Optimization of preparation process of cationic liposome nanoparticles containing Survivin-siRNA and osthol
Qi-Chao Sun,Hao-Tian Bai,Ya-Lan Li,Jian-Nan Meng,Yu-Wei Guo,Rui Wang✉
1.The First Affiliated Hospital of Heilongjiang University of Chinese Medicine,Harbin 150040,China
2.Heilongjiang University of Traditional Chinese Medicine,Harbin 150040,China
Keywords:siRNA Cationic liposomes Film dispersion method Preparation technology
ABSTRACT Objective:To prepare cationic liposome nanoparticles loaded with survivin -siRNA and Cnidium monnieri based on the ability of liposomes to contain both water-soluble and lipid soluble components.Methods:The preparation technology of Osthol cationic liposomes was optimized by orthogonal test with membrane material ratio,drug lipid ratio,ultrasonic time and steaming temperature as factors.The volume ratio of HA-siRNA to protamine and the ratio of HA-siRNA protamine complex to liposome were investigated by control variable method with potential and particle size as indexes.The particle size and zeta potential were measured by potentiometric particle size analyzer,and the shape was observed by transmission electron microscope;The absorbance of different concentrations of FAM-Survivin-siRNA standard solution was measured by microplate analyzer,and the entrapment efficiency of cationic liposomes loaded with FAM-Survivin-siRNA and osthole was calculated.Results:The optimum preparation conditions of osthole loaded cationic liposomes were as follows:the ratio of membrane to material was 3:1,the ratio of drug to lipid was 1:5,the steaming temperature was 30℃,the ultrasonic time was 70 min,and the encapsulation efficiency was 78.34%.The optimum preparation conditions of osthole loaded cationic liposomes loaded FAMSurvivin-siRNA were as follows:the volume ratio of Survivin-siRNA to protamine was 1:1,Protamine complex 25μg.Add 50μL cationic liposomes.The particle size is 132.3±0.2nm,zeta potential is 43.15±0.05mv,and its shape is irregular round;According to the standard curve,the entrapment efficiency of cationic liposome nanoparticles co loaded with Survivin-siRNA and osthole was 81.34 ± 0.041%.Conclusion:The prepared cationic liposome nanoparticles loaded with Survivin-siRNA and osthole have good encapsulation efficiency,particle size and zeta potential.
1.Introduction
SiRNA,a kind of double RNA molecule,is also known as small interfering RNA.Small interfering RNA is a double stranded microRNA molecule,which is made of dsDNA by processing.Dicer enzyme can recognize and unpack dsRNA,which is a RNA of certain length and structure,namely siRNA [1-3].SiRNA has many disadvantages,such as high molecular weight,low transfection efficiency,poor cell targeting,and it is easy to be inhibited and degraded by human glomerular filtration and endogenous enzymes when it exists in human blood tissue or human cytoplasm.It may even produce strong skin inflammation and low bioavailability[4-5].Osthol is a fat soluble component in the traditional Chinese medicine,which has been proved to have the function of regulating immunity and inhibiting tumor growth[6].The cationic liposomes have relatively high encapsulation rate for siRNA,and have good ability of cell defense against anions and strong chemical stability of serum [7-8].Cationic liposomes can reduce the toxicity of drugs,but also have cell affinity and targeting.Liposomes can also be loaded with water-soluble substances and lipid soluble substances[9].Because siRNA is water-soluble material and ostazone is lipid soluble substance,the cationic liposome nanoparticles can be prepared by using the advantages of cationic liposomes.In this paper,the preparation of Survivin-siRNA and cnidin cationic liposomes nanoparticles was prepared by membrane dispersion method,and the preparation process was optimized by single factor and orthogonal experiment.
2.Materials and methods
2.1 Materials
Osb-2200 (Japanese eyela Society),ultrasonic cell crusher (Sonics company of USA),synergy H1 (Berton Instrument Co.,Ltd.),nanozs90 (Malvin company,UK) were used as potential particle size instrument.
DOTAP(Shanghai Sisin biological Co.,Ltd.batchNo.:rd-01173),cholesterol (China Pharmaceutical Group Chemical Reagent Co.,Ltd.batchNo.:20130618),hyaluronic acid (Shanghai Yuanye Biotechnology Co.,Ltd.batch No.:h02n8j47121),protamine (Shanghai Yuanye Biotechnology Co.,Ltd.batch No.:z31o8h47046),siRNA/ FAM-siRNA (Suzhou Gema gene Co.,Ltd.),DEPC (cas:1609-47-8) of Beijing bolotoda Technology Co.,Ltd.,dspe-peg2000 (batch No.of Shanghai Yuanye Biotechnology Co.,Ltd.:p31m8s32960).Characteristics of ostazone (Xi'an Lvtian Biotechnology Co.,Ltd.):white fine powder water:<1.00% heavy metal:<10ppm arsenic salt:<2ppm content: 98.0%.The product is subject to the relevant project inspection methods in the appendix of the 2020 edition of Chinese Pharmacopoeia.It is in accordance with the regulations and can be used for medicine.
