Taurine in semen extender modulates post–thaw semen quality, sperm kinematics and oxidative stress status in mithun (Bos frontalis) spermatozoa
Perumal Ponraj, Kobu Khate, Kezhavituo Vupru
1ICAR-National Research Centre on Mithun, Medziphema, Nagaland – 797 106, India
2ICAR-Central Island Agricultural Research Institute, Port Blair, 744 105, Andaman and Nicobar Islands, India
ABSTRACT
Objective: To assess the effect of taurine on post-thaw semen quality parameters, sperm kinematics, antioxidant and oxidative stress profiles and sperm cholesterol efflux in mithun (Bos frontalis).Methods: A total of 50 ejaculates (n=25 samples) were selected based on biophysical parameters. Each sample was split into four equal aliquots after dilution with the Tris-citrate-glycerol extender.GroupⅠ, Ⅱ, Ⅲ and Ⅳ contained 0 mM (the control), 25 mM, 50 mM and 100 mM of taurine, respectively. Frozen-thawed samples were analysed for motility parameters (progressive forward and in bovine cervical mucus penetration test), kinetic and velocity parameters by computer-assisted sperm analyzer, viability, sperm and nuclear abnormalities, acrosome integrity, plasma membrane and nuclear integrities, sperm enzymatic leakage and biochemical (sperm cholesterol and oxidative stress) profiles.
Results: The extender containing 50 mM taurine led to a significant enhancement in viability, acrosomal integrity, plasma membrane integrity, motility (progressive and in cervical mucus), and sperm cholesterol content and notably reduced sperm morphological and nuclear abnormalities, and leakage of intracellular enzymes compared to other taurine treated and untreated control groups(P<0.05). Moreover, in addition to significant improvement in kinetic and velocity profiles, 50 mM taurine protected the integrity of acrosome and biochemical membranes than in the untreated control and other taurine treated groups. Inclusion of 50 mM taurine held a clear advantage over the control or 25 mM or 100 mM taurine in cryopreservation of mithun semen.
Conclusions: Taurine (50 mM) supplementation in semen extender can be effectively utilized to reduce oxidative stress and improve post-thaw semen quality in mithun.
KEYWORDS: Antioxidants; Cryopreservation; Kinematic profiles; Mithun; Oxidative stress; Spermatozoa; Taurine; Semen quality; Post-thaw
Significance
Cryopreservation triggers osmotic, chemical and oxidative stresses on the spermatozoa, which leads to deterioration of sperm quality and unfits for long term preservation and artificial insemination. Taurine is a sulfonic amino acid and non-enzymatic scavenger, protecting the spermatozoa against lipid peroxide. This study showed that taurine (50 mM)prevented the sperm damage and improved sperm profiles by modulating oxidative and cryo stresses. In conclusion, taurine can protect the sperm against cryo and oxidative stresses.
1. Introduction
Mithun is a unique magnificent domestic bovine species available in North Eastern hilly region of India. Earlier reports suggested that mithun suffers intensive inbreeding depression because of lack of suitable breeding bulls and breeding management. Mithuns are reared under extensive free-range system with natural service which is the preferred breeding practice. This natural breeding has various limitations which lead to loss of production and reproductive performances. These limitations would be overcome by implementation of semen collection and artificial breeding programmes. Preliminary research on basic semen quality profiles in liquid preservation revealed that 50 mM taurine is suitable for mithun liquid semen preservation[1]. Artificial insemination contributes in genetic improvement, in which a single ejaculate from an elite male is used to impregnate many females. Various stages of cryopreservation or freezing process triggers physical,osmotic and chemical stresses on the sperm membrane associated with oxidative stress induced by free radicals[2]. All these deleterious effects result in loss of semen quality and large number of sperms incapable to fertilize the ovum, leading to fertilization failure[3].High polyunsaturated fatty acids content in mammalian sperm membranes and lack of significant cytoplasmic antioxidants make the spermatozoa highly susceptible to lipid peroxidation[4]. Effects of reactive oxygen species (ROS) on spermatozoa are irreparable loss of motility, sperm DNA disintegration and reduced fertilizing ability[5]. Therefore, supplementation of exogenous antioxidants in the semen extender[6-12] or feeding of antioxidants[13] or flaxseed oil[14] or implantation of slow release melatonin[15] can reduce the deleterious effects of oxidative and cryo stresses during semen cryopreservation[5].
