骨源性因子ucOCN在运动抗抑郁中的作用机制*
陈祥和 李文秀 刘 波 殷荣宾
骨源性因子ucOCN在运动抗抑郁中的作用机制*
陈祥和1李文秀1刘 波1殷荣宾2
(1扬州大学体育学院, 江苏 扬州 225127) (2苏州大学体育学院, 江苏 苏州 215000)
羧化不全骨钙素(ucOCN)是骨中成骨细胞分泌的特异性蛋白, 因其在调控神经发育、神经可塑性等中的重要角色而受到神经科学领域关注。“骨−脑”串联“对话”是骨内分泌−神经介导的应答系统, ucOCN透过血脑屏障后介导单胺类神经递质、神经内分泌、神经免疫、神经再生及基因表达等机制, 进而作用于海马CA3区、扣带回等脑区功能发挥来调节抑郁发生及改善。而ucOCN作为骨源性力学刺激敏感基因, 运动上调其表达后进入血液循环, 通过介导5-HT/GABA分泌、HPA轴功能、炎症反应、神经营养因子(BDNF等)表达或信号途径(如GSK3β/β-catenin、TLR4/miR-223/NLRP3等)激活等来实现“骨串联脑”, 发挥运动抗抑郁作用。通过对骨源性因子ucOCN介导脑区功能变化从而实现运动抗抑郁的作用机制进行探讨、梳理, 一方面有助于更深入了解骨内分泌功能, 另一方面为抑郁发生、改善和运动抗抑郁研究提供新的理论基础和研究思路。
羧化不全骨钙素, 骨, 脑, 运动, 抑郁症
抑郁症不仅是社会重大焦点问题, 其发病机制探究亦是神经科学领域的研究热点。研究发现, 单胺类神经递质分泌、下丘脑−垂体−肾上腺轴(Hypothalamic–pituitary–adrenal axis, HPA)、细胞因子表达等功能的失调, 可通过作用于眶皮质区、海马扣带回及下丘脑等脑内结构, 调控中缝核及其轴突路径相关结构内神经元中神经递质如5-羟色胺(5-hydroxytryptamine, 5-HT))分泌、关键因子表达等, 进而影响抑郁的发生、发展。现已证实的抑郁症发病机制主要有:单胺类神经递质假说、基因−环境相互作用、神经发生、神经可塑性、免疫激活与抑制等(Frye & Walf, 2009)。骨钙素(Osteocalcin, OCN)作为骨中成骨细胞(Osteoblast, OB)分泌的特异性非胶原蛋白(Lin et al., 2020), 其活化形式羧化不全骨钙素(Uncarboxylated osteocalcin, ucOCN)经生物信号刺激后, 由骨分泌入血后作用于胰岛β细胞、睾丸、脂肪、脑等组织器官, 通过靶细胞膜受体引起级联反应调控能量代谢、精子形成、认知功能障碍等(Vella & Kumar, 2013)。
OB具有内分泌功能, 将OB上的ucOCN基因敲除后, ucOCN-/-小鼠记忆力和空间学习能力下降且出现抑郁样行为; 而注射外源性ucOCN后, ucOCN-/-小鼠抑郁样行为显著改善(Khrimian et al., 2017)。提示, OB分泌的特异性ucOCN在抑郁发生上具有“骨−脑Crosstalk”调控作用。运动是改善抑郁的有效手段, 但相关研究焦点集中于中枢神经系统可塑性适应变化上。近来发现, OB中的ucOCN作为运动敏感基因, 其活化入血穿过血脑屏障后与G蛋白偶联受体158 (G protein-coupled receptors 158, Gpr158)结合调节靶基因脑源性神经营养因子(Brain-derived neurotrophic factor, BDNF)表达, 调控抑郁发生、改善(Khrimian et al., 2017)。国内刘微娜团队在探究运动抗抑郁时提出了:“脑−肠互动”、“肌−脑Crosstalk”、脂肪细胞因子等理论(薛香莉等, 2019) (夏杰等, 2017) (刘文彬等, 2018)。但基于整合生物学理论和骨内分泌功能, 本论文将着重探究ucOCN介导“骨−脑Crosstalk”发挥运动抗抑郁的生物学效应和可能机制, 进而深层次揭示骨内分泌活动与运动抗抑郁的分子关系, 为运动健脑提供思路和理论依据。
1 ucOCN介导“骨−脑Crosstalk”在抑郁发生中的作用机制
1.1 ucOCN调节多巴胺、γ-氨基丁酸等神经递质
5-HT、多巴胺(Dopamine, DA)、γ-氨基丁酸(γ-aminobutyric acid, GABA)等单胺类神经递质快速再摄取和抑制降解改善脑区突触间隙信息传递, 调控抑郁发生及其遗传易感性(Andolina et al., 2014)。5-HT浓度快速升高后, 与5-HT1B受体相互作用的钙结合蛋白p11在海马扣带回中表达下调,而5-HT特异性再摄取抑制剂——SSRIs干预后, 其在海马扣带回表达上调, 介导抗抑郁(Wolf et al., 2018)。SSRIs还可抑制5-HT转运体(5-Hydroxytamine transporter, SERT)表达及功能, 提高突触间隙5-HT水平来发挥抗抑郁作用(Baudry et al., 2010)。随着研究深入, 除经典5-HT外, 学者们开始关注谷氨酸能和DA能递质系统在抑郁发生中的作用。临床研究证实, 抗抑郁药物可降低抑郁症患者血清中升高的谷氨酸(Zhang et al., 2013)。而动物研究发现, 抑郁小鼠海马释放的谷氨酸显著增加(Ding et al., 2017)。氯胺酮作为非竞争性N-甲基-D-天冬氨酸受体(N-Methyl-D-aspartic receptor, NMDAR)拮抗剂, 对顽固型抑郁症患者静脉注射亚麻醉剂量氯胺酮后可在2 h内快速产生抗抑郁作用(Zanos et al., 2019)。提示, 脑谷氨酸能递质系统调控抑郁发生。进一步研究发现, 其分子机制与以下两方面有关:谷氨酸激活哺乳动物雷帕霉素靶蛋白(Mammalian target of rapamycin, mTOR)途径增加前额叶皮层神经元的突触数量; 氯胺酮下调沉默状态NMDAR表达后抑制真核起始因子2 (Eukaryotic initiation factor 2, eEF2)激酶(CaMKIII)活性, 导致eEF2去磷酸化并促进皮层BDNF翻译(Gu et al., 2017)。DA调控慢性应激导致的大鼠抑郁样行为, 其机制与DA能神经元突触传递被抑制, 且右侧下额叶、纹状体、海马齿状回等脑区DA浓度显著降低密切相关; 而DA受体激动剂则能改善应激型大鼠的抑郁样行为(Kowiański et al., 2018)。最近研究显示, 人脑组织中多巴胺D1和D2受体结合增多, 而干预抑郁样小鼠脑区两受体结合则产生显著抗抑郁效应(Zhao, Ying, et al., 2019)。单胺类神经递质在介导抑郁和运动抗抑郁中的作用已被证实, 而随着研究深入, 有学者对此假说提出异议。但是, 不可否认的是单胺类神经递质调控抑郁发生和抗抑郁中的作用可能与其他神经生物学机制存在密切关系。