2.2 Experimental methods
2.2.1 Reagent preparation
0.1 % DEPC water:take 1000ml of deionized water,add depcml accurately,seal the bottle mouth of fresh-keeping film,stir it overnight by magnetic force,and configure it as 0.1% DEPC water.200 μg/ml protamine:weigh 2mg of protamine accurately,put it into the 10ml volumetric flask after treatment,add 0.1% DEPC water to dissolve and fix the volume.
Two hundred μg/ml hyaluronic acid:weigh 2mg of hyaluronic acid accurately,put it into the 10ml volumetric flask after treatment,add 0.1% DEPC water to dissolve and fix the volume.
10mg/ml DSPE-PEG2000:weigh 50mg of dstearoyl phosphatidylethanolamine polyethylene glycol 2000 (Dspe-peg2000)accurately,put it into the 5ml volumetric flask after treatment,and add 0.1% DEPC water to dissolve and fix the volume.
2.2.2 Preparation process
Weigh the amount of dosap and cholesterol in a 100ml round bottom flask,and add the appropriate chloroform to dissolve.The solution is completely to be dissolved,and the organic solvent is removed on the rotary evaporation instrument until the liposome forms a film on the wall of the vessel.Put in vacuum drying box to completely remove organic solvent.Add a proper amount of 0.1% DEPC to wash the film.The ultrasonic treatment was performed for 30min,adding the prescription amount of ostazone,and then ultrasonic treatment according to the time required by the prescription.The ultrasonic was performed for 60s by cell crusher,and then slowly over 0.45μl microporous membrane was repeatedly filtered for 5-10 times to obtain the emulsion white uniform suspension.Take Survivin-siRNA out of refrigerator,centrifuge at 4000rpm for 1min,add 0.1% DEPC water to shake and dissolve well.The HA-siRNA protamine complex was prepared by mixing siRNA solution with hyaluronic acid of equal volume,shaking evenly,adding a proper amount of protamine,and then standing at room temperature for 10 minutes.The cationic liposomes of the saturated ostazone and HA-siRNA protamine complex were mixed fully.The liposomes were put into the water bath at 50℃ for 10 minutes.The nanoparticles containing Survivin-siRNA and ostazone cationic liposomes were prepared.
2.2.3 Single factor experiment
2.2.3.1 Investigate the influence of membrane material ratio on the encapsulation efficiency
When other conditions were fixed,the mass ratio of DOTAP to cholesterol was 5:1,4:1,3:1,2:1,1:1.Osthole loaded liposomes were prepared according to "1.2.2",and the change of entrapment efficiency of liposomes was investigated.
2.2.3.2 Investigate the effect of drug lipid ratio on encapsulation efficiency
When the mass ratio of osthole to liposome was 1:3,1:4,1:5,1:6 And 1:7,osthole loaded liposomes were prepared according to"1.2.2",and the change of entrapment efficiency of liposomes was investigated.
2.2.3.3 The effect of ultrasonic time on encapsulation efficiency was investigated
Other conditions were fixed and the ultrasonic time was changed to 40min,50min,60min,70min and 80min respectively.Osthole loaded liposomes were prepared according to "1.2.2",and the change of entrapment efficiency of liposomes was investigated.
2.2.3.4 The effect of rotary steaming temperature on encapsulation efficiency was investigated
Other conditions were fixed and the rotary steaming temperature was changed to 20℃,30℃,40℃,50℃ and 60℃,respectively.Osthole loaded liposomes were prepared according to "1.2.2",and the change of entrapment efficiency of liposomes was investigated.
2.2.4 Optimization of osthole liposome formulation by orthogonal experiment
According to the results of single factor experiment analysis,the effect of rotary steaming temperature on osthole entrapment efficiency was not significant (P>0.05).Therefore,based on single factor experiment,membrane material ratio (a),drug lipid ratio (b)and ultrasonic time (c) were set as investigation factors,and osthole entrapment efficiency (y) was used as evaluation index.L9(34)orthogonal experiment table was used,with 3 levels for each factor.The experimental factors and level arrangement are shown in Table 1.
