportional to the square of the crystallite size and provides information about the strength of the material.[29,30]The calculated values of the dislocation density of the prepared nanoparticles revealed that the sample withx=0.1 had the minimum value of dislocation density.

Table 1. Concentrations of Ni2+ and calculated structural parameters of Ba0.8La0.2Fe12−xNixO19 hexaferrites.

Fig.1. XRD patterns of the synthesized Ba0.8La0.2Fe12−xNixO19 hexaferrite powders.

Fig. 2. Plot of the lattice parameters (a & c) and unit cell volume (V) as a function of the Ni2+ content in the synthesized hexaferrite samples.
3.2. Elemental composition analysis
The elemental composition of the synthesized samples was analyzed quantitatively using EDX spectroscopy; the results are presented in Table 2. The observed composition of all the elements was a good match for the expected stoichiometric composition.The results confirmed that La3+and Ni2+cations had been substituted for Ba and Fe cations at the appropriate ratios.

Table 2. Elemental composition of Ba0.8La0.2Fe12−xNixO19 M-type hexaferrites obtained by EDX.
3.3. Functional group analysis
The FTIR spectra of the prepared powder were recorded in the range of 4000 cm−1—400 cm−1and are presented in Fig.3. The absorption bands around 430 cm−1and 580 cm−1are the two featured bands of M-type hexaferrites,which were observed in all the samples, with minor variations in the positions and relative intensities. The band observed at around 430 cm−1relates to the octahedral Fe—O bond,while the band at around 580 cm−1is due to the stretching vibrations of the tetrahedral Ba—O bond.[31,32]The presence of these bonds confirmed the formation of the BaM hexagonal structure. The doublet peak at 2361 cm−1originates from ambient CO2gas.

Fig.3. FTIR spectra of the synthesized hexaferrite samples.
3.4. Raman analysis
The Raman spectra of the hexaferrite samples are shown in Fig.4. According to group theory,42 active Raman modesΓRaman=11A1g+14E1g+17E2garise due to the vibrations of 64 atoms at various sites in the unit cell.[33]In our samples,active Raman modes are observed at around 284.71, 335.22,410.28,457.21,522.13,613.52,and 684.87 cm−1,which confirms the formation of the BaM phase. The peaks present at around 684.87 cm−1are due to theA1gvibration of Fe—O bonds at the octahedral 4f1site and the bipyramidal 2bsite.Furthermore, the peaks at 457.21 cm−1are also due to theA1gvibration of Fe—O bonds at the octahedral 2a site. The peaks at around 613.52 cm−1and 410.28 cm−1are attributedto theA1gvibration of Fe—O bonds at the octahedral 4f2and 12ksites respectively. The Raman modes observed at around 522.13 cm−1and 284.71 cm−1are attributed toE1gvibration. Moreover, the peaks at 335.22 cm−1are attributed toE2gvibrations. The small variations found in the peak positions are attributed to the substitution of Ni2+ions in the BaM lattice.[31,34—36]

Fig.4. Raman spectra of the Ni2+ substituted Ba-La hexaferrite samples.
3.5. UV-vis analysis
The absorption spectra and optical energy bandgap are related,as given by Tauc’s relation:α(hv)=A(hv−Eg)n,[37—39]whereAandαrepresent a constant and the absorption coefficient, respectively, andntakes values of 1/2 for direct and 2 for indirect transitions. Tauc plots of the prepared samples are presented in Fig. 5. The obtained values of the optical energy bandgaps lie in the range of 1.21 eV—3.39 eV and are plotted as a function of the Ni2+ion content, as shown in Fig. 6. It can be seen from Fig. 6 that the optical bandgap is drastically decreased by the incorporation of a small amount of Ni2+at values ofxup tox=0.2, however, a further increase in the Ni2+concentration does not have a significant effect on the bandgap values.[26,31,40—44]These results suggest that the synthesized samples are potential candidates for use in various optoelectronic applications.
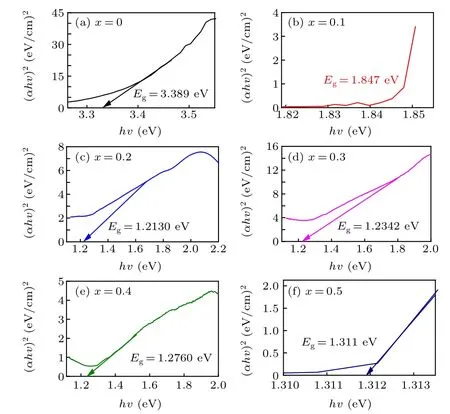
Fig.5. Plot of(αhυ)2 versus the photon energy(hυ)for the prepared hexaferrite nanoparticles.

