Tomato SlPti5 plays a regulative role in the plant immune response against Botrytis cinerea through modulation of ROS system and hormone pathways
TANG Qiong,ZHENG Xiao-dong,GUO Jun,YU Ting
1 College of Biosystems Engineering and Food Science,National Engineering Laboratory of Intelligent Food Technology and Equipment/Key Laboratory for Agro-Products Postharvest Handling of Ministry of Agriculture/Zhejiang Key Laboratory for Agro-Food Processing,Zhejiang University,Hangzhou 310058,P.R.China
2 State Key Laboratory of Food Nutrition and Safety/Key Laboratory of Food Nutrition and Safety,Ministry of Education/Key Laboratory of Food Quality and Health of Tianjin,Tianjin University of Science and Technology,Tianjin 300457,P.R.China
Abstract While SlPti5 has been shown to play a crucial role in the regulation of antagonistic genes in Solanum lycopersicum and Arabidopsis against pathogen infection,there have been no comprehensive studies on the effects of SlPti5 on the regulatory response mechanism of reactive oxygen species (ROS) system and hormone pathways during growth and disease resistance of tomato plants. Here,we investigated the function of SlPti5 in the defense response of tomato against Botrytis cinerea utilizing a virus-induced gene silencing (VIGS)-based system. Expression profile analysis showed that SlPti5 was significantly induced upon B.cinerea infection,with high expression levels in the leaves and fruit of tomato. VIGS-based silencing of SlPti5 inhibited early vegetative growth,increased the plant’s susceptibility to infection,promoted the development of ROS,affected the expression of genes involved in the ROS scavenging system,and attenuated the expression of genes associated with pathogenesis and the ethylene/jasmonic acid signaling pathways. In sum,our data demonstrated that SlPti5 stimulates the immune response of tomato plant to Botrytis cinerea infection by involving the ethylene (ET)-and jasmonic acid (JA)-mediated pathways and modulating the expression of some key pathogenesis-related (PR) genes.
Keywords:tomato (Solanum lycopersicum),Botrytis cinerea,SlPti5,immune response,ET-and JA-mediated signaling pathways,pathogenesis-related proteins
1.Introduction
Higher plants,which are sessile organisms,have evolved an intricate and complex innate immune system that consists of two types of responses,namely,effectortriggered immunity (ETI) and pathogen-or microbialassociated molecular pattern (PAMP/MAMP)-triggered immunity (PTI) to defend themselves from pathogen attack (Bernouxet al.2011). However,only PTI is thought to be effective against necrotrophic pathogens such asBotrytiscinerea(Ouyanget al.2016). There are also two levels of inducible immunity,systemic acquired resistance (SAR) and induced systemic resistance(ISR),that can be activated by various stimuli through specific signaling pathways (Zhang H Jet al.2015). The plant is alerted to the presence of a pathogen when the cell surface receptors of plant detect PAMPs or other pathogen-related effectors (Osakabeet al.2013). The receptor–PAMP interaction then triggers a series of defense-related signaling pathways (Pieterseet al.2009),leading eventually to transcriptional reprogramming that regulates and controls the expression of a large set of genes (Buscaill and Rivas 2014). This transcriptional reprogramming is the result of the coordinated action of numerous transcription factors (TFs). TFs are divided into different families according to their conserved DNA-binding domains. A variety of TFs belonging to the AP2/ERF,MYB,NAC,bZIP,and WRKY families have been shown to be involved in plant immune responses against various pathogens (Alveset al.2013;Zhang L Met al.2018).