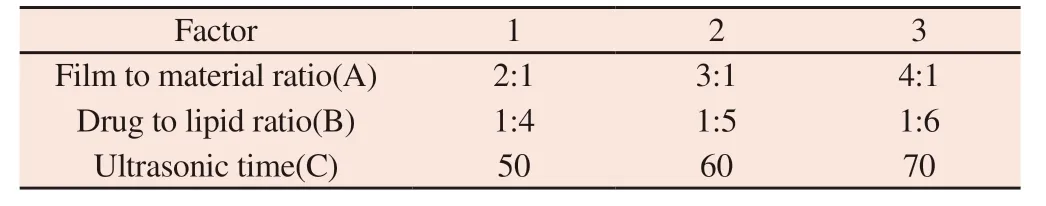
Table 1 Factor level table of orthogonal experiment
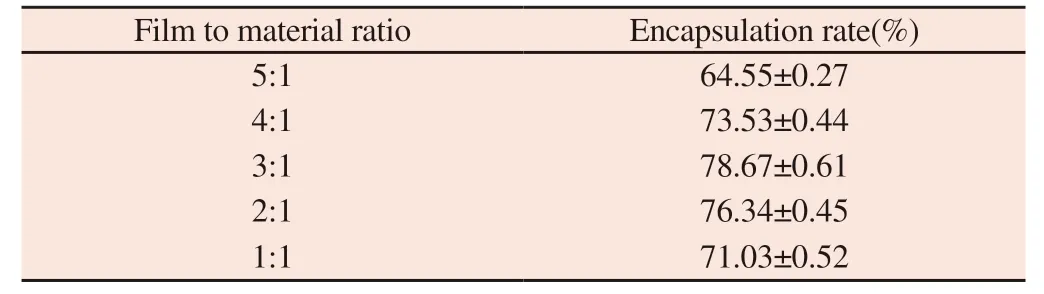
Table 2 Influence of film to material ratio on encapsulation rate
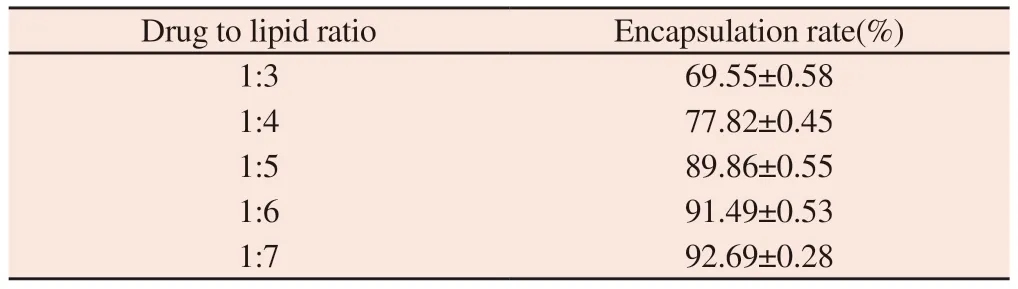
Table 3 Influence of drug to lipid ratio on encapsulation rate
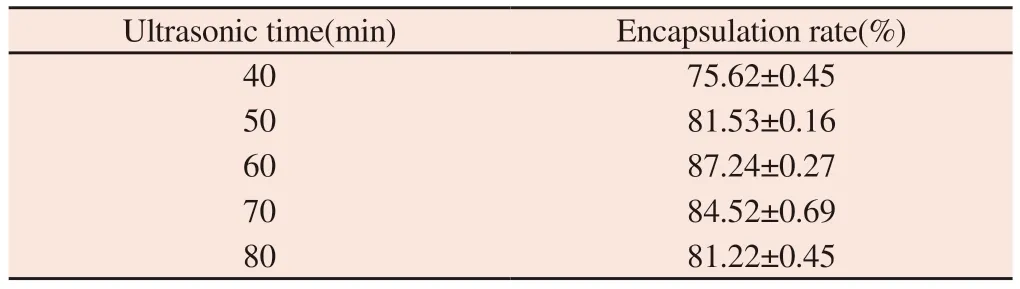
Table 4 Influence of ultrasonic time on encapsulation rate
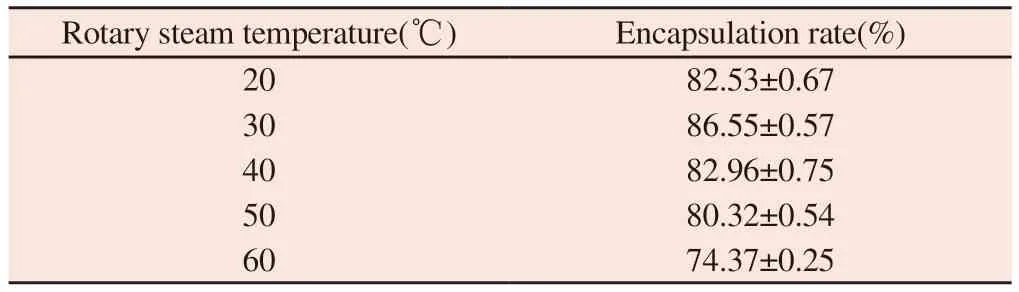
Table 5 Influence of rotary steam temperature on encapsulation rate
2.2.5 Volume ratio of HA-siRNA to protamine
According to the volume ratio of HA-siRNA to protamine of 0.80:1.00,0.85:1.00,0.9:1.00,0.95:1.00,1.0:1.00,1.05:1.00,the hasirna and protamine were added to the pre-labeled centrifuge tube,and the mixture was shaken and mixed.After 10 minutes at room temperature,the particle size and zeta potential of the complexes were determined by potentiometric particle size analyzer.
2.2.6 Ratio of HA-siRNA protamine complex to liposome
Take the non zymogen centrifuge tube and add 25μg HA-siRNA protamine complex of survivin siRNA was labeled with 10μg HAsiRNA protamine 10μl、20 μl、30 μl、40 μl、50 μl、60 μl、70 μl The HA-siRNA protamine complex was mixed with the prepared cationic liposome,and the liposome was allowed to stand at room temperature for 10min.the particle size and potential of the obtained liposome nanoparticles were determined by potentiometric particle size analyzer.