OCN经OB分泌后穿过血脑屏障, 在海马和中脑中与神经元相结合, 促进单胺类神经递质合成的同时抑制GABA合成, 进而改善抑郁; 且怀孕期间向OCN-/-母体小鼠注射OCN可有效预防OCN-/-后代小鼠的抑郁表征出现(Oury et al., 2013)。后续研究发现, OCN发挥作用是以ucOCN的形式调控大脑发育和脑功能, 其缺失导致空间学习能力和记忆力严重缺陷, 加剧抑郁样行为(Rentz et al., 2020)。磁共振扫描发现, ucOCN-/-小鼠大脑比其同窝WT小鼠小, 海马裂和海马伞之间的齿状回CA4区覆盖区域减少30%, 并且海马体半球间胼胝体消失(Shan et al., 2019)。而ucOCN抑制GABA合成, 导致脑和脑干中DA和NE降低20%~50%; OB上特异性敲除ucOCN后, ucOCN–/–小鼠空间学习和记忆能力显著下降, 且出现焦虑和抑郁样行为。注射外源性ucOCN后, 其穿过血脑屏障并与中脑腹侧A11细胞群、海马颞横回和脑干中神经元结合, 促进神经递质合成、分泌并抑制GABA合成, 从而促进学习和记忆能力及抗焦虑、抑郁作用(Liu et al., 2018)。在探究氯胺酮抗抑郁时, 发现ucOCN表达上调能增加前额叶皮质(Prefrontal cortex, PFC)突触神经小体中突触前蛋白SynapsinⅠ及突触后致密蛋白95 (Postsynaptic density protein 95, PSD95)和谷氨酸受体1 (Glutamate receptor 1, GLuR1)表达, 改善抑郁样行为; 靶向性注射ucOCN siRNA可降低SynapsinⅠ等突触蛋白表达, 抑制氯胺酮的抗抑郁作用(aan het Rot et al., 2010)。快感缺失作为抑郁症典型症状, 是大脑奖赏系统功能障碍的直接体现, 其大脑的中脑腹侧被盖区(Ventral tegmental area, VTA)内DA能神经的放电频率明显增加, ucOCN穿过血脑屏障进入VTA后降低DA能神经元放电频率, 通过“骨−脑Crosstalk”来改善抑郁表征(Krishnan et al., 2007)。
ucOCN可通过调节神经递质基因表达来改善抑郁。如ucOCN下调NMDA后促进谷氨酸受体AMPAR表达, 抑制CaMKIII活性, 导致eEF2去磷酸化并上调皮层中BDNF表达, 促进谷氨酸产生, 减少抑制性神经递质GABA表达, 发挥“骨−脑Crosstalk”快速抗抑郁作用(周婵娟, 2016)。后续研究发现, 骨源性ucOCN通过血液途径穿过血脑屏障后与海马CA3区神经元膜上Gpr158结合, 作用于长链非编码RNA浆细胞瘤变体易位基因1 (Long-chain non-coding RNA plasmacytic variant transposable gene 1, PVT1)表达来实现“骨−脑Crosstalk”调控抑郁, 当Gpr158活化后可激活IP3Rs和靶基因BDNF表达, 进而促进神经递质表达, 改善抑郁(Khrimian et al., 2017)。慢性应激压力亦可导致前额叶皮层(Prefrontal cortex, PFC)中Gpr158表达上调, 通过调节改变AMPA受体活性的突触强度来诱导抑郁样行为(Sutton et al., 2018)。在此通路中, Gpr158被证实是ucOCN的脑神经元靶受体, 且ucOCN和Gpr158在调控抑郁发生中的作用关系已被发现和证实。
1.2 ucOCN调节神经内分泌
神经内分泌是调控抑郁症发生的主因之一。研究显示, 抑郁症小鼠血清糖皮质激素(Glucocorticoid, GC)和促肾上腺皮质激素释放激素(Corticotropin releasing hormone, CRH)均显著升高, 且正常小鼠长期注射GC和/或CRH均会出现抑郁样行为(Edvinsson et al., 2020)。基于此, 学者们开始关注HPA轴在抑郁发生中的作用。该轴在长期慢性应激下会反复持续被激活, 导致GC、促肾上腺皮质激素(Adrenocorticotropic hormone, ACTH)和皮质醇等分泌异常, 而过量GC、ACTH等通过激活其受体可抑制小鼠海马齿状回颗粒下区(Subgranular zone, SGZ)神经干细胞的增殖并导致海马萎缩(Rubin et al., 1987)。这也揭示了抑郁症患者脑内海马组织体积萎缩的机制。并且, HPA轴异常导致脑神经元细胞丧失、树突萎缩、可塑性受损, 降低海马对HPA轴的负反馈调节(Eyre & Baune, 2012)。在探究胰岛素与抑郁的发生关系时, 发现胰岛素敏感性改变激活免疫炎症网络, 损伤神经元或神经胶质细胞, 降低大脑皮质兴奋(Mcintyre et al., 2007); 胰岛素抑制大脑NE再摄取, 逆向抑制下丘脑儿茶酚胺和海马中磷酸肌醇表达, 抑制NE和DA转运蛋白, 锂盐代谢失衡, 导致抑郁发生(周婵娟, 2016; Gould & Manji, 2005)。
骨中ucOCN通过血液循环作用于肾上腺皮质束状带细胞内质网, 导致小鼠血清GC浓度降低, 而敲除ucOCN后GC长期处于较高水平并引发抑郁表现(Mar et al., 2020)。另一研究中, 抑郁症患者血清ucOCN浓度升高的同时伴随GC分泌异常(Eyre & Baune, 2012)。ucOCN抑制GC合成、分泌, 进而抑制前额叶皮层和海马区核转录因子- κB (Nuclear factor kappa-B, NF-κB)途径, 小鼠抑郁样行为被改善(Edvinsson et al., 2020)。并且, 在探究ucOCN调控抑郁发生机制时, 发现抑郁症患者血清ucOCN表达下调激活HPA轴, 导致ACTH和皮质醇浓度异常升高(Rubin et al., 1987)。而GC、ACTH和皮质醇分泌异常导致脑部神经元受损、可塑性下降, 抑制海马对HPA轴的负反馈调节(Eyre & Baune, 2012)。GPRC6A是ucOCN在脑内海马、扣带回、齿状回等和胰腺b细胞中的靶受体, 敲除ucOCN后, GPRC6A失活会抑制小鼠胰岛素分泌产生(de Toni et al., 2019); 临床研究亦发现, 2型糖尿病(Type 2 diabetes mellitus, T2DM)患者血清ucOCN与胰岛素分泌呈显著正相关(Liang et al., 2016)。T2DM小鼠血清ucOCN下降导致抑郁样行为出现, 而外源性ucOCN可显著改善ucOCN-/-小鼠的抑郁行为。其分子机制与胰岛素分泌减少激活海马炎症反应和提高IR水平, 导致Ca2+通道被抑制并下调膜蛋白Ezrin表达, 引起小胶质细胞、星型胶质细胞等功能失常和神经元细胞受损、死亡有关(牛望等, 2020)。综上所述, 抑郁发生与骨源性ucOCN介导的胰岛素信号途径密切相关, 并且这在一定程度上也揭示了T2DM共病抑郁症发生的分子机制。
1.3 ucOCN介导神经免疫机制
抑郁发生与压力应激和T淋巴细胞、B淋巴细胞、自然杀伤细胞(Natural killer cell, NK)等免疫细胞数量和活性降低, 促炎因子[肿瘤坏死因子- α (Tumor necrosis factor-α, TNF-α)、白介素6 (Interleukin-6, IL-6)等]释放等多种免疫功能异常密切相关。应激状态下, CRH增加、交感神经系统和HPA轴激活引起皮质醇等激素释放, 抑制正常免疫反应(胡亮等, 2019)。近几年, 神经免疫在抑郁发生中的作用机制受到重点关注。研究表明, 神经炎症通过调控神经再生、HPA轴功能等来影响抑郁和抑郁样神经病变发生。IL-10作为抗炎因子, 其介导抑郁发生, 当对抑郁大鼠注射IL-10后可显著改善其神经功能; 同时, 海马中IL-1β和TNF-α表达下调, 实质中积聚的中性粒细胞数量减少, 神经保护增强(Knoblach & Faden, 1998)。人体研究发现, 抑郁发生后T细胞募集, 通过对抑郁症患者脑组织分析, 发现海马区CD3+、CD4+T淋巴细胞高表达, 而75%样本中存在CD8+T淋巴细胞高表达(Holmin et al., 1998)。但该研究样本数量仅9例, 结果存在一定局限性。有研究却发现, 少突胶质细胞、小胶质细胞和星型胶质细胞等在神经系统中均可形成补体, 抑郁发生后募集活化的T淋巴细胞、巨噬细胞和中性粒细胞等来合成和分泌补体蛋白(Woodruff et al., 2010; Hansen & Malcangio, 2013)。小胶质细胞激活补体受体3 (Complement receptor 3, CR3)在抑郁神经系统病变中发挥突触修剪作用(Kettenmann et al., 2013)。小胶质细胞激活CR3后引起海马长时程突触抑制, 在神经炎症导致抑郁等相关脑功能障碍中导致突触损坏及记忆损伤(Zhang et al., 2014)。LIAN等发现, 神经炎症因子NF-κB激活星型胶质细胞补体C3来参与抑郁发生(Lian et al., 2015)。并且, NF-κB激活后促进小胶质细胞释放C3, 并作用于神经细胞上C3受体引起突触功能改变; 此外, NF-κB/C3/C3aR途径还参与神经细胞内钙电流调节, 增强兴奋状态下突触后电流。然而, 突触后电流受AMPAR调节, 介导抑郁的突触重塑(丘玥等, 2016)。表明, 神经系统自身合成补体参与抑郁的发生、发展。抑郁发生还与IL-1等细胞因子透过血脑屏障刺激脉络丛和第四脑室周围丘脑、下丘脑、海马、室旁核等器官组织中白细胞增多, 进而引起的免疫反应有关。