Fig.6. Plot of the energy bandgap as a function of the Ni2+ ion content.
3.6. Magnetic hysteresis loop analysis
The magnetic hysteresis(M—H)loops of the synthesized nanohexaferrite samples were recorded at 300 K and are depicted in Fig. 7. TheM—Hloops of all the samples show a hard magnetic nature. Various magnetic parameters,including the saturation magnetization (Ms), magnetic retentivity (Mr),coercivity (Hc), and squareness ratio (Mr/Ms) are measured from theM—Hloops and are given in Table 3. The maximum observed value of saturation magnetization is 99.92 emu/g for the sample withx=0, which reduced to 40.8 emu/g for the sample withx=0.5. According to previous research, Fe3+ions exhibit various magnetic moments at different sites. As a result, the preferred sites of magnetic Ni2+ions determine the material’s overall magnetization. The Ni2+has a magnetic nature, but a low magnetic moment compared to Fe3+.It was reported previously that Ni2+ions prefer to substitute for Fe3+ions at 4f2and 12kat low concentrations and at the 12ksites for higher concentrations. The substitution of Ni2+(2µB)ions at the Fe3+(5µB)ion sites is responsible for the decrease in saturation magnetization with increasing Ni2+concentration.[15,20,31,45]The saturation magnetization values that are achieved in this study for Ni2+free samples are compared with the values reported in the literature[4,9,20,45—47]and are found to be significantly higher. The higher values of saturation magnetization achieved are attributed to the vacancy free nature of the samples and the presence of paramagnetic La3+cations at the spin-up(12k, 2a, and 2b)sites.[48,49]The magnetic retentivityMr, coercivityHc, and squareness ratio(Mr/Ms) also decreased with an increase in the Ni2+content. A graphical representation of the Ni2+contents versus the saturation magnetization (Ms) and the magnetic retentivity (Mr) are shown in Fig. 8. Magneto-crystalline anisotropyis known to be responsible for the variations in the magnetic retentivity, coercivity, and squareness ratio values of ferrite materials.[20]The microwave frequency (ωm) is determined using the relationωm=8π2Msγ, whereγis a gyromagnetic fraction with the significance of 2.8 MHz/Oe, andMsis the saturation magnetization.[50]The applied field versus the microwave operating frequency(ωm)of the prepared hexaferrites samples is plotted in Fig. 9. It can be seen in Fig. 9 that the range of microwave operating frequencies(ωm)is 8.80 GHz—22.2 GHz, indicating that the prepared hexaferrites are applicable in longitudinal recording media, data storage magnetic devices,and microwave absorption.

Table 3. Magnetic parameters of the prepared hexaferrites.

Fig.7. The M—H loops for the Ni2+ doped Ba—La hexaferrites.

Fig. 8. Plot of the saturation magnetization and remanent magnetization of the synthesized samples as a function of the Ni content.

Fig.9. Applied field versus microwave operating frequency.
4. Conclusion
The BaM nanoferrites with the general formula Ba0.8La0.2Fe12−xNixO19(0≤x ≤0.5)were synthesized by the co-precipitation route. The prepared powders were densified using conventional sintering processing at 1000°C for 4 h.A phase confirmation and a structural analysis were carried out using XRD and the average crystallite size of the samples was found to be in the range of 25.2±0.1 nm—28.5±0.1 nm.The optical properties of the prepared samples were investigated using UV-vis spectroscopy and it was found that the optical energy bandgap lies in the range 1.21 eV—3.39 eV. A high value of saturation magnetizationMs(99.92 emu/g)was achieved for the sample withx= 0, which makes La-BaM hexaferrites suitable for operation in the significantly higher frequency range of up to 22.2 GHz. The magnetic parameters(Ms,Mr,Hc, andMr/Ms)of the prepared samples show a decreasing trend with an increase in the Ni content. The optical and magnetic properties of the Ni2+substituted BaM hexaferrites suggest that these samples are potential candidates for use in magnetic storage, microwave frequency absorption devices,and magneto-optical devices.
Acknowledgment
Project supported by the Taif University Researchers Supporting Project number (TURSP-2020/293), Taif University,Taif,Saudi Arabia.
- Chinese Physics B的其它文章
- High sensitivity plasmonic temperature sensor based on a side-polished photonic crystal fiber
- Digital synthesis of programmable photonic integrated circuits
- Non-Rayleigh photon statistics of superbunching pseudothermal light
- Refractive index sensing of double Fano resonance excited by nano-cube array coupled with multilayer all-dielectric film
- A novel polarization converter based on the band-stop frequency selective surface
- Effects of pulse energy ratios on plasma characteristics of dual-pulse fiber-optic laser-induced breakdown spectroscopy