2.2.7 Establishment of a method for determination of entrapment efficiency of liposome nanoparticles
2.2.7.1 Establishment of standard curve
The fluorescent labeled Survivin-siRNA (FAM-Survivin-siRNA)was dissolved in 0.1% DEPC water to prepare FAM-siRNA stock solution.Take 0.1% DEPC water to dilute the stock solution to 0.05 μg/ μl,0.10 μg/ μl,0.15 μg/μl,0.20 μg/ μl,0.25 μg/ μl,0.30 μg/μl standard solutions with different concentration gradients.The absorbance of each group was measured by ELISA at 492 nm.
2.2.7.2 Precision test
FAM-Survivin-siRNA solution was prepared according to the optimal process conditions.The absorbance value was determined at 492 nm.The absorbance value was recorded after 5 parallel determinations.2.2.7.3 Repeatability test
Six copies of cationic liposome nanoparticles containing FAMSurvivin-siRNA and osthole were prepared according to the optimum conditions.The absorbance values were measured at 0 min,15 min,30 min,45 min,60 min and 75 min at 492 nm.
2.2.7.4 Sample recovery test
Six copies of FAM-Survivin-siRNA and osthole loaded cationic liposome nanoparticles with known concentration were prepared according to the optimal process conditions.The standard samples were added respectively.The absorbance value was determined according to“1.2.7.1”.The concentration of FAM-Survivin-siRNA was calculated and the sample recovery was calculated.
2.2.7.5 Content determination
The cationic liposome nanoparticles co loaded with FAM-SurvivinsiRNA and osthole were prepared under the optimal conditions.Take appropriate amount of solution for centrifugation,take the supernatant,at 492 nm,use the multi-functional enzyme reader to measure the absorbance value,calculate the free FAM-SurvivinsiRNA concentration according to the standard curve,and record it as C.
Drug loading formula:drug loading=drug contained in the particle preparation total amount of microparticles × 100%
The formula of encapsulation efficiency:EE(%)=(M-C) ×V÷ M ×100%.EE is the encapsulation efficiency,V is the sample volume,and M is the total content of FAM-Survivin-siRNA in the solution.
3.Results
3.1 Influence of membrane material ratio on encapsulation efficiency
It can be seen from table 2 that the membrane material ratio increases with the decrease of the ratio in the range of 5:1 to 3:1.But then it decreased.The encapsulation efficiency of liposomes was higher when the membrane material ratio was 4:1,3:1 and 2:1,so 4:1,3:1 and 2:1 were selected as the three levels of membrane material ratio.
3.2 Influence of drug lipid ratio on encapsulation efficiency
It can be seen from table 3 that the greater the drug lipid ratio,the greater the encapsulation efficiency;On the contrary,the smaller the encapsulation efficiency is.Considering the drug loading and entrapment efficiency,1:4,1:5 and 1:6 were selected as the three levels of drug lipid ratio.
3.3 Influence of ultrasonic time on encapsulation efficiency
It can be seen from table 4 that the encapsulation efficiency increases with the increase of time when the ultrasonic time is between 40 min and 60 min.The encapsulation efficiency decreased gradually after 60 min.The encapsulation efficiency of liposomes was higher when the ultrasonic time was 50 min,60 min and 70 min,so the ultrasonic time was 50 min,60 min and 70 min.
3.4 Effect of rotary steaming temperature on encapsulation efficiency
It can be seen from table 5 that before the spinning temperature reaches 30℃,the packing rate increases with the increase of temperature,and the sealing rate decreases with the increase of temperature after 30℃.The encapsulation rate of liposomes is higher when the spinning temperature is 20℃,30℃ and 40℃,and the highest at 30℃,so 30℃ is the best spinning temperature.
3.5 Analysis of orthogonal experiment results
The results of orthogonal experiment design and variance analysis are shown in Table 6 and table 7 respectively.From table 6,the extreme deviation r of C is the largest,followed by B and A.The greater the extreme difference of factors,the higher the influence on the sealing rate.Therefore,the effect of three factors,a,B and C on the encapsulation rate was C>B>A,that is,ultrasonic time>membrane material ratio >drug fat ratio.From the level of each factor (k-value analysis),factor A is K2>K1>K3,factor B is K2>K1>K3,factor C is K3>K1>K2.The variance analysis in Table 3 shows that a,B and C have a certain influence on the packing rate,among which C has a significant impact on the packing rate.The optimal scheme can be determined by the above analysis:the film material ratio is 3:1,the drug fat ratio is 1:5,and the ultrasonic time is 70min.