mTOR是调节免疫反应的关键因子, 可通过调控APP剪切酶如:淀粉蛋白前β位分解酶1 (β-site APP-cleaving enzyme l, BACE1)、Disintegrin和金属蛋白酶结构域15 (Metalloproteinase domain 15, ADAM15)表达来维持海马突触可塑性和记忆形成的关键因子, 而阻断ucOCN介导的丝氨酸/苏氨酸激酶(Serine/threonine kinase, AKT)/mTOR/ NF-κB途径后, ucOCN调控抑郁发生的作用消失(Stepanichev et al., 2014)。星型胶质细胞是一种神经元功能辅助细胞, 参与炎症反应, 抑郁发生后星型胶质细胞数量和密度减少、形态萎缩(Halassa et al., 2009; Jun et al., 2014)。在探究不同浓度ucOCN对C57BL/6乳鼠星型胶质细胞增殖影响时,发现相较于其它浓度, 30 ng/mL ucOCN可显著上调CyclinD2、D3、E及B1表达, 促进G1和G1/S期星型胶质细胞增殖(王新发, 2017)。然而, ucOCN功能缺失导致星型胶质细胞能量代谢紊乱,三磷酸腺苷(Adenosine triphosphate, ATP)不能正常合成, 缺乏肌醇1, 4, 5-三磷酸受体2型(Inositol 1, 4, 5-trisphosphate receptors 2, IP3Rs2)和转基因阻滞的囊泡胶质细胞诱导星型胶质细胞ATP释放缺陷, 而补充ATP可刺激星型胶质细胞的内源性ATP释放在小鼠抑郁症模型中诱导抗抑郁样效应, 进而在内侧PFC中的三磷酸腺苷受体(Adenosine Triphosphate Receptor, P2X2)受体介导ATP的抗抑郁样作用(Liu et al., 2004)。并且, 星型胶质细胞通过局部K+摄取和空间K+缓冲星型胶质细胞阻止细胞外K+的积累以及兴奋性氨基酸转运蛋白1 (Excitatory amino acid transporter 1, EAAT1)、EAAT2 Na+依赖转运蛋白的谷氨酸积累, 从而影响抑郁的神经元兴奋性(Stepanichev et al., 2014)。另外, ucOCN也可通过调节神经免疫相关基因表达来调节抑郁。ucOCN可上调海马组织中PVT1表达, 降低TNF-α、IL-1β和IL-6表达后通过作用于海马组织蛋白激酶C (Protein kinase C, PKC)进而上调BDNF表达, 促进神经元再生及星型胶质细胞活化, 减少TUNEL阳性细胞数量(Zhao, Ding, et al., 2019); 亦可激活AKT/mTOR炎症途径从而调节AKT等蛋白表达, 促进星型胶质细胞、小胶质细胞增加并抑制神经元细胞凋亡, 改善抑郁样行为。并且, 体外实验也证实, ucOCN对Aβ-42损伤PC12细胞的保护作用是通过AKT/mTOR途径进行调控(单畅, 2019)。以上直接证据表明, 骨源性ucOCN通过介导AKT/mTOR/NF-κB炎症途径和炎症细胞来调节海马扣带回星型胶质细胞功能, 进而调控抑郁发生。然而, ucOCN也可直接作用于IL-6、IL-10、IL-1β等表达(Millar et al., 2019), 并通过影响HPA轴、中性粒细胞数量等来调控抑郁发生, 那么ucOCN能否通过介导IL-6、IL-10等炎症因子进而调控抑郁发生尚值得后续探究。
1.4 ucOCN调节神经再生
重度抑郁症患者的脑成像发现, 海马齿状回(Dentate gyrus, DG)等脑组织微细结构退化和海马体积变小, 这一研究验证了中枢神经结构退化在抑郁发生中的作用, 也催生了“抑郁症神经再生假说” (Bremner et al., 1995)。中枢侧脑室室管膜下区(Subventricular, SVZ)和海马DG颗粒下层区是目前研究证实的两个存在神经前体细胞和具有神经再生潜能的区域(石旺清等, 2013)。海马神经再生障碍是抑郁发生的重要机制, 调控海马神经功能及促进神经干细胞增殖、分化、成熟后整合参与神经环路可抗抑郁或改善抑郁样行为(Santarelli et al., 2003)。后续研究发现, 海马为主的齿状回颗粒下层区神经发生减退被认为是抑郁发生的最终通路(Duman, 2004)。慢性应激是抑郁发生的诱导因素, 动物研究中, 社交失败等应激源抑制神经发生, 而抗抑郁可促进海马神经再生(郭雨欣等, 2012)。但有研究却发现, 抑制神经发生并不能导致抑郁发生。习得性无助抑郁模型的脑室壁下回神经发生减退与抑郁行为表现并不相关(Vollmayr et al., 2003); 经颅磁治疗可逆转应激对抑郁症大鼠HPA轴的影响, 但对中侧脑室神经元发生作用不显著(Czéh et al., 2002); 并且, 阻断神经发生后, 单胺类抗抑郁药物、ACTH释放因子和血管升压素药物的效果不受影响(David et al., 2009)。在一项临床研究中, 发现抗抑郁药物对情绪的改善作用并不依赖于神经再生(Bessa et al., 2009)。神经干细胞主要存在于中侧脑室壁的脑室下区和海马齿状回颗粒下层, 以上研究发现海马区神经再生是调控抑郁的关键, 而中侧脑室壁的脑室下区的神经再生却无效。近年来, 探究T2DM神经再生与认知障碍相关性受到国内学者关注。T2DM大鼠骨中Runx2表达下调后抑制ucOCN分泌, 进而导致中脑和海马神经再生被抑制, 神经元结构和功能改变, 认知功能(空间探索和学习记忆能力)受损(Gu et al., 2017)。T2DM加速认知障碍及迅速发展为痴呆症, 导致智力活动、执行能力和处理速度下降(Yu et al., 2020)。然而, T2DM患者血清ucOCN水平显著下降, 这是引起认知功能障碍, 甚至抑郁症、痴呆症及其他精神疾病的原因机制。
抑郁发生后大脑神经再生障碍会导致学习记忆能力下降等表征(López et al., 2016)。ucOCN作为骨源性特异蛋白, ucOCN–/–小鼠双皮质素(Dipcortin, DCX)+和BrdU+神经元数量均显著减少, 当母体基因型为ucOCN–/–时, 这种减少更为严重; 发现ucOCN的敲除促进海马神经细胞凋亡, 减缓神经发生是成年抑郁样小鼠海马依赖性学习能力下降的主因(Oury et al., 2015)。ucOCN基因敲除导致的神经元数量减少、再生能力下降是导致抑郁发生的主因。其分子机制与骨中ucOCN表达下调入血后抑制海马齿状回Notch途径(袁萍, 2020)和磷酸二酯酶9 (Phosphodiesterase 9, PDE9)-环磷酸鸟苷(Cyclic guanosine phosphate, cGMP)-cGMP依赖性蛋白激酶(cGMP dependent protein kinase, PKG)途径(校欢, 2020)密切相关。两条信号途径被抑制后下调靶基因BDNF等表达, 导致小鼠海马齿状回中BrdU、DCX和BrdU/NeuN阳性细胞数量下降, 并显著抑制NSCs细胞活力及其增殖, 导致抑郁发生, 实现“骨−脑Crosstalk”, 上述机制汇总可见表1。