Addition of taurine to ovine[16], feline[17] and rabbit sperm[18]has protected against the deleterious effects of ROS and improved the semen quality profiles during liquid storage. Taurine is one of the sulfonic amino acid and is non-enzymatic scavenger that significantly protected the spermatozoa against lipid peroxide under the liquid (4 ℃) storage or in cryopreservation[16-18]. In recent years, taurine is used in semen extenders for cryopreservation of boar[19], bull[20], ram[21] and buck[22] sperm to improve the semen quality parameters and fertility by inhibiting lipid peroxidation and protecting sperm cells against accumulation of ROS. No information is available with regard to the effect of taurine in Tris based semen extender on fertility of cryopreserved semen in mithun. Therefore,it was hypothesized that application of taurine in semen extender would improve the post-thaw quality of spermatozoa in mithun.With this, the objective of the present study was to assess the effect of different concentrations of taurine in semen diluents on semen quality parameters, kinematic and velocity profiles, oxidative stress profiles and leakage of intracellular enzymes of the cryopreserved semen in mithun.
2. Materials and methods
2.1. Location of the study
The proposed study was conducted at the mithun breeding farm, ICAR-National Research Centre on Mithun, Medziphema,Nagaland, India. It is located between 25°54′30′ North latitude and 93°44′15′ East longitude and at an altitude range of 250-300 m above mean sea level. The temperature humidity index ranges from 54.41±1.09 in Winter (November to January), 63.51±1.85 in Spring(February to April), 74.00±1.77 in Autumn (August to October) to 76.06±1.74 in Summer (May to July) season. Similarly, sunshine hours also differed significantly (P<0.05) among Winter (4.11±0.36),Spring (4.81±0.28), Summer (6.55±0.15) and Autumn (6.32±0.28)seasons with an average of 5.45±0.34.
2.2. Experimental animals
Ten apparently healthy (body condition score 5-6 of 10, classified as good) mithun bulls of 4-6 years of age were selected. The average body weight of the bulls was 510 kg (range: 495-520 kg).Experimental animals were maintained under uniform feeding,lighting, housing and other managemental conditions as per the farm schedule. Experimental animals were offered ad libitum potable drinking water, 30 kg mixed jungle forages (18.40% and 10.20% dry matter and crude protein, respectively) and 4 kg concentrates (87.10% and 14.50% dry matter and crude protein, respectively) fortified with mineral mixture and salt. The concentrate feed consisted of maize:35%, rice polishing: 25%, wheat bran: 25%, groundnut oil cake:12%, salt: 0.80%, mineral mixture: 2% and vitamin mixture: 0.2%.Mithun sheds were made up of asbestos-roof and half of the wall made up of wire mesh and half was by the bricks wall in all four sides of the shed which was surrounded by the trees. At 09: 00 h,mithun bulls were removed from the shed for grazing and at 14: 00 h the animals were restrained in their original positions in the shed.
2.3. Extender preparation
The extender used in this study contained: Tris (hydroxymethyl)aminomethane: 3.028 g; citric acid: 1.675 g; fructose: 1.250 g;glycerol (7%): 7 mL; streptomycin sulphate (μ/mL): 1 000; penicillin G sodium (IU/mL): 1 000; egg yolk (20%): 20 mL and different concentrations of taurine (25 mM, 50 mM and 100 mM, in Group Ⅱ, Ⅲ and Ⅳ, respectively) for 100 mL deionized water.In bovine (50 mM[23]) and ovine (25 or 50 mM[21]), taurine yielded significantly higher beneficial effects on semen quality profiles and in-vitro fertility profiles in cryopreserved semen. Mithun (Bos frontalis) is a bovine species and suffers similar cryo-stress effect on sperm as domestic bovine or ovine species. Therefore, taurine with different concentrations were selected to select the suitable or optimum dosage for semen cryopreservation in mithun. The extender for the control group (GroupⅠ) contained no taurine. The final pH of extender used in all three groups was adjusted to 6.8-7.0.