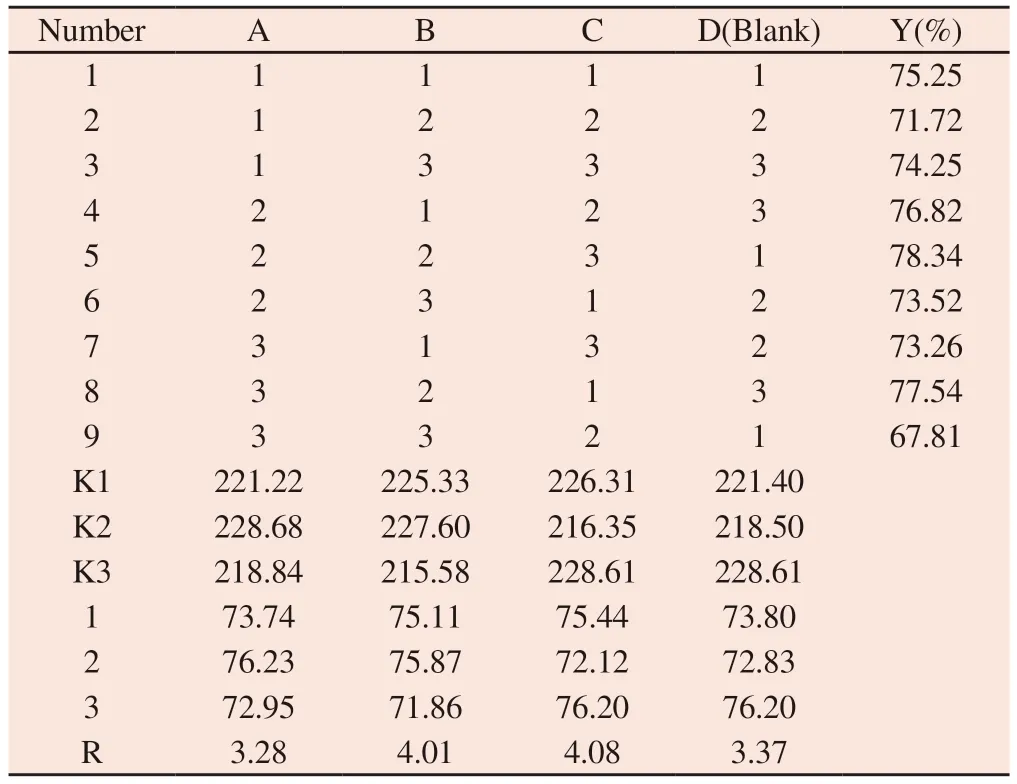
Table 6 Arrangement results of orthogonal experiment
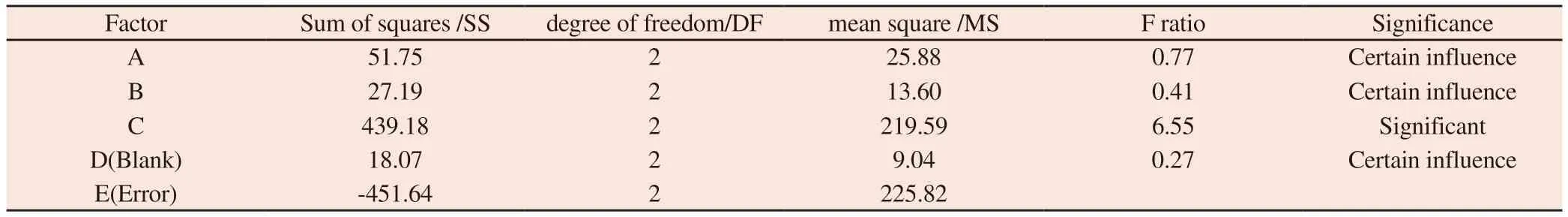
Table 7 Analysis of variance results
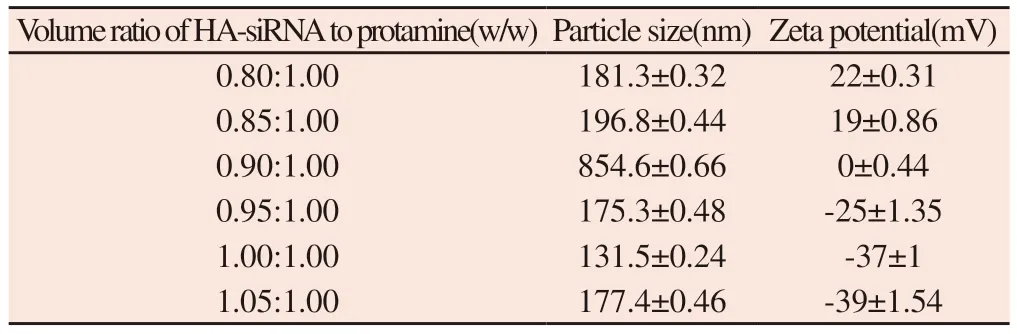
Table 8 Size and potential of HA-siRNA and protamine complexes
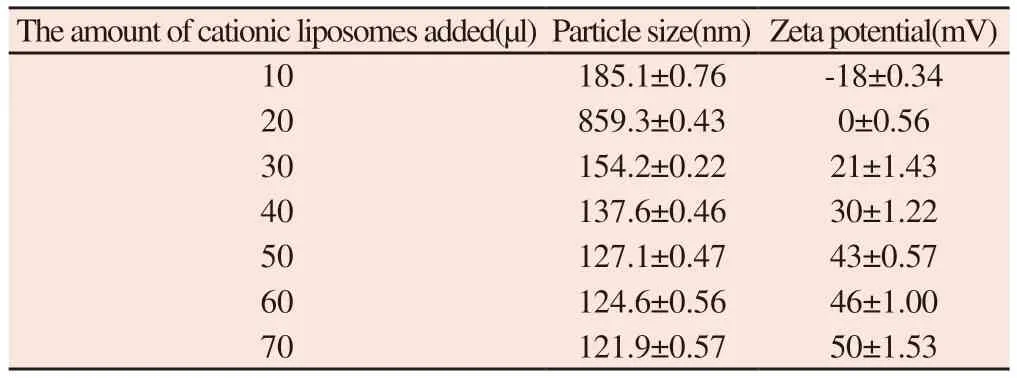
Table 9 Size and potential of HA-siRNA-protamine complex binding with different amounts of liposomes

Table 10 Verification experimental results table
3.6 Volume ratio of HA-siRNA to protamine
It can be seen from table 8 that when the volume ratio of HAsiRNA-protamine is 0.9,the particle size of the composite is the largest,generally neutral.With the increase of HA-siRNA proportion,the particle size and zeta potential of the complex decreased rapidly.Therefore,we think that 1.0 is the best volume ratio of HA-siRNA to protamine,in which the complex is a small negatively charged particle.