2 ucOCN介导“骨−脑Crosstalk”在运动抗抑郁中的作用机制
2.1 ucOCN调节神经递质在运动抗抑郁中的作用
整合生理学的观点认为, 机体各器官组织之间是相互作用、影响。骨既是运动器官, 亦是重要代谢器官, 因此运动抗抑郁的积极效应均可从骨中找到答案。运动抗抑郁中单胺类神经递质分泌增加(Lee et al., 2019)。有研究发现, 慢性中等不可预知应激抑郁大鼠不同脑区内单胺类神经递质(如NE、DA、5-HT和5-羟吲哚乙酸)水平显著下降, 而长期游泳训练在改善大鼠抑郁表征的同时也会显著恢复和增加单胺类神经递质的分泌(崔冬雪, 2005)。基于代谢组学分析, 不同运动促进CUMS抑郁模型大鼠血浆中5-HT、NE、DA等分泌从而改善抑郁(张波, 2019)。而ucOCN是力学刺激敏感基因, 运动促进骨中ucOCN表达并进而调控抑郁改善, 临床研究中, 每周3~5次、每次35min的慢跑有氧运动使得成年抑郁症患者血清GABA含量下降且谷氨酸含量升高, 进而改善抑郁样行为; 并且, 血清ucOCN浓度升高与GABA浓度下降和谷氨酸浓度升高呈显著正相关(杜远, 2019)。表明, 运动促进抑郁症患者血清ucOCN高表达同时亦抑制GABA并促进谷氨酸等神经递质分泌, 改善抑郁样行为。随着对ucOCN研究深入, 发现运动上调抑郁症大鼠骨中ucOCN表达并促进血清5-HT、DA分泌, 从而改善其抑郁样行为(Zoch et al., 2016)。Obri等(2018)研究也发现, 8周跑台训练上调CUMS大鼠骨中ucOCN表达并释放入血后, 促进5-HT并抑制GABA等分泌, 从而改善其抑郁样行为。另外, 2个月跑台训练(30min/天)可显著提高T2DM合并抑郁症小鼠血清ucOCN水平, 改善其抑郁样行为(Rentz et al., 2020)。其机制与运动激活ucOCN及其介导Gpr158/BDNF途径, 促进神经递质分泌, 进而调控抑郁等认知障碍发生密切相关。提示, ucOCN介导的单胺类神经递质分泌实现了“骨−脑Crosstalk”从而介导运动抗抑郁。基于骨中ucOCN介导抗抑郁的作用及其运动敏感性, 运动诱导骨中ucOCN表达或激活后, 促进单胺类神经递质分泌进而抗抑郁的机制与ucOCN促进IL-6表达及激活胰岛素−胰岛素受体途径有关, IL-6及胰岛素分泌增加经过血液循环、血脑屏障进入脑组织促进5-HT、DA并抑制GABA等单胺类神经递质分泌, 进而改善抑郁(Fordahl & Jones, 2017; Muhammad et al., 2013)。
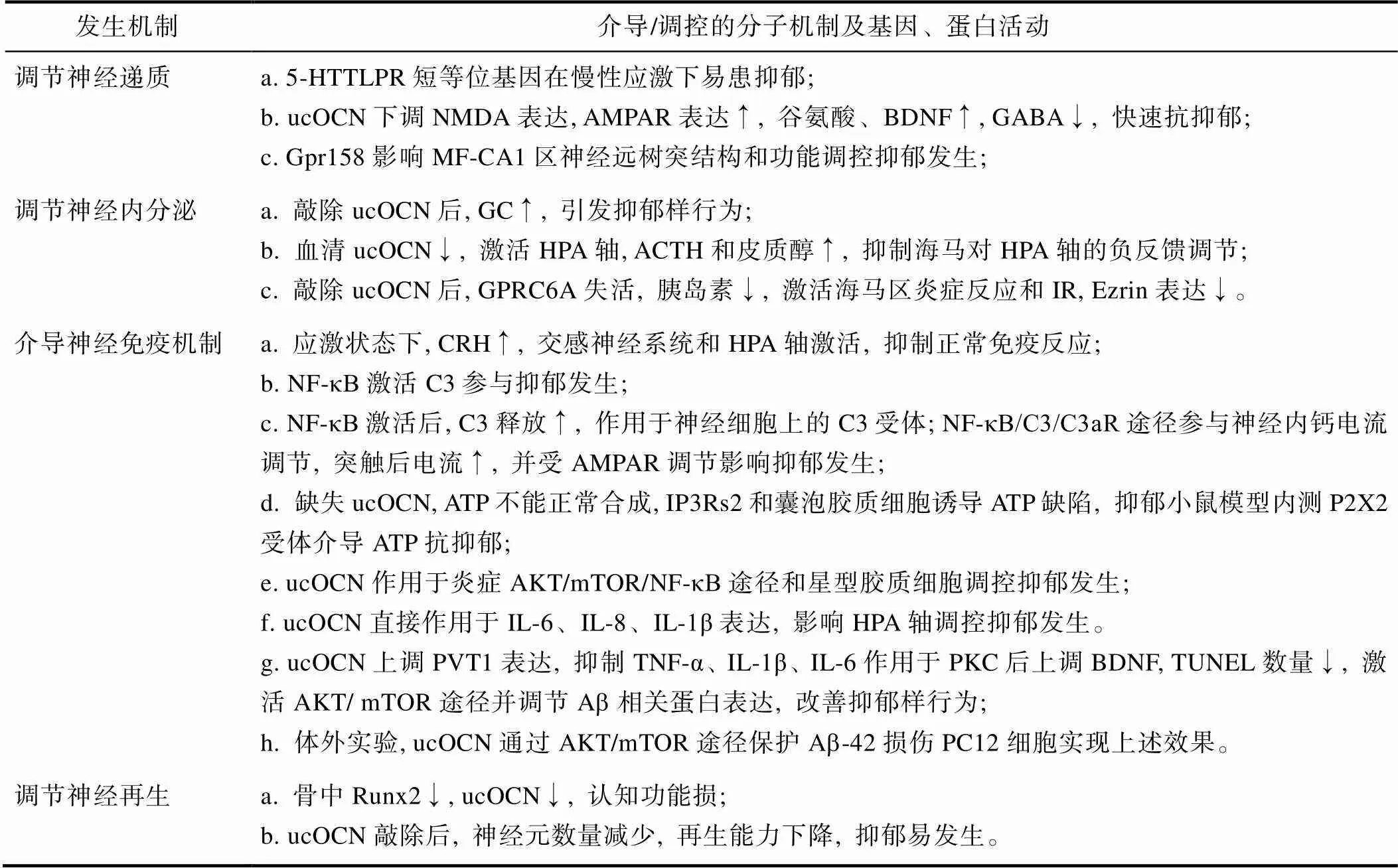
表1 ucOCN介导“骨−脑Crosstalk”在抑郁发生中的作用机制汇总表
注:羧化不全骨钙素(ucOCN), 血清糖皮质激素(GC), 下丘脑−垂体−肾上腺轴(HPA轴), 促肾上腺皮质激素(ACTH), G蛋白偶联受体C家族A成员6(GPRC6A), 胰岛素抵抗(IR), 促肾上腺皮质激素释放激素(CRH), 核转录因子-κB (NF-κB), 星形胶质细胞补体C3, 谷氨酸受体AMPAR受体, 肌醇1, 4, 5-三磷酸受体2型(IP3Rs2), 三磷酸腺苷受体(P2X2), 5-HT转运体基因连锁多态性区域(5-HTTLPR), N-甲基-D-天冬氨酸(NMDA)。
2.2 ucOCN调节神经内分泌在运动抗抑郁中的作用
神经内分泌介导抑郁发生和减缓, 而运动作为一种应激反应可激活HPA轴促进GC、ACTH等激素分泌, 同时亦可增加HPA轴对慢性应激的适应(Hegberg et al., 2019)。Pang等(2013a)在研究体育锻炼对酒精戒断康复期间的抑郁样行为影响时, 发现6周自主跑轮运动可显著改善酒精戒断康复期间小鼠的抑郁样行为; 后续, Pang等利用游泳训练(6周、6天/周、每次50 min)对该小鼠进行运动干预, 发现酒精戒断康复阶段小鼠HPA轴被激活, 血清中ACTH、GC、CRH和促视神经黑皮质激素分泌增加, 小鼠抑郁行为得到显著改善(Pang et al., 2013b)。HPA轴调控抑郁发生、改善, 4周跑轮运动加快小鼠在约束压力下皮质醇、ACTH、盐皮质激素到达峰值时间及加快其衰减; 自主跑轮运动改善全身性注射地塞米松引起的肾上腺变小和ACTH、GC、皮质醇降低, 进而改善抑郁(Hare et al., 2014)。临床研究中, 对80名肥胖青少年进行3个月有氧运动减肥同时, 发现肥胖青少年血清ucOCN水平与尿皮质醇和抑郁样行为改善呈显著正相关(Okbay Güneş et al., 2017)。6周有氧运动训练可显著激活重度抑郁症患者HPA轴, 提高血清皮质醇浓度, 改善抑郁(Gerber et al., 2020)。而对接受过乳腺癌手术且伴有抑郁样行为的85名女性进行6个月运动干预后, 发现HPA轴功能显著改善, 唾液皮质醇显著增加, 且伴随总白细胞、中性粒细胞和淋巴细胞等显著下降(Saxton et al., 2014)。以上动物和临床研究均提示, 运动通过激活HPA轴, 促进ACTH、GC、盐皮质激素、皮质醇等分泌进而改善抑郁行为。其机制与心钠素(Atrial natriuretic peptide, ANP)受体、GC受体、盐皮质激素受体等mRNA表达增加密切相关。ucOCN介导抑郁且在运动应激下表达显著上调, 其表达上调后促进ACTH、GC和盐皮质激素分泌, 血清浓度增加(Nella et al., 2016); 亦可激活HPA轴功能, 提高血清皮质醇、ACTH浓度(Shobana et al., 2019)。而17名抑郁症患者进行12周跑步训练后, 血清ucOCN显著升高且GC、盐皮质激素和ACTH浓度亦显著升高(Tsikirai et al., 2020); 但也有研究发现, 12周跑步运动不能显著提高肺癌患者血清ucOCN和GC、ACTH等浓度, 抑郁样行为变化不明显(Cavalheri et al., 2019)。以上结果差异, 说明运动在上调抑郁症患者ucOCN表达及GC等相关激素分泌上存在时间差异, 并与被试对象不同有关。现有研究证实ucOCN介导ACTH、GC、HPA轴等实现了运动抗抑郁中的“骨−脑Crosstalk”, 但目前运动方式单一, 多种运动抗抑郁作用的对比或联合干预研究尚待补充。
2.3 ucOCN介导神经免疫机制在运动抗抑郁中的作用