The cherry tomato (Solanumlycopersicumvar.cerasiforme) is one of the major horticultural product worldwide. The plant is,however,susceptible to a variety of fungal pathogens,in particular,Botrytiscinerea(Minet al.2018;Tanget al.2019).Botrytis cinereais an airborne necrotrophic pathogen that attacks more than 200 species of crops causing severe economic losses. The interaction between tomato andB.cinereais a valuable plant–pathogen model for investigating the molecular mechanisms of plant defense against necrotrophic fungal pathogens (Mengiste 2012;Ouyanget al.2016). Recent evidence shows that ethylene (ET),salicylic acid (SA),and jasmonic acid (JA) signaling pathways and transcriptional reprogramming are critical in the tomato immune defense againstB.cinerea(Nieet al.2017). The phytohormones ET,JA,and SA are closely linked to physiological states in which plants can react productively and efficiently to pathogen infections(Harelet al.2014). ET and JA normally regulate defense reactions to necrotrophic pathogens and modulate ISR,while SA activates the SAR response and mediates basic defense reactions through a complex signaling cascade(Alazem and Lin 2015). The balance between these three hormones depends on the specific pathogen and is crucial to defense responses (Dong 1998;Dangl and Jones 2001). It has been found that genes encoding the SlHUB1 and SlHUB2 enzymes involved in the monoubiquitination of histone H2B (Zhang Y Fet al.2015),phosphatidylinositol phospholipase (SlPLC2) (Gonorazkyet al.2016) and mitogen-activated protein kinase kinase(SlMKK2 and SlMKK4) (Liet al.2014) participate in the immune response of tomato toB.cinereapossibly by modulation of the SA-and JA/ET-mediated signaling pathways.
The AP2/ERF superfamily of TFs is specific to plants.Recent genome-wide bioinformatic analyses identified 146 AP2/ERF genes in tomato,of which 77 belong to the ERF family (Liuet al.2016). ERF proteins contain a 58–59 residue DNA-binding domain that is strongly conserved and many tomato ERF genes have been shown to be differentially induced bystress factors,including hormones (Ohmetakagi and Shinshi 1995). The phytohormone ET,the simplest alkene (C2H4),may act on ERFs to modulate the expression of pathogenesisrelated (PR) genes. ERFs bind to the GCC-box,either activating or repressing transcription,and are known to regulate immune defense (Ouyanget al.2016). Among the characterized tomato ERF genes,SlPti4(Sl-ERF.A3),SlPti5(Sl-ERF.C5),SlERF1(Sl-ERF.H1),SlERF4(Sl-ERF.B3),SlTSRF1(Sl-ERF.C4),SlTERF1(Sl-ERF.C1),andSlERF5(Sl-ERF.C6) are members of the B3 group(also known as the IX group) that play significant roles in modulating defense against pathogens (Alazem and Lin 2015). In addition,the ERF genes,SlPti4,SlPti5,andSlPti6,have been identified by the yeast twohybrid system as binding partners of the resistance (R)genePtokinase. These ERFs can be induced by thePseudomonasbacterium and have been shown to bind specifically to the GCC-box in the promoter region of severalPRgenes (Zhouet al.1997). Study has shown that ERF may also participate in resistanceviaROS signaling;overexpression ofSlERF01not only increases the accumulation of H2O2but also leads to HR-induced cell death in transgenic tomato plants compared with control plants (Yanget al.2020).SlPti5is expressed during exposure to biotic stresses and is independent of ET,JA,and SA signaling pathways,suggesting that this gene plays a specific role in plant defense (Tharaet al.1999). Moreover,SlPti5overexpression in tomato demonstrates thatSlPti5functions positively in defense gene regulation and disease resistance (Heet al.2001).The expression ofSlPti4,SlPti5,andSlPti6inArabidopsisactivates the expression of the SA-regulated genesPR1andPR2. However,each of these tomato ERFs affects the expression of the JA-and ET-regulated genesPR3,PR4,PDF1.2,andThi2.1differently (Guet al.2002).AlthoughSlPti5expression is known to be induced by aphids,contributing to potato aphid resistance in tomatoes,this is not dependent on ET signaling (Wuet al.2015). To sum up,in all the studies onPti5,the regulatory relationship and interaction betweenPti5and hormone pathway during the resistance process have not been reported before.
Although many researchers have analyzed the functional properties ofSlPti5in plants against biotrophic pathogens,its role in defense against necrotrophic pathogens such asB.cinereain tomato remains unknown.Moreover,at present,most studies onPti5function mainly focus onPti5overexpression and exogenous induction,its regulation of tomato growth and resistance is not comprehensive. In addition,ROS scavenging system is related to the resistance of tomato (Mengiste 2012;Huet al.2017),and the effect ofPti5on the accumulation and scavenging of ROS has not been reported yet.Therefore,the present study investigated the functions and involvement ofSlPti5in disease resistance of tomato againstB.cinereaby analyzing the defense responses and disease phenotypes in theSlPti5-RNAi line through virus-induced gene silencing (VIGS) and explored the possible molecular mechanisms. Our results revealed thatSlPti5positively regulates the immune response of tomato againstB.cinereathrough the ROS scavenging system and hormone signal pathways.