3.7 Ratio of HA siRNA protamine complex to liposome
It can be seen from table 9 that with the increase of lipid content,the total particle size first increases and then decreases,while zeta potential continues to increase.50 μl was the best amount of cationic liposome;The size of the nanoparticles is 127.1 nm,and the zeta potential is 43mV.
3.8 Optimum process
The method of film dispersion was adopted.The ratio of DOTAP to cholesterol was 3:1,and then it was added into a round bottom flask.Appropriate amount of chloroform was added.The liposome film was formed at 30℃ until the bottom was flat.After vacuum drying for 2 hours,the organic solvent was removed.Then,10ml deionized water was added.After ultrasonic treatment for 30 minutes,osthole was added according to the ratio of osthole to liposome of 1:5,and ultrasonic treatment for 70 minutes,The liposomes containing osthole were obtained.
The cryopreserved Survivin-siRNA was centrifuged at 4000rpm for 1min,and then dissolved by adding DEPC water.It contains 25μg HA-siRNA was formed by adding 1g Survivin-siRNA solution into the same mass of hyaluronic acid solution and standing at room temperature for 10 min.HA-siRNA protamine complex was formed by mixing HA-siRNA and protamine in a volume ratio of 1:1 and standing at room temperature for 10 minutes.In the HA-siRNA protamine complex,50μl 1% of osthole loaded liposomes,standing at room temperature for 10 minutes.Add 50 μ1 Dspe-peg 2000,standing in 50 ℃ water bath for 10 min.Survivin-siRNA and osthole loaded cationic liposome nanoparticles were prepared.
3.9 Best process validation
The best preparation process of osthole loaded liposomes was selected according to the orthogonal experiment method.The encapsulation efficiency was determined three times in parallel according to the best preparation process.The measured value was compared with the predicted value,and the error between the two values was calculated.The experimental results are shown in table 10.
Results:the relative deviation between the predicted value and the actual value of the entrapment efficiency of osthole loaded liposomes was less than 5%,which proved that the process was accurate,reliable and reproducible.
3.9.1 Characterization of liposome nanoparticles
3.9.1.1 Potential and particle size of liposome nanoparticles
The samples of cationic liposomes loaded with Survivin-siRNA and osthole were prepared under the optimal conditions.The appropriate amount of samples were added into the cuvette and sample pool respectively,and the particle size and potential were determined by particle size analyzer.
The particle size of cationic liposome nanoparticles loaded with Survivin-siRNA and osthole was 132.3 ±2nm,the potential is 43.15±0.05mV.
3.9.1.2 The appearance of liposome nanoparticles was observed by transmission electron microscope
The cationic liposome nanoparticles co-loaded with SurvivinsiRNA and osthole were prepared according to the optimal process of "2.8" .Add l drop on the copper mesh and let it stand at room temperature until dry.The morphology of liposomes was observed under transmission electron microscope.The results are shown in Figure 1.
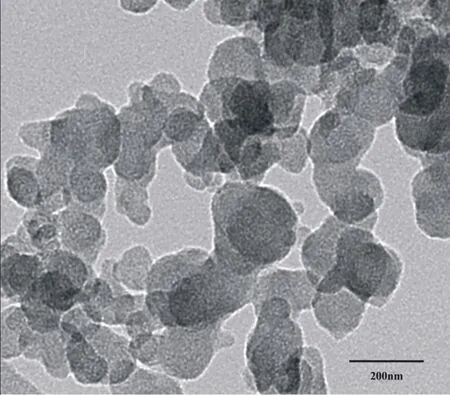
Figure 1 Transmission electron microscopy of liposomes
As shown in the figure above,the nanoparticles co loaded with Survivin-siRNA+osthole cationic liposomes are irregular round like.