2.4. Semen collection and processing
Semen was collected not more than twice per week from any animal through standardized trans-rectal massage method during summer season (May to July; temperature humidity index:76.06±1.74; sunshine hours: 6.55±0.15). Semen samples with mass activity of 3+ or above were selected for the experiment[1].Immediately after collection, the ejaculates were kept in a water bath at 35°℃ and evaluated the preliminary semen quality parameters. After discarding the ejaculates with wide variation in pH (i.e. <6.7 and >7.2), colour or too low volume (<0.5 mL),the rest were evaluated microscopically. The ejaculates having concentration >500 million/mL, mass activity >3+, individual motility >70% and total morphological abnormalities <10% or below were processed further. Following the above-screening protocol,50 ejaculates were selected. After the preliminary evaluations, two consecutive ejaculates of a same bull were pooled together (termed“sample” hereafter, n=25) and subjected to the two-fold initial dilution with pre-warmed (35°℃) Tris-citrate-glycerol extender.Thus, 50 selected ejaculates were pooled to make 25 samples for the experiment. The partially diluted samples were brought to the laboratory in an insulated flask containing warm water (35°℃) for further processing.
Each sample was split into four equal aliquots and diluted (to get final concentration of 60 million spermatozoa per mL) with the Triscitrate-glycerol extender containing either 0 mM or 25 mM or 50 mM or 100 mM taurine (GroupⅠ, Ⅱ, Ⅲ or Ⅳ, respectively).Diluted semen samples of each group were filled in polyvinyl chloride straws (0.5 mL; IMV, L’Aigle, France) by manual sucking and sealed with polyvinyl alcohol powder (IMV, L’Aigle, France). These straws were cooled from 35 ℃ to 5 ℃ at a rate of 0.2 ℃–0.3 ℃ per min in a cold cabinet (IMV, L’Aigle, France) and maintained at 5 ℃ for 4 h. Subsequently, these straws were wipe-cleaned, dried and spread over the freezing rack. The rack containing straws was kept in biological programmable freezer (IMV, L’Aigle, France) for freezing (final temperature maintained at −124 ℃, 12 min) followed by plunging straws into the liquid nitrogen (−196 ℃) and were stored therein.
2.5. Post thaw semen evaluation
At the time of evaluation, the stored semen straws were taken out of the cryocans and thawed in water at 37 ℃ for 30 s. Semen quality parameters, viz. post thaw sperm motility[24], kinetic, velocity and motility parameters by computer assisted sperm analyzer(CASA; Hamilton Thorne Sperm Analyser, HTM-IVOS, version IVOS 11, Hamilton Thorne Research, USA), viability and total sperm morphological abnormality by Eosin–Nigrosin staining[25],acrosomal integrity by Giemsa staining[26], plasma membrane integrity by hypo-osmotic swelling test[27], nuclear integrity by Feulgen’s staining technique[14] and vanguard distance travelled by sperm in the bovine cervical mucus penetration test[28] were determined.
2.6. Biochemical assays
An aliquot of semen from each sample was centrifuged at 800×g for 10 min; seminal plasma siphoned out and sperm pellets were separated and washed by resuspending in phosphate buffer saline and centrifugation (thrice). After final centrifugation, 1 mL of deionized water was added to the spermatozoa. The seminal plasma and sperm pellets were snap-frozen and stored in sterilized cryovials in deep freezer at −80 ℃ until further analysis. At the time of estimation,concentration of spermatozoa was determined and then re-diluted to contain 100×106cells/mL. Biochemical profiles such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), superoxide dismutase (SOD), catalase,glutathione (GSH) and total antioxidant capacity (TAC) in seminal plasma of frozen-thawed sample and malondialdehyde (MDA) and total cholesterol in frozen thawed sperm pellet were estimated.
2.6.1. Leakage of intracellular enzymes
Intracellular enzymes such as AST (μmol/dL), ALT (μmol/dL) and LDH (IU/dL) were estimated in the seminal plasma by commercially available assay kits (Span Diagnostics Ltd., India).
2.6.2. Antioxidant and oxidative stress profiles
Antioxidants such as TAC (K274; Bio Vision, CA, USA; μmol/L),SOD (U/mL), GSH (μmol/mL) and catalase (nmol/min/mL) were estimated using commercially available enzyme-linked immunosorbent assay kits (706002, 703002 and 707002, Cayman Chemical Co., USA, respectively) at optical density (570, 440-460, 405-424 and 540 nm, respectively). These antioxidants were estimated with use of microplate spectrophotometer (Thermo Scientific Multiskan GO Microplate Spectrophotometer, USA). Lipid peroxidation level of spermatozoa was measured by determining the MDA production at 535 nm using thiobarbituric acid-trichlroacetic acid as per the method of Marbut et al[29].