2.Materials and methods
2.1.SlPti5 expression profile analysis
Samples of the root,stem,leaf,flower,and immature fruit were collected from uninfected wild-type tomato plants during the same growth period,immediately frozen in liquid nitrogen and stored at–80°C before total RNA extraction and analysis of gene expression. Different tissue samples from the same tomato plant were used as one sample,with a total of 20 samples.
2.2.Plant materials and VIGS of SlPti5 in tomato
Tomato (Solanumlycopersicum) ‘Alisa Craig’,which was generously provided by Dr.Yu Jingquan (College of Agriculture and Biotechnology,Zhejiang University,Hangzhou,China),was used for all the following experiments. Tomato seeds,soaked and germinated in advance,were sown in a sterile 2:1 (v/v) mixture of peat and vermiculite to which Hoagland nutrient solution had been added in 50-well trays. The germination conditions included continuous 300 μmol m–2s–1photosynthetic photon flux density,a 14 h light/10 h dark photoperiod,22°C/20°C day/night air temperature,and 88% relative humidity. VIGS was conducted using bipartite tobacco rattle virus (TRV) vectors in accordance with the protocol described by Liuet al.(2002) and Zhang Het al.(2018).Ten to fourteen-day-old cotyledons of fully expanded tomato seedlings were infiltrated with TRV vectors using a mix of pTRV1 and pTRV2. A PCR-amplified cDNA fragment of tomatoSlPti5was cloned into pTRV2 using a multiple cloning site to silence the target gene. The empty pTRV2 vector functioned as a negative control,and the pTRV2:SlPDSvector was used as a marker for silencing in tomato,which caused the silencing ofSlPDSand induced a photo bleaching phenotype. The acquired plasmid was introduced by electroporation intoAgrobacteriumtumefaciensGV3101. The gene-silenced tomato plants were grown under the conditions described above. After 3–4 weeks,the transcript abundance ofSlPDSwas analyzed by quantitative real-time (qRT)-PCR in the pTRV2:SlPDSplants to evaluate the silencing efficiency. Only tomato plants exhibiting evidentSlPDSsilencing were used for subsequent experiments. About 10 4–5-week-old tomato plants at the 4th-to 6th-leaf stage were used for every treatment in all the VIGS and non-VIGS experiments. The primers used for both VIGS cloning and qRT-PCR are listed in Appendix A.
2.3.Pathogen preparation,inoculation,and assays of disease symptoms
The pathogenB.cinereawas cultured on potato-dextrose agar (PDA) medium (Wanget al.2008) in darkness and at 25°C for 7 d before use. Firstly,B.cinereaspores were obtained by flooding the culture with sterile distilled water,then the mycelia ofB.cinereahas been removed by filtration,after which the concentration of the suspended spores was estimated in a hemocytometer and adjusted to 2×105spores mL–1. Two methods forB.cinereainoculation were used:in vivo,the whole leaf portion of six tomato plants of each treatment group was sprayed uniformly with the spore suspension while leaves of the mock group were sprayed with distilled water;in vitro,2 μL of theB.cinereaspores were spotted on the upper surface of the 20 detached tomato leaves of each treatment group and media buffer on the leaves of the mock group. The plants were placed in a plant factory at 22°C/20°C under a 14 h/10 h (day/night) photoperiod cycle with 90% relative humidity. Leaves from the treated tomato plants were harvested at 0,12,24,and 48 h and quickly frozen in liquid nitrogen. Disease symptoms were photographed after two-day inoculation and assessed by quantifying the actin transcript abundance ofB.cinereathrough qRT-PCR (Zhang Set al.2015).