3.9.2 Detection of encapsulation efficiency of liposome nanoparticles
3.9.2.1 Making of standard curve
According to the method under "1.2.7.1",the standard curve was established with the absorbance as the ordinate and the solution of FAM-Survivin-siRNA and osthole cationic liposome nanoparticles as the abscissa.The results are shown in Table 11 and Figure 2.
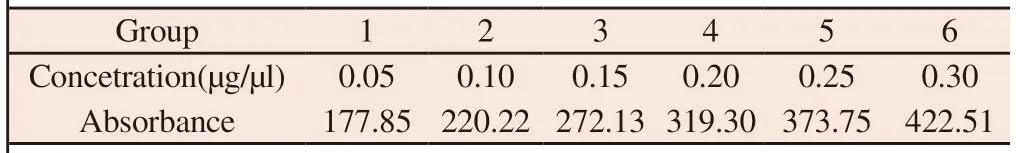
Table 11 Absorbance values of standard solutions of different concentrations
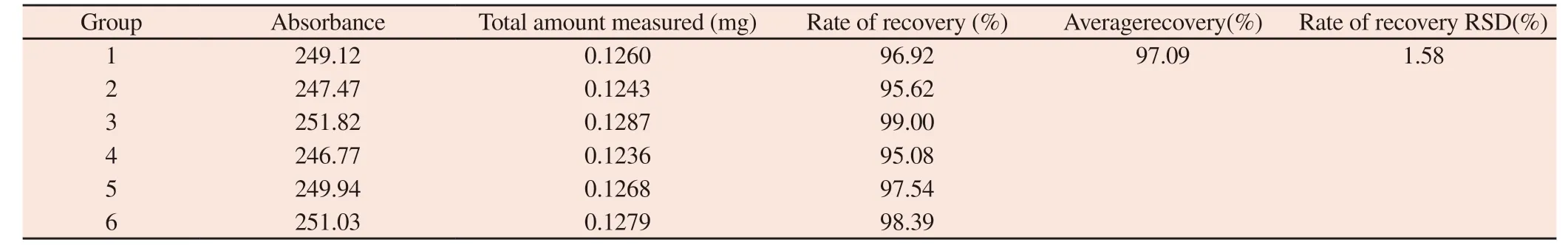
Table 12 Determination results of recovery of added sample
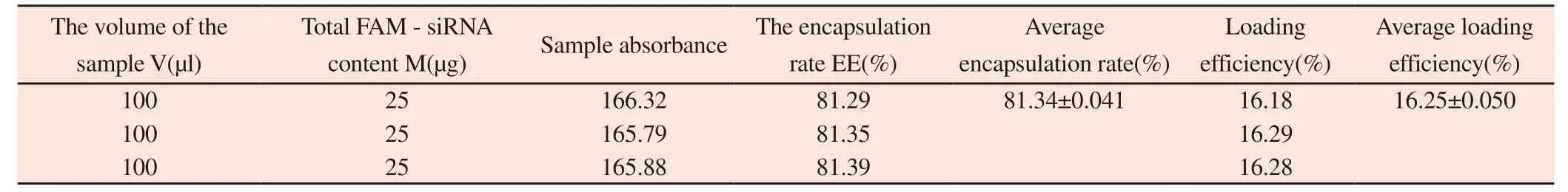
Table 13 Encapsulation rate of Survivin-siRNA and osthol cationic liposome nanoparticles
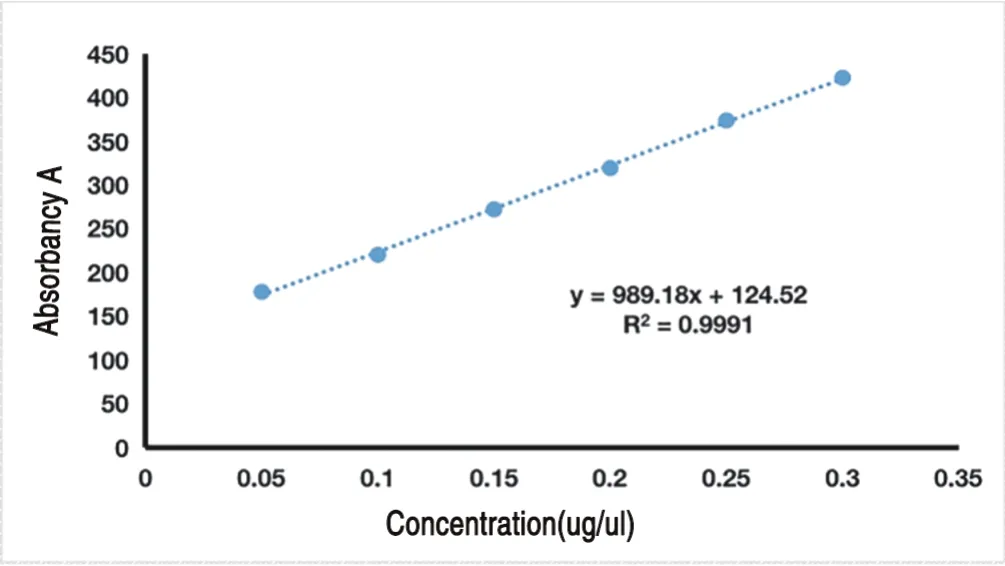
Figure 2 Standard curve
Draw the standard curve of absorbance (y) for FAM-SurvivinsiRNA concentration (x),and get the standard curve equation:y=989.18x+124.52 (R2=0.9991).The results showed that the absorbance and FAM-Survivin-siRNA concentration were in the range of 0.05-0.30 μg/ μl.There was a good linear relationship in the range of 1.