2.7. Sperm cholesterol content
Total cholesterol content was estimated in spermatozoa. Hundred million washed spermatozoa were taken in a 10 mL vial. The sperm pellet was extracted with 20 volumes of chloroform: methanol (1:1 v/v) solution and vortexed for 20 s. Thereafter, it was centrifuged at 800×g for 5 min. Spermatozoa was evaporated to dryness under liquid nitrogen and kept at −20 ℃. At the time of estimation, 0.5 mL of chloroform was added to each vial, cholesterol was estimated by cholesterol estimation assay kit (Span Diagnostics Ltd., India) and results were expressed as μg cholesterol/108spermatozoa.
2.8. Statistical analysis
The statistical analysis of the data was performed as per standard procedures with Statistical Analysis System for Windows, SAS Version 9.3 (SAS Institute, Inc., Cary, NC, 2001). The data used in the study were all in normal distribution according to Shapiro-Wilk test and were expressed as mean±standard deviation (mean±SD).Data were analyzed by one-way analysis of variance followed by the Tukey’s post hoc test to determine significant differences among the treatments and control groups on these sperm quality parameters using the SAS software. Differences with values of P<0.05 were considered to be statistically significant.
2.9. Ethics statement
The experimental procedure was approved by the Institutional Animal Ethics Committee of ICAR-National Research Centre on Mithun [CPCSEA-NRCM (RPM) 4/2010 (Vol I)-6], Medziphema,Nagaland, India with approval No. IXX08094. All animal experiments were performed according to the International Guidelines on Ethical Use of Animals.
3. Results
3.1. Characteristics of semen
Mithun semen samples (n=25) were mostly creamy white to thick creamy in colour, with an average semen volume of (2.35±0.12) mL and an average sperm concentration of (865.14±8.94) million per mL. Statistical analysis revealed a significant enhancement in quality parameters in ejaculates diluted with extender containing 50 mM taurine (P<0.05).
3.2. Post thaw semen quality
Spermatozoa treated with 50 mM taurine had significantly higher post thaw motility, viability, acrosomal intactness, plasma membrane integrity, nuclear integrity and vanguard distance travelled by sperm in cervical mucus than those in the control and taurine 25 mM or 100 mM treated groups (P<0.05). On the other hand, the total sperm morphological abnormality was significantly reduced in the 50 mM taurine treated group than those in the control and other taurine treated groups (P<0.05) (Figure 1).
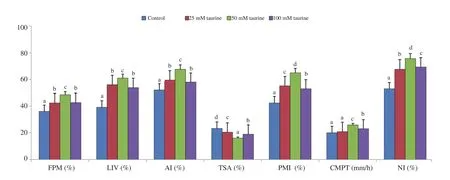
Figure 1. Effect of taurine on post thaw semen quality profiles in mithun. Data are expressed as mean±SD. FPM: forward progressive motility; LIV:livability; AI: acrosomal integrity; TSA: total sperm abnormality; PMI: plasma membrane integrity (HOST test used); CMPT: cervical mucus penetration test (vanguard distance travelled by sperm); NI: nuclear integrity. Vertical bar with small letters (a, b, c, d) indicates significant difference among the different experimental groups (P<0.05). Number of semen samples for the control and treatment groups: n=25.
Forward progressive motility and total motility of sperm measured by CASA were significantly higher in the 50 mM taurine treated group than those in the control and other taurine treated groups(P<0.05). On the other hand, static motility was reduced significantly in the 50 mM taurine treated group than those in the control or other taurine treated groups (P<0.05). Non-progressive motility was significantly higher in the 50 mM taurine treated group than the untreated control group (P<0.05) (Figure 2).