神经免疫可直接参与运动抗抑郁, 亦可介导HPA轴等神经内分泌来发挥作用, 由此其在运动抗抑郁中的作用得到特别关注(胡亮等, 2019)。近年来, 运动抗抑郁的神经免疫机制集中在:细胞或体液免疫介导氧化应激或炎症反应进而改善抑郁行为上(Kohut et al., 2006)。如有氧跑台运动可通过诱导内源性硫化氢(Hydrogen sulfide, H2S)气体信号, 并通过抑制Toll样受体4 (Toll-like receptors 4, TLR4)介导的髓样分化因子(Myeloid differentiation factor 88, MyD88)/NF-кB炎症信号途径, 从而改善慢性不可预知应激(CUMS)抑郁小鼠抑郁样行为, 促进海马神经元修复; 并且TLR4抑制剂的效果与跑台运动效果一致, 均可抑制抑郁小鼠血液与海马组织炎症反应(屈红林, 2019)。Kohut等发现, 有氧运动通过降低血清中IL-6、IL-18、TNF-α、C-反应蛋白(C-reactive protein, CRP)等炎症因子来改善慢性应激引发的抑郁(Eyre et al., 2013)。6次/周、共18周的自行车运动可显著降低血清IL-6和IL-18水平(Zhao et al., 2016); 12周慢跑亦可显著抑制慢性堵塞性肺疾病(Chronic obstructive pulmonary disease, COPD)合并抑郁症患者血清中TNF-α、IL-4、IL-6和CRP炎症因子水平(ABD EL-KADER & Al-Jiffri, 2016), 进而改善抑郁样行为。动物研究中, Algaidi等(2019)利用2周强迫游泳建立抑郁症Wistar大鼠模型, 3周自主跑轮运动干预后利用免疫组化对大鼠齿状回、内侧前额叶皮层等部位的巨噬细胞迁移抑制因子(Macrophage migration inhibitory factor, MIF)、IL-6和BDNF表达进行检测, 发现MIF和IL-6表达下调后, 靶基因BDNF等激活并改善大鼠抑郁样行为。说明, 运动改善抑郁与炎症因子表达下调显著相关, 但以上研究集中在有氧运动, 而抗阻训练或有氧运动联合抗阻训练在改善抑郁上的作用效果尚不清楚。ucOCN介导神经免疫调节抑郁发生和减缓, 体育科学领域内, 运动通过提高OB活性来上调ucOCN表达, 进而抑制IL-6、IL-18和TNF-α等炎症因子的mRNA表达, 可显著改善抑郁样行为(Napoli et al., 2014)。其机制与ucOCN表达上调可通过激活ERK途径和STAT途径来下调海马中IL-6和IL-8mRNA及蛋白表达, 进而通过丙二醛(Malondialdehyde, MDA)/超氧化物歧化酶(Superoxide dismutase, SOD)/核因子类红细胞衍生的2-样2 (Nuclear factor erythroid- derived 2-like 2, Nrf2)/血红素加氧酶1 (Heme oxygenase 1, HO1)途径上调VGF和BDNF表达密切相关(Millar et al., 2020)。当利用8周有氧运动对伴随有抑郁表现的大学生进行干预时, 发现血清中表达上调的ucOCN与下调的TNF-α和CRP呈显著负相关, 且其抑郁行为被显著改善(Huang et al., 2020; 许静等, 2016)。以上研究揭示, ucOCN通过调控脑组织神经免疫实现了“骨−脑Crosstalk”, 从而介导运动抗抑郁。
ucOCN介导神经免疫实现“骨−脑Crosstalk”发挥运动抗抑郁, 其机制与ucOCN介导的PFC抗炎能力激活神经生长因子(Nerve growth factor, NGF)−酪氨酸蛋白激酶A (Tyrosine protein kinase A, TrkA) (崔建梅等, 2020)、过氧化物酶体增殖物激活受体-γ共激活因子-1α (Peroxisome proliferator- activated receptorγcoactivator-1α, PGC-1α)/Ⅲ型纤连蛋白域蛋白5 (type Ⅲ domain-containing protein 5, FNDC5)/BDNF (陈蓉, 2019)、miRNAs/ PGC-1α/赖氨酸乙酰转移酶(Lysine Acetyltransferase, KATs) (罗佳, 2019)等信号途径调控抑郁发生、改善密切相关。而ucOCN作为力学刺激敏感基因, 运动上调ucOCN表达后可下调抑郁小鼠海马中IL-6、PGC-1α、miRNA-130b等mRNA表达进而调控抑郁。
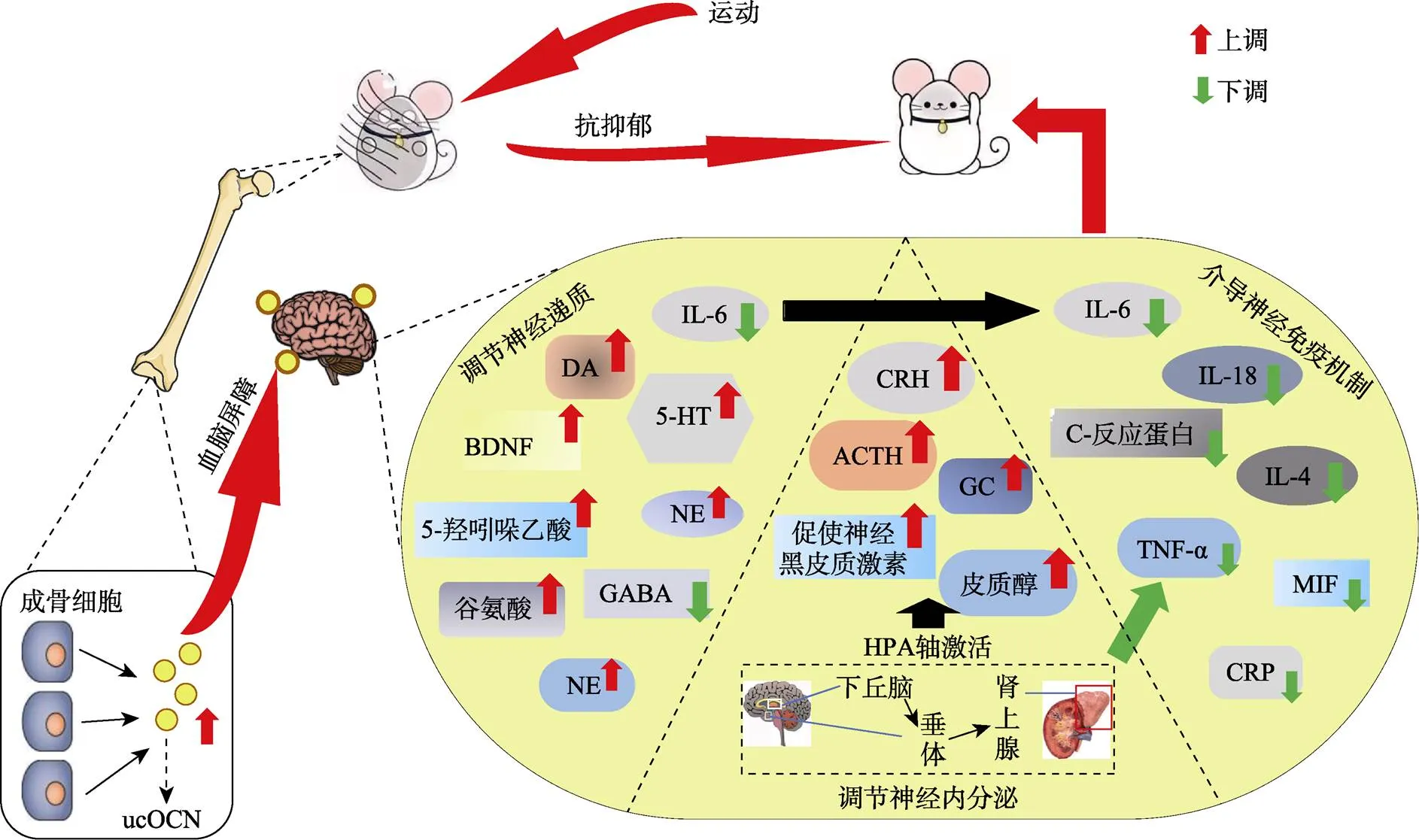
图1 ucOCN介导“骨−脑Crosstalk”在运动抗抑郁中的作用机制示意图
注:ucOCN是OB中分化的特异性蛋白。神经元5-羟色胺(5-HT), 脑源性神经营养因子(BDNF), 多巴胺(DA)、γ-氨基丁酸(GABA), 糖皮质激素(GC), 促肾上腺皮质激素释放激素(CRH), 巨噬细胞迁移抑制因子(MIF), C-反应蛋白(CRP), 白介素6 (IL-6), 肿瘤坏死因子-α (TNF-α)。运动促进骨中ucOCN表达, 通过血脑屏障入脑后, 作用于脑中海马、前额叶等脑区, 促进BDNF、DA、5-HT、NE, 抑制GABA等神经递质表达, 同时减少IL-6水平; ucOCN通过作用于下丘脑等脑区来激活HPA轴, 促进ACTH、CRH等基因表达, 同时通过神经内分泌循环作用降低炎症因子水平, 减缓神经炎症反应。
3 小结与展望
ucOCN作为骨源性力学刺激敏感基因, 运动上调骨中ucOCN表达或活性, 以转录辅激活作用调节单胺类神经递质分泌、神经内分泌功能和神经免疫等, 进而通过多种途径作用于海马等脑组织, 以“骨−脑Crosstalk”形式改善HPA轴功能、减缓中枢炎症反应、促进神经元细胞再生, 进而发挥抗抑郁效应。基于目前相关研究, 对ucOCN介导的“骨−脑Crosstalk” “对话”模式进行分析, 提出了运动抗抑郁中的ucOCN介导途径, 部分机制如图1所示。虽然综述目前相关研究, 发现了“骨−脑Crosstalk”的有利证据, 但仍存在几个问题亟待进一步的探究:(1) ucOCN介导单胺类神经递质或神经内分泌进而调控运动抗抑郁的具体分子机制网络尚待揭示。(2)运动促进神经再生, ucOCN能否介导神经再生从而介导运动抗抑郁?(3)骨骼分泌物或特异性表达因子较多, 目前仅确认了ucOCN在运动抗抑郁中的作用, 未来研究需要进一步筛选可能透过血脑屏障进入脑组织发挥作用的骨分泌或表达特异性小分子物质; 或进一步探讨血脑屏障上的相应受体, 并探讨其机制。(4)运动激活骨中多种基因表达, 但研究发现可介导“骨−脑Crosstalk”的基因并不多。相信, 深入探究和验证以上问题, 将从“骨−脑Crosstalk”的视角上更深层次的解析运动抗抑郁及运动健脑的生物学机制网络。
陈蓉. (2019).(硕士学位论文). 湖南师范大学. https://kns.cnki.net/KCMS/detail/detail.aspx? dbname=CMFD201902&filename=1019671533.nh
崔冬雪. (2005).(博士学位论文). 华东师范大学. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFD9908&filename=2005086214.nh
崔建梅, 王卓琳, 郭燕兰, 李中华, 于芳, 李洪涛, 苏晓云. (2020). 自愿转轮运动对慢性应激大鼠焦虑及抑郁样行为、前额叶皮质炎症因子及NGF/TrkA信号通路的影响.(3), 321−327.doi: 10.13297/j.cnki. issn1005-0000.2020.03.012
杜远. (2019).(硕士学位论文). 山东大学. https:// kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202001&filename=1020013505.nh
郭雨欣, 邢国刚. (2012). 抑郁症的生物学机制研究进展.(1), 57−60.