3.9.2.2 Precision test results
FAM-Survivin-siRNA solution was prepared according to the method in "1.2.7.1" and the absorbance value was determined at 492nm by the method in "1.2.7.2" for 5 times.The RSD was 1.28%(n=5).The results showed that the precision was good.
3.9.2.3 Repeatability test results
Six copies of cationic liposome nanoparticles containing FAMSurvivin-siRNA and osthole were prepared according to the optimal process conditions.The absorbance value was determined at 492 nm by the method in "1.2.7.3",and the RSD was 1.80%,indicating good repeatability.
3.9.2.4 Test results of sample recovery
The average recovery of FAM-Survivin-siRNA was 97.09% and RSD was 1.58%,which indicated that the method was accurate.
3.9.2.5 Content determination results
According to the method under "1.2.7.5",the test results are shown in table 13.
The entrapment efficiency of FAM-Survivin-siRNA loaded osthole cationic liposome nanoparticles was 81.34±0.041%,which proved that the entrapment efficiency of Survivin-siRNA in liposomes was good.
4.Discussion
Cationic liposomes are widely used as non viral vectors.The cationic liposomes have a strong affinity with the cell membrane because of the negative charge of the cell membrane,which is beneficial for the cationic liposomes to enter the cell for drug transport.The film dispersion method is commonly used in the preparation of liposomes [10-11].It mainly dissolves phospholipids,cholesterol and other film-forming materials directly in a small amount of organic solvents,removes the excess organic solvents by vacuum rotary evaporation,forms a layer of liposome dispersion film at the bottom of a round bottom flask,and uses distilled water and other mediators to heat the hydrated liposome dispersion film to obtain large chamber liposomes,Liposomes with different particle sizes were obtained by ultrasound[12].It has the advantages of simple preparation and high encapsulation efficiency.In the preparation of liposomes,in order to prevent siRNA enzymolysis,the experimental equipment should be soaked overnight with 0.1% DEPC water and sterilized at high temperature.Based on the principle of charge self loading,the negatively charged siRNA and positively charged cationic liposomes are combined by the effect of electric load,but the molecular weight of siRNA is too small,when it is combined with cationic liposomes alone,it will produce unstable polymerization.Therefore,hyaluronic acid and siRNA were selected to form a negatively charged conjugate,and ha-sirna protamine complex was formed by charge interaction with positively charged protamine [13].When determining the entrapment efficiency of liposomes loaded with Survivin-siRNA and osthole,we should pay attention to the easy degradation of siRNA.When using the microplate reader,the standard solution should be prepared and used immediately.
Particle size is an important physical and chemical factor affecting the passive targeting effect of liposomes in vivo [14].Desai et al.[15-17] found that particles with a diameter of 100nm have high membrane permeability,and particles with a diameter of 100-200nm have high membrane permeability,while particles with a diameter larger than 500nm are difficult to penetrate the epithelial cell membrane.Zeta [18-20] potential is one of the important indexes to evaluate the stability of liposomes.In general,when the absolute value of zeta potential is more than 60mV,liposomes are in the most stable state;The absolute value of zeta potential is stable at 30-60mV;However,when the absolute value of zeta potential is usually lower than 30mV,the liposomes are unstable and prone to coagulation rapidly.
Author's contribution
The design ideas of this paper are provided by Professor Wang Rui.Lecturer Meng Jianan provides guidance for the theoretical knowledge of this paper;The paper was written by Sun Qichao.The specific experimental process was completed by Sun Qichao,Li Yalan and Guo Yuwei.Data analysis and result processing were completed by Baihaotian.
 Journal of Hainan Medical College2022年1期
Journal of Hainan Medical College2022年1期
- Journal of Hainan Medical College的其它文章
- Study on the in vitro activity of Hehuan Yin aqueous extract against hepatitis C 2a virus
- Effect of Majie Pingchuan cataplasm on epithelial-derived cytokines in asthmatic mice based on the "lung-skin-intestine" axis
- Intervention effect of Danbei Yifei formula on pulmonary fibrosis based on urine metabolomics by UHPLC-Q-Exactive
- Clinical characteristics,GRACE score,TIMI score and prognosis of patients with type 2 diabetes mellitus complicated with acute coronary syndrome
- Effect of intradermal needle at five-zang Back-Shu points on treatment of chronic fatigue syndrome
- Clinical effect of Chinese medicine aerosol fumigation on demodex infection related meibomian gland dysfunction