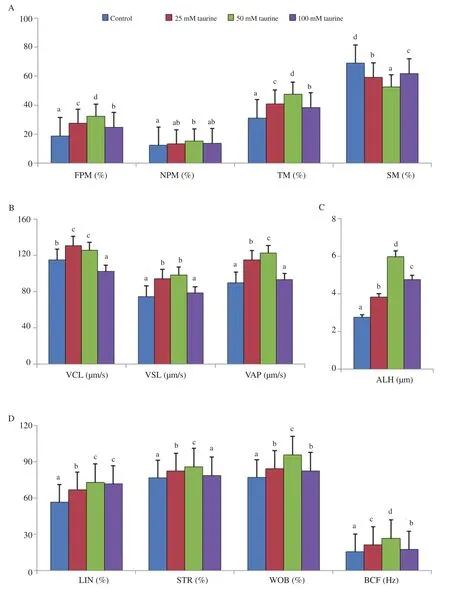
Figure 2. Effect of taurine on post thaw motility and velocity parameters by computer assisted sperm analyser in mithun. Data are expressed as mean±SD. A:motility parameters, including forward progressive motility (FPM), non-progressive motility (NPM), total motility (TM), and static sperm (SM). B: velocity parameters, including curvilinear velocity (VCL), straight line velocity (VSL), and average path velocity (VAP). C: amplitude of lateral head displacement (ALH).D: linearity and frequency parameters, including linearity (LIN), straightness (STR), wobbling (WOB) and beat/cross frequency (BCF). Vertical bar with small letters (a, b, c, d) indicates significant difference among the different experimental groups (P<0.05). Number of semen samples for the control and treatment groups: n=25.
Velocity profiles, such as curvilinear motility (VCL) and average path velocity (VAP) were significantly differed among the experimental groups. VCL was higher in the 25 mM taurine treated group and and VAP were higher in the 50 mM taurine treated group than other experimental groups (P<0.05). Straight line velocity(VSL) was higher in both 25 and 50 mM taurine groups, but they were not significantly different. Similarly, amplitude of lateral head displacement (ALH), linearity (LIN), straightness (STR), wobbling(WOB) and beat cross frequency (BCF) were significantly higher in the 50 mM taurine treated group than those in the control or other taurine treated groups (P<0.05) (Figure 2).
3.3. Intracellular enzymes leakage
Leakage of intracellular enzymes such as AST, ALT and LDH were significantly reduced in the 50 mM taurine treated group than those in the untreated control or other taurine treated groups (P<0.05),whereas the ratio between AST:ALT was significantly higher in three taurine treated groups than in untreated control group (P<0.05)(Figure 3).
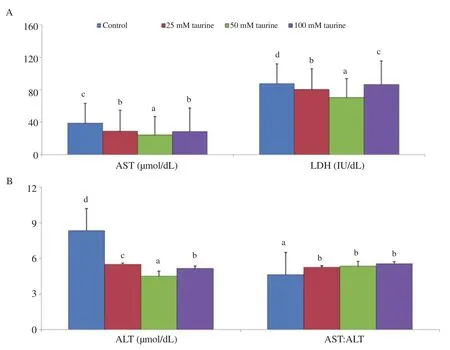
Figure 3. Effect of taurine on intracellular enzymes of sperm in post thaw stage in mithun. Data are expressed a(AST) and lactate dehydrogenase (LDH). B: alanine aminotransferase (ALT) and the ratio of AST and ALT (AS b, c, d) indicates significant difference among the different experimental groups (P<0.05). Number of semen sam n=25.s mean±SD. A: aspartate aminotransferase T:ALT). Vertical bar with small letters (a,ples for the control and treatment groups:
3.4. Antioxidant/oxidative parameters and sperm cholesterol
Antioxidants such as GSH, SOD, catalase and TAC were significantly higher and oxidative stress marker such as MDA was significantly lower in the 50 mM taurine treated group than those in other taurine treated or untreated control groups (P<0.05) (Figure 4).Similarly, sperm cholesterol was significantly higher in the 50 mM taurine treated group than those in other taurine (25 or 100 mM)treated or untreated control groups (P<0.05) (Figure 4).
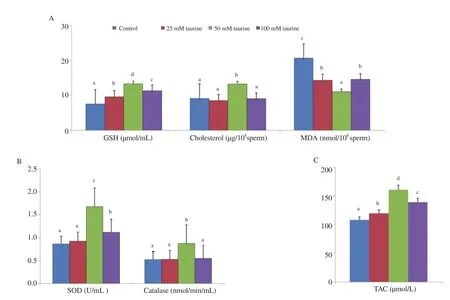
Figure 4. Effect of taurine on antioxidant profiles in mithun. Data are expressed as mean±SD. A: glutathione (GSH B: superoxide dismutase (SOD) and catalase. C: total antioxidants (TAC). Vertical bar with small letters (a, b, c, d different experimental groups (P<0.05). Number of semen samples for the control and treatment groups: n=25.), cholesterol, and malondialdehyde (MDA).) indicates significant difference among the
4. Discussion
Taurine inclusion in the semen extender improved the semen quality parameters, level of antioxidants and total cholesterol of sperm and reduced the leakage of intracellular enzymes, free radical formation and sperm morphological and nuclear abnormalities in mithun. Thus,it protected the structures and functions of spermatozoa efficiently.Perusal of available literature revealed no information on taurine inclusion on semen quality parameters and oxidative stress markers in mithun semen cryopreservation. Though several authors reported taurine has significant beneficial effects in semen quality parameters and oxidative stress markers in different species like bovine[20],ovine[16,21], caprine[22], porcine[19], feline[17], and rabbit[18] sperm,similar studies in mithun were lacking.
The beneficial effects of taurine in semen cryopreservation are due to it being a potent antioxidant[23]. The sulfonated amino acid,taurine, present in both epididymal and oviduct fluid, is an important protector of cells against the ROS when exposed to aerobic conditions[18]. In our study, taurine improved these semen quality parameters, which was similar to the results obtained by other researchers in unfrozen rabbit[18] and ram[16] semen and frozenthawed ram sperm[30].
Mammalian sperm membrane has higher polyunsatured fatty acids and it renders the sperm very susceptible to lipid peroxidation,which occurs as a result of the oxidation of the membrane lipids by partially reduced oxygen molecules. Lipid peroxides impair the sperm function through altered sperm motility, membrane integrity and damage to sperm DNA and fertility through oxidative stress and production of cytotoxic aldehydes[31]. In addition, antioxidant system of seminal plasma and spermatozoa is compromised during semen processing[32]. Therefore, inclusion of exogenous antioxidants may modulate the antioxidant system of semen.
Results of the present study showed that addition of 50 mM taurine improves cryopreservability of mithun sperm which were preserved at liquid nitrogen. Similar results reported that taurine improved the sperm motility and intactness of acrosomal membrane in bull semen[23,33].
Taurine helps to maintain the normal acrosomal integrity[33] and stabilizes the plasmalemma of spermatozoa; thus it increased the sperm motility. Taurine is able to react with many ROS directly for protecting the mammalian cells against oxidative stress and thus enhances the sperm motility[21]. Therefore, taurine improved the motility and viability of the sperm cells in liquid storage[17,18] and frozen semen[23].
Moreover, taurine protects the plasma membrane integrity,nuclear membrane integrity, mitochondrial membrane integrity as well as the cytoskeleton structure of flagella of sperm as cell protecting mechanism. Further, taurine protects antioxidants (SOD and catalase) in semen extender[21], which in turn improves the membrane transportation as well as the fertility rate. The axosome and associated dense fibers of the middle pieces in sperm are covered by mitochondria that generate energy from intracellular stores of adenosine triphosphate for sperm motility[34]. Based on our results,we can hypothesize that taurine displayed protective effects on the functional integrity of the axosome and mitochondria; thus taurine improved the sperm motility in cryopreserved mithun semen.
Intracellular enzymes (AST and ALT) are important for sperm metabolic processes which in turn provide energy for sperm viability, motility, membrane integrity and fertility and the activities of these transaminases in semen are best and good markers of semen quality because these enzymes measure the membrane stability of sperm[35]. Thus, increasing the abnormal spermatozoa in storage causes high concentration of transaminase enzyme in the extra cellular fluid due to sperm membrane damage and ease of leakage of enzymes from spermatozoa[36]. Moreover, decrease of AST and ALT in the seminal plasma of taurine treated semen may be due to it maintaining the structural stability of the sperm[37].Similar result was obtained in the present study that taurinetreated semen had lower level of AST and ALT as it stabilizes the membrane of acrosome, plasma membrane, mitochondria and flagella of the sperm.
GSH is the most abundant non-protein thiol in mammalian cells and is present mainly in reduced form and only a small amount is in oxidized form. In our study, higher GSH was observed in the seminal plasma of taurine treated semen[21,33], which indicates that taurine protects the antioxidant system in mithun semen.