胡亮, 韩雨晴. (2019). 运动抗抑郁的神经生物学机制研究新进展.),(3), 9−20+ 125. doi: 10.15983/j.cnki.jsnu.2019.03.232
刘文彬, 刘微娜, 漆正堂. (2018). 神经营养因子介导运动的抗抑郁作用.(10), 54−66. doi: 10.16469/ j.css.201810007
罗佳. (2019).(硕士学位论文). 湖南师范大学. https://kns.cnki.net/KCMS/detail/detail. aspx?dbname=CMFD201902&filename=1019671532.nh
牛望, 李茜, 蒋若天. (2020). 胶质细胞在术后认知功能障碍发生发展中的研究进展.(4), 708−713+720.
丘玥, 王之遥, 黄宇光. (2016). 神经病理性疼痛的补体相关神经免疫机制的研究进展.(3), 214−218.
屈红林. (2019).HS(博士学位论文). 湖南师范大学. https://kns.cnki.net/KCMS/ detail/detail.aspx?dbname=CDFDLAST2020&filename= 1019672967.nh
单畅. (2019).(博士学位论文). 上海交通大学. https://kns.cnki.net/ KCMS/detail/detail.aspx?dbname=CDFDLAST2020&filename=1020619276.nh
石旺清, 郑关毅, 陈晓东, 朱元贵, 张静, 江琼. (2013). 大鼠脑缺血/再灌注后bFGF和GAP-43的表达与神经再生.(1), 63-67+98-100. doi: 10.13459/j.cnki.cjap.2013.01.023
王新发. (2017).(硕士学位论文). 重庆医科大学. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201801&filename=1017844417.nh
夏杰, 刘微娜, 漆正堂, 季浏. (2017). PGC-1α介导的“肌脑Crosstalk”与运动的抗抑郁机制——基于整合生物学的反思与展望.(4), 57−64. doi: 10.16099/j.sus.2017.04.010
校欢. (2020).(硕士学位论文). 重庆医科大学. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202002&filename=1020765079.nh
许静, 房辉, 李玉凯, 张丹丹, 张谷月, 田骆冰, ... 周莉. (2016). 2型糖尿病男性患者血清羧化不全骨钙素水平与抑郁状态的相关性研究.(11), 127−131.
薛香莉, 刘微娜, 漆正堂, 娄淑杰. (2019). 基于“脑-肠互动”理论探究脑肠肽在运动抗抑郁中的作用机制.(12), 76−85. doi: 10.16469/j.css.201912008
袁萍. (2020).(博士学位论文). 重庆医科大学. https://kns.cnki.net/KCMS/detail/detail. aspx?dbname=CDFDLAST2021&filename=1020764528.nh
张波. (2019).(硕士学位论文). 山西大学. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202001&filename=1019247331.nh
周婵娟. (2016).(博士学位论文). 重庆医科大学. https://kns.cnki.net/ KCMS/detail/detail.aspx?dbname=CDFDLAST2017&filename=1017843350.nh
aan het Rot, M., Collins, K. A., Murrough, J. W., Perez, A. M., Reich, D. L., Charney, D. S., & Mathew, S. J. (2010). Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression.(2), 139–145. https://doi.org/10.1016/j.biopsych.2009.08.038
Abd El-Kader, S. M., & Al-Jiffri, O. H. (2016). Exercise alleviates depression related systemic inflammation in chronic obstructive pulmonary disease patients.(4), 1078–1088. https://doi.org/10. 4314/ahs.v16i4.25
Algaidi, S. A., Eldomiaty, M. A., Elbastwisy, Y. M., Almasry, S. M., Desouky, M. K., & Elnaggar, A. M. (2019). Effect of voluntary running on expression of myokines in brains of rats with depression., https://doi.org/10.1177/2058738419833533
Andolina, D., Maran, D., Viscomi, M. T., & Puglisi-Allegra, S. (2014). Strain-dependent variations in stress coping behavior are mediated by a 5-HT/GABA interaction within the prefrontal corticolimbic system.(3), https://doi.org/10.1093/ijnp/pyu074
Baudry, A., Mouillet-Richard, S., Schneider, B., Launay, J. M., & Kellermann, O. (2010). miR-16 targets the serotonin transporter: A new facet for adaptive responses to antidepressants.(5998), 1537–1541. https://doi.org/10.1126/science.1193692
Bessa, J. M., Ferreira, D., Melo, I., Marques, F., Cerqueira, J. J., Palha, J. A., Almeida, O. F., & Sousa, N. (2009). The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling.(8), 764–739. https://doi.org/10.1038/mp.2008.119
Bremner, J. D., Randall, P., Scott, T. M., Bronen, R. A., Seibyl, J. P., Southwick, S. M., Delaney, R. C., McCarthy, G., Charney, D. S., & Innis, R. B. (1995). MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder.(7), 973–981. https://doi.org/10.1176/ajp.152.7.973
Cavalheri, V., Burtin, C., Formico, V. R., Nonoyama, M. L., Jenkins, S., Spruit, M. A., & Hill, K. (2019). Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer.(6), CD009955. https://doi.org/10.1002/14651858.CD009955.pub3
Czéh, B., Welt, T., Fischer, A. K., Erhardt, A., Schmitt, W., Müller, M. B., Toschi, N., Fuchs, E., & Keck, M. E. (2002). Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis.(11), 1057–1065. https://doi.org/ 10.1016/s0006-3223(02)01457-9
David, D. J., Samuels, B. A., Rainer, Q., Wang, J. W., Marsteller, D., Mendez, I., Drew, M., Craig, D. A., Guiard, B. P., Guilloux, J. P., Artymyshyn, R. P., Gardier, A. M., Gerald, C., Antonijevic, I. A., Leonardo, E. D., & Hen, R. (2009). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression(4), 479–493. https://doi.org/10.1016/j.neuron. 2009.04.017
de Toni, L., Guidolin, D., de Filippis, V., Peterle, D., Rocca, M. S., di Nisio, A., de Rocco Ponce, M., & Foresta, C. (2019). SHBG141-161 Domain-Peptide Stimulates GPRC6A-Mediated Response in Leydig and β-Langerhans cell lines.(1), 19432. https://doi.org/ 10.1038/s41598-019-55941-x
Ding, X. F., Li, Y. H., Chen, J. X., Sun, L. J., Jiao, H. Y., Wang, X. X., & Zhou, Y. (2017). Involvement of the glutamate/glutamine cycle and glutamate transporter GLT-1 in antidepressant-like effects of Xiao Yao san on chronically stressed mice.(1), 326. https://doi.org/10.1186/ s12906-017-1830-0
Duman, R. S. (2004). Depression: A case of neuronal life and death?.(3), 140–145. https://doi.org/10.1016/j.biopsych.2004.02.033
Edvinsson, Å., Hoyer, A., Hansson, M., Kallak, T. K., Sundström-Poromaa, I., Skalkidou, A., & Lager, S. (2020). Placental glucocorticoid receptors are not affected by maternal depression or SSRI treatment.(1), 30–36. https://doi.org/10.1080/ 03009734.2019.1702126
Eyre, H., & Baune, B. T. (2012). Neuroplastic changes in depression: A role for the immune system.(9), 1397–1416. https://doi. org/10.1016/j.psyneuen.2012.03.019
Eyre, H. A., Papps, E., & Baune, B. T. (2013). Treating depression and depression-like behavior with physical activity: An immune perspective., 3. https://doi.org/10.3389/fpsyt.2013.00003
Fordahl, S. C., & Jones, S. R. (2017). High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling.(2), 290–299. https://doi.org/10.1021/acschemneuro.6b00308
Frye, C. A., & Walf, A. A. (2009). Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites.(2), 266–269. https://doi.org/ 10.1016/j.physbeh.2009.02.022
Gerber, M., Imboden, C., Beck, J., Brand, S., Colledge, F., Eckert, A., Holsboer-Trachsler, E., Pühse, U., & Hatzinger, M. (2020). Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial.(5), 1419. https://doi.org/10.3390/jcm9051419
Gould, T. D., & Manji, H. K. (2005). Glycogen synthase kinase-3: A putative molecular target for lithium mimetic drugs.(7), 1223–1237. https://doi.org/10.1038/sj.npp.1300731
Gu, P. Y., Yu, F., Jin, S., Yang, Q., Su, J., Chen, Y., Zhao, L., & Hu, S. L. (2017). Analysis of serum undercarboxylated osteocalcin level in rats with type 2 diabetes mellitus and the correlation with cognitive impairment.(3), 2603–2607. https://doi.org/10.3892/etm.2017.4838
Halassa, M. M., Florian, C., Fellin, T., Munoz, J. R., Lee, S. Y., Abel, T., Haydon, P. G., & Frank, M. G. (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss.(2), 213–219. https://doi.org/10.1016/j.neuron.2008.11.024
Hansen, R. R., & Malcangio, M. (2013). Astrocytes-- multitaskers in chronic pain.(1-3), 120–128. https://doi.org/10.1016/ j.ejphar.2013.03.023
Hare, B. D., Beierle, J. A., Toufexis, D. J., Hammack, S. E., & Falls, W. A. (2014). Exercise-associated changes in the corticosterone response to acute restraint stress: Evidence for increased adrenal sensitivity and reduced corticosterone response duration.(5), 1262– 1269. https://doi.org/10.1038/npp.2013.329
Hegberg, N. J., Hayes, J. P., & Hayes, S. M. (2019). Exercise intervention in PTSD: A narrative review and rationale for implementation., 133. https://doi.org/10.3389/fpsyt.2019.00133
Holmin, S., Söderlund, J., Biberfeld, P., & Mathiesen, T. (1998). Intracerebral inflammation after human brain contusion.(2), 291–298. https://doi.org/ 10.1097/00006123-199802000-00047
Huang, T. H., Lin, J. C., Ma, M. C., Yu, T., & Chen, T. C. (2020). Acute responses of bone specific and related markers to maximal eccentric exercise of the knee extensors and flexors in young men.(2), 206–215.