2.4.RNA isolation and qRT-PCR analysis of gene expression
Total RNA was isolated using an RNA Extraction Kit RNAiso Plus (9108,TaKaRa,Beijing,China) and its quality and concentration measured with a Nano Drop 1000 Spectrophotometer (Thermo-Fisher,USA).Reverse transcription of the RNA was carried out immediately using 1 μg of total DNA-free RNA to firststrand cDNA with the PrimeScript™ RT Reagent Kit with gDNA Eraser (RR047A,TaKaRa,Beijing,China)in accordance with manufacturer’s protocol. The cDNA was diluted 5-fold,and 2 μL of the diluted cDNA was used as the template for qRT-PCR analysis (Tanget al.2019).
qRT-PCR was performed with the SYBR Premix Ex TaqTM(Tli RNaseH Plus) (RR420A,TaKaRa,Beijing,China) using a StepOneTMReal-Time PCR instrument(v.2.2.2,Applied Biosystems) for mRNA quantification.Each reaction system (20 μL) consisted of 10 μL of SYBR Green PCR Mater Mix,0.4 μL of ROX Reference Dye,6.8 μL of sterile water,and 0.4 μL of each of forward and reverse primers. The thermal cycling condition were 95°C for 30 s,followed by 40 cycles of 5 s at 95°C,and 30 s at 60°C. Melting curve analysis from 60°C to 95°C was used to determine product specificity (Tanget al.2019). The gene-specific primers used (designed by and purchased from Sangon Biotech,Shanghai,China) are shown in Appendix A. The housekeeping geneSlActinwas used as the internal standard for data normalization.Fold changes were calculated according to the 2–ΔΔCTformula (Livak and Schmittgen 2001). Three biological replicates and three technical replicates were used for all qRT-PCR analyses.
2.5.Chlorophyll fluorescence analysis and trypan blue staining
Chlorophyll fluorescence analysis was conducted using an Imaging-PAM chlorophyll fluorometer (IMAG-MAXI,Heinz Walz,Germany) equipped with a computer-operated PAM-control unit. The photochemical quantum efficiency of PSII (ΦPSII) was determined as previously described(Gentyet al.1989). TheΦPSIIvalue was calculated asΦPSII=(Fm´–Fs)/Fm´.
Trypan blue staining was carried out as previously described (Huet al.2018). In short,the lactophenoltrypan blue solution was pre-heated in a water bath at 65°C first,and then 1-cm tomato leaf disks with different treatments were immersed in the dye solution respectively. Six tomato plants were selected for each treatment,and three leaves were selected for each tomato plant. Vacuum several times with a 10-mL syringe to ensure complete staining. Then leaf disks were boiled at 100°C for 10 min and covered with plastic wrap. After that,the shaking table was used for overnight dyeing at 100 r min–1. After the dyeing was finished,the decoloring solution was changed every 6 h for 3–4 times until all the chlorophyll was washed away. Cell death was observed using a microscopy and photographs were taken using a CCD camera.
2.6.In situ detection of reactive oxygen species(ROS) accumulation
Insitudetection of H2O2and the superoxide anion in leaf tissues ofB.cinerea-and mock-inoculated plants was carried out by 3,3-diaminobenzidine (DAB)staining (Thordalet al.1997) tetranitroblue tetrazolium chloride (NBT) staining (Doke 1983),respectively. ROS accumulation in different tomato leaves was photographed by a digital camera.
2.7.Statistical analyses
All treatments were performed at least three times,ending in a completely randomized design. Data were obtained from single experiments and represented three replicates with similar results. Data were analyzed by SPSS Statistics version 20.0 (IBM Corp,Armonk,USA) using one-way analysis and Ducan’s multiple range tests. Data are presented as mean±standard deviation (SD) of three independent biological replicates of each determination.Differences withP<0.05 were considered statistically significant.
3.Results
3.1.Expression profile of SlPti5 in different tissues of uninfected wild-type tomato plants
The expression ofSlPti5in different tissues of uninfected wild-type tomato plants was measured by qRT-PCR.As shown in Fig.1-A,considerable differences inSlPti5expression in the different tissues were observed.Expression was the highest in the fruit,which was 4.56-fold of that in the roots. High expression was also detected in leaves as compared to fruit,which was 4.25-fold the expression in roots. There was no significant difference between the fruit and leaf expression. The expression ofSlPti5in the stems and flowers was 3.27-and 2.21-fold of that in the roots.
3.2.Expression analysis of SlPti5 in B.cinereainfected wild-type tomato plants
After inoculation withB.cinerea,the expression ofSlPti5increased with time reaching its peak value (7.89-fold) at 24 hours post-inoculation (hpi) (Fig.1-B) before gradually decreasing. This result indicated thatB.cinereainfection inducedSlPti5expression.

Fig.1 Tissue-specific expression of SlPti5 in tomato (A) and relative expression of SlPti5 in tomato leaf inoculated with Botrytis cinerea infection (B). Error bars