Catalase scavenges both extracellular and intracellular superoxide anion and prevents lipid peroxidation of the plasma membrane. In the present study, concentration of SOD and catalase was higher in taurine-treated semen. Taurine at a dose of 50 mM improved the sperm motility in cryopreserved semen and displayed antioxidative properties and elevated the catalase level in association with higher SOD concentration. Similar results were reported in rabbit[18]and bull semen[33]. Further, taurine, a permeating cryoprotectant acts as an antioxidant and causes membrane lipid and protein rearrangement, which results in increased membrane fluidity, greater dehydration at lower temperature and therefore increased ability of spermatozoa to survive during the cryopreservation[38]. This could be one of the reasons for improved motility, viability and membrane integrity of spermatozoa in taurine-treated semen.
Taurine prevents cholesterol efflux from the sperm membrane and MDA production in diluents, which indicates that it prevents premature capacitation and acrosomal reaction. Along with phospholipids, cholesterol is necessary for cell physical integrity and ensures fluidity of the cell membrane. Cholesterol plays a crucial role in the sperm membrane as its release from the sperm membrane initiates the key step in the process of capacitation and acrosome reaction that is crucial for fertilization[39]. In the present study, cholesterol efflux and MDA production were decreased in the taurine-treated group than in the untreated control group[21,23,40,41].Therefore, the semen samples treated with taurine had higher cryoresistance power than untreated sperm.
In this study, semen quality parameters were improved in taurinetreated extender, which may be due to the fact that taurine prevents excessive generation of free radicals produced by spermatozoa themselves by means of its antioxidant property.
However, the present study has some limitations. In this study, we examined the effect of taurine on in-vitro semen quality profiles,antioxidant and oxidative stress profiles and biochemical profiles.We used limited groups (25, 50 and 100 mM taurine) and revealed 50 mM is optimum for mithun. Therefore, we need further studies that effects of taurine on in-vitro or in-vivo fertility rate are warranted to confirm the present findings. Furthermore,different divided concentrations between 25 mM and 100 mM with the interval of 10 mM are to be tested to select more suitable or optimum dosage for mithun to get higher post thaw semen quality and fertility rate.
In conclusion, the possible protective effects of taurine supplementation are enhancing the antioxidant enzyme content and preventing efflux of cholesterol and phospholipids from the cell membrane and MDA production. Thus, it protects the spermatozoa during cryopreservation and enhances the in-vitro sperm functional properties in this mithun species.
Conflict of interest statement
Authors declare that there is no conflict of interest involved in the present work.
Acknowledgements
This research work was supported by a grant from the Department of Biotechnology, Ministry of Science and Technology, CGO Complex, Lodhi Road, Government of India, New Delhi-110003,India for the project entitled “Evaluation of melatonin as fertility marker in Mithun (Bos frontalis) bulls: Effect on circadian rhythm and seasonal variation in semen quality parameters” (Project No.BT/PR9590/AAQ/1/562/2013 dated 05.12.2014).
Authors’ contributions
Perumal Ponraj contributed to conceptualization, data curation and formal analysis. Kobu Khate, Kezhavituo Vupru and Perumal Ponraj contributed to investigation. Perumal Ponraj and Kobu Khate contributed to methodology. Perumal Ponraj contributed to project administration. Perumal Ponraj, Kobu Khate, Kezhavituo Vupru contributed to writing original draft. Perumal Ponraj contributed to writing review and editing.
 Asian Pacific Journal of Reproduction2022年1期
Asian Pacific Journal of Reproduction2022年1期
- Asian Pacific Journal of Reproduction的其它文章
- Successful live birth after intracytoplasmic sperm injection using testicular sperm in non-mosaic Klinefelter syndrome
- Effects of enzymatic and non-enzymatic antioxidants in diluents on cryopreserved bull epididymal sperm
- Blepharis persica increases testosterone biosynthesis by modulating StAR and 3 β-HSD expression in rat testicular tissues
- Recurrent ovarian endometrioma after conservative surgery: A retrospective study
- Uptake of in-vitro fertilization among couples attending fertility clinic in a tertiary health institution
- An implementation study of barriers to universal cervical length screening for preterm birth prevention at tertiary hospitals in Thailand: Healthcare managers’ perspectives