Jun, C., Choi, Y., Lim, S. M., Bae, S., Hong, Y. S., Kim, J. E., & Lyoo, I. K. (2014). Disturbance of the glutamatergic system in mood disorders.(1), 28–35. https://doi.org/10.5607/en.2014.23.1.28
Kettenmann, H., Kirchhoff, F., & Verkhratsky, A. (2013). Microglia: New roles for the synaptic stripper.(1), 10–18. https://doi.org/10.1016/j.neuron.2012.12.023
Khrimian, L., Obri, A., Ramos-Brossier, M., Rousseaud, A., Moriceau, S., Nicot, A. S., Mera, P., Kosmidis, S., Karnavas, T., Saudou, F., Gao, X. B., Oury, F., Kandel, E., & Karsenty, G. (2017). Gpr158 mediates osteocalcin's regulation of cognition.(10), 2859–2873. https://doi.org/10.1084/ jem.20171320
Knoblach, S. M., & Faden, A. I. (1998). Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury.(1), 143–151. https://doi.org/ 10.1006/exnr.1998.6877
Kohut, M. L., McCann, D. A., Russell, D. W., Konopka, D. N., Cunnick, J. E., Franke, W. D., Castillo, M. C., Reighard, A. E., & Vanderah, E. (2006). Aerobic exercise, But not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults.(3), 201–209. https://doi.org/10.1016/ j.bbi.2005.12.002
Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., & Moryś, J. (2018). BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity.(3), 579–593. https://doi.org/10.1007/s10571-017-0510-4
Krishnan, V., Han, M. H., Graham, D. L., Berton, O., Renthal, W., Russo, S. J., Laplant, Q., Graham, A., Lutter, M., Lagace, D. C., Ghose, S., Reister, R., Tannous, P., Green, T. A., Neve, R. L., Chakravarty, S., Kumar, A., Eisch, A. J., Self, D. W., Lee, F. S., … Nestler, E. J. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions.(2), 391–404. https://doi.org/10.1016/j.cell.2007. 09.018
Lee, J. M., Kim, T. W., Park, S. S., Kim, C. J., Shin, M. S., Lee, S. J., Kim, S. H., & Baek, S. S. (2019). Wnt signaling pathway is implicated in the alleviating effect of treadmill exercise on maternal separation-induced depression.(2), 200–205. https://doi.org/10.12965/jer.1938148.074
Lian, H., Yang, L., Cole, A., Sun, L., Chiang, A. C., Fowler, S. W., Shim, D. J., Rodriguez-Rivera, J., Taglialatela, G., Jankowsky, J. L., Lu, H. C., & Zheng, H. (2015). NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease.(1), 101–115. https://doi.org/10.1016/j.neuron.2014.11.018
Liang, Y., Tan, A., Liang, D., Yang, X., Liao, M., Gao, Y., Jiang, Y., Yao, Z., Lin, X., Lu, Z., Wu, C., Zhang, S., Hu, Y., Qin, X., Mo, Z., Li, H., & Zhang, H. (2016). Low osteocalcin level is a risk factor for impaired glucose metabolism in a Chinese male population.(4), 522–528. https://doi.org/10. 1111/jdi.12439
Lin, X., Patil, S., Gao, Y. G., & Qian, A. (2020). The Bone Extracellular Matrix in Bone Formation and Regeneration., 757. https://doi.org/10. 3389/fphar.2020.00757
Liu, C., Zhu, R., Liu, H., Li, L., Chen, B., Jia, Q., Wang, L., Ma, R., Tian, S., Wang, M., Fu, M., Niu, J., Orekhov, A. N., Gao, S., Zhang, D., & Zhao, B. (2018). Aqueous extract of mori folium exerts bone protective effect through regulation of calcium and redox homeostasis via PTH/VDR/CaBP and AGEs/RAGE/Nox4/NF-κB signaling in diabetic rats., 1239. https://doi.org/10.3389/fphar.2018.01239
Liu, Q. S., Xu, Q., Arcuino, G., Kang, J., & Nedergaard, M. (2004). Astrocyte-mediated activation of neuronal kainate receptors.(9), 3172–3177. https://doi.org/10.1073/pnas.0306731101
López, A. J., Kramár, E., Matheos, D. P., White, A. O., Kwapis, J., Vogel-Ciernia, A., Sakata, K., Espinoza, M., & Wood, M. A. (2016). Promoter-specific effects of DREADD modulation on hippocampal synaptic plasticity and memory formation.(12), 3588–3599. https://doi.org/10.1523/JNEUROSCI. 3682-15.2016
Mar, A. D., Nick, O., Jan-Paul, B., Arul, R. N., Barbara, B., Irina, P., … Joseph, M. M. (2020). Mon-722 cross-species glucocorticoid-sensitive posterior dentate gyrus gene network: Developing a polygenic score associated to susceptibility to depression after early life adversity exposure in humans.(Supplement_1).
McIntyre, R. S., Soczynska, J. K., Konarski, J. Z., Woldeyohannes, H. O., Law, C. W., Miranda, A., Fulgosi, D., & Kennedy, S. H. (2007). Should depressive syndromes be reclassified as "metabolic syndrome type II"?.(4), 257–264. https://doi.org/10.1080/10401230701653377
Millar, S. A., Anderson, S. I., & O'Sullivan, S. E. (2019). Osteokines and the vasculature: A review of the in vitro effects of osteocalcin, fibroblast growth factor-23 and lipocalin-2., e7139. https://doi.org/10.7717/ peerj.7139
Millar, S. A., Zala, I., Anderson, S. I., & O'Sullivan, S. E. (2020). Osteocalcin does not influence acute or chronic inflammation in human vascular cells.(4), 3414–3424. https://doi.org/10.1002/ jcp.29231
Muhammad, S. I., Maznah, I., Mahmud, R., Zuki, A. B., & Imam, M. U. (2013). Upregulation of genes related to bone formation by γ-amino butyric acid and γ-oryzanol in germinated brown rice is via the activation of GABAB-receptors and reduction of serum IL-6 in rats., 1259–1271. https:// doi.org/10.2147/CIA.S45943
Napoli, N., Strollo, R., Paladini, A., Briganti, S. I., Pozzilli, P., & Epstein, S. (2014). The alliance of mesenchymal stem cells, bone, and diabetes., 690783. https://doi.org/10.1155/ 2014/690783
Nella, A. A., Mallappa, A., Perritt, A. F., Gounden, V., Kumar, P., Sinaii, N., Daley, L. A., Ling, A., Liu, C. Y., Soldin, S. J., & Merke, D. P. (2016). A phase 2 study of continuous subcutaneous hydrocortisone infusion in adults with congenital adrenal hyperplasia.(12), 4690–4698. https://doi.org/10.1210/jc.2016-1916
Obri, A., Khrimian, L., Karsenty, G., & Oury, F. (2018). Osteocalcin in the brain: From embryonic development to age-related decline in cognition.(3), 174–182. https://doi.org/10.1038/ nrendo.2017.181
Okbay Güneş, A., Alikaşifoğlu, M., Şen Demirdöğen, E., Erginöz, E., Demir, T., Kucur, M., & Ercan, O. (2017). The relationship of disordered eating attitudes with stress level, bone turnover markers, and bone mineral density in obese adolescents.(3), 237–245. https://doi.org/ 10.4274/jcrpe.3794
Oury, F., Ferron, M., Huizhen, W., Confavreux, C., Xu, L., Lacombe, J., Srinivas, P., Chamouni, A., Lugani, F., Lejeune, H., Kumar, T. R., Plotton, I., & Karsenty, G. (2015). Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis.(5), 2180. https://doi.org/10. 1172/JCI81812
Oury, F., Khrimian, L., Denny, C. A., Gardin, A., Chamouni, A., Goeden, N., Huang, Y. Y., Lee, H., Srinivas, P., Gao, X. B., Suyama, S., Langer, T., Mann, J. J., Horvath, T. L., Bonnin, A., & Karsenty, G. (2013). Maternal and offspring pools of osteocalcin influence brain development and functions.(1), 228–241.
Pang, T. Y., Du, X., Catchlove, W. A., Renoir, T., Lawrence, A. J., & Hannan, A. J. (2013b). Positive environmental modification of depressive phenotype and abnormal hypothalamic-pituitary-adrenal axis activity in female C57BL/6J mice during abstinence from chronic ethanol consumption., 93. https://doi.org/10.3389/fphar.2013.00093
Pang, T. Y., Renoir, T., Du, X., Lawrence, A. J., & Hannan, A. J. (2013a). Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running.(11), 1803–1810. https://doi.org/10.1111/ejn.12195
Rentz, J., Winberg, J., Swardfager, W., & Mitchell, J. (2020). Sat-293 osteocalcin and exercise improve mood and cognition in female mice with high-fat diet induced type 2 diabetes.(Supplement_1).
Rubin, R. T., Poland, R. E., Lesser, I. M., Winston, R. A., & Blodgett, A. L. (1987). Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls.(4), 328–336. https://doi.org/10.1001/ archpsyc.1987.01800160032006
Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., Weisstaub, N., Lee, J., Duman, R., Arancio, O., Belzung, C., & Hen, R. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants.(5634), 805–809. https://doi. org/10.1126/science.1083328
Saxton, J. M., Scott, E. J., Daley, A. J., Woodroofe, M., Mutrie, N., Crank, H., Powers, H. J., & Coleman, R. E. (2014). Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: A randomised controlled trial.(2), R39. https://doi.org/10.1186/bcr3643
Shan, C., Ghosh, A., Guo, X. Z., Wang, S. M., Hou, Y. F., Li, S. T., & Liu, J. M. (2019). Roles for osteocalcin in brain signalling: Implications in cognition- and motor-related disorders.(1), 23. https://doi.org/ 10.1186/s13041-019-0444-5
Shobana, A., Danae, D., Sundeep, K., Matthew, D., & Irina, B. (2019). Sat-366 the impact of mild autonomous cortisol secretion on bone metabolism.(Supplement_1).
Stepanichev, M., Dygalo, N. N., Grigoryan, G., Shishkina, G. T., & Gulyaeva, N. (2014). Rodent models of depression: Neurotrophic and neuroinflammatory biomarkers., 932757. https://doi.org/ 10.1155/2014/932757
Sutton, L. P., Orlandi, C., Song, C., Oh, W. C., Muntean, B. S., Xie, K., Filippini, A., Xie, X., Satterfield, R., Yaeger, J., Renner, K. J., Young, S. M., Jr, Xu, B., Kwon, H., & Martemyanov, K. A. (2018). Orphan receptor GPR158 controls stress-induced depression., e33273. https://doi.org/10.7554/eLife.33273
Tsikirai, T. M., Ramirez, F., & Nedley, N. (2020). Light and exercise therapy improves depression in women with premenstrual syndrome.(S1), SUN-006.
Vella, A., & Kumar, R. (2013). Osteocalcin and the Regulation of Glucose Metabolism.(1), 11–16. https://doi.org/10.1007/s12018-012-9126-x
Vollmayr, B., Simonis, C., Weber, S., Gass, P., & Henn, F. (2003). Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness.(10), 1035–1040. https://doi.org/10.1016/s0006-3223(03)00527-4
Wolf, D., Klasen, M., Eisner, P., Zepf, F. D., Zvyagintsev, M., Palomero-Gallagher, N., Weber, R., Eisert, A., & Mathiak, K. (2018). Central serotonin modulates neural responses to virtual violent actions in emotion regulation networks.(7), 3327–3345. https://doi.org/10.1007/s00429-018-1693-2
Woodruff, T. M., Ager, R. R., Tenner, A. J., Noakes, P. G., & Taylor, S. M. (2010). The role of the complement system and the activation fragment C5a in the central nervous system.(2), 179–192. https://doi.org/10.1007/s12017-009-8085-y
Yu, H., Li, H., Liu, X., Du, X., & Deng, B. (2020). Levels of serum S100B are associated with cognitive dysfunction in patients with type 2 diabetes.(5), 4193–4203. https://doi.org/10.18632/aging.102873
Zanos, P., Highland, J. N., Stewart, B. W., Georgiou, P., Jenne, C. E., Lovett, J., Morris, P. J., Thomas, C. J., Moaddel, R., Zarate, C. A., Jr, & Gould, T. D. (2019). (2R, 6R)-hydroxynorketamine exerts mGlu2 receptor- dependent antidepressant actions.(13), 6441–6450. https://doi.org/10.1073/ pnas.1819540116
Zhang, J., Malik, A., Choi, H. B., Ko, R. W., Dissing-Olesen, L., & MacVicar, B. A. (2014). Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase.(1), 195–207. https:// doi.org/10.1016/j.neuron.2014.01.043
Zhang, J., Narr, K. L., Woods, R. P., Phillips, O. R., Alger, J. R., & Espinoza, R. T. (2013). Glutamate normalization with ECT treatment response in major depression.(3), 268–270. https://doi.org/ 10.1038/mp.2012.46
Zhao, C., Ma, H., Yang, L., & Xiao, Y. (2016). Long-term bicycle riding ameliorates the depression of the patients undergoing hemodialysis by affecting the levels of interleukin-6 and interleukin-18., 91–100. https://doi.org/10.2147/NDT. S124630
Zhao, J., Ying, L., Liu, Y., Liu, N., Tu, G., Zhu, M., Wu, Y., Xiao, B., Ye, L., Li, J., Guo, F., Zhang, L., Wang, H., & Zhang, L. (2019). Different roles of Rac1 in the acquisition and extinction of methamphetamine-associated contextual memory in the nucleus accumbens.(23), 7051–7071. https://doi.org/10.7150/thno.34655
Zhao, T., Ding, Y., Li, M., Zhou, C., & Lin, W. (2019). Silencing lncRNA PVT1 inhibits activation of astrocytes and increases BDNF expression in hippocampus tissues of rats with epilepsy by downregulating the Wnt signaling pathway.(9), 16054–16067. https://doi.org/10.1002/jcp.28264
Zoch, M. L., Clemens, T. L., & Riddle, R. C. (2016). New insights into the biology of osteocalcin., 42–49. https://doi.org/10.1016/j.bone.2015.05.046
The potential role of bone-derived factor ucOCN in the anti-depressive effects of exercise
CHEN XiangHe1, LI WenXiu1, LIU Bo1, YIN RongBin2
(1College of Physical Education, Yangzhou University, Yangzhou 225127, China)(2College of Physical Education, Soochow University, Suzhou 215000, China)
Undercarboxylated osteocalcin (ucOCN) is a specific protein secreted by osteoblasts in bone. It has attracted attention in the field of neuroscience because of its important role in regulating neurodevelopment and neuroplasticity. “Bone-Brain Crosstalk” is a bone endocrine-nerve mediated response system. ucOCN modulate the monoamine neurotransmitters, neuroendocrine, neuroimmunity, nerve regeneration and gene expression after passing through the blood-brain barrier. ucOCN further acts on the hippocampus CA3 area, cingulate gyrus and other brain areas to regulate the occurrence and reduction of depression. As a bone-derived mechanical stimulation sensitive gene, ucOCN enters the blood circulation after exercise upregulating its expression, and mediates 5-HT/GABA secretion, HPA axis function, inflammation, neurotrophic factor (BDNF, etc.) expression or signal pathways (such as GSK3β/β-catenin, TLR4/miR- 223/NLRP3, etc.) activation to achieve the “Bone-mediated brain” and mediate the antidepressant effect of exercise. This review emphasized the anti-depression effect of exercise through the discussion and sorting out of the mechanism of ucOCN-mediated brain function changes. On one hand, it is helpful to have a deeper understanding of bone endocrine function, on the other hand, it provides a new theoretical basis and research ideas for the occurrence and improvement of depression and the study of exercise anti-depression.
undercarboxylated osteocalcin, bone, brain, exercise, depression
B845
2021-04-28
* 国家社会科学基金教育学青年课题《精准运动改善青少年抑郁症的模式构建及应用研究》(CLA200279)。
陈祥和, E-mail: huashixh@163.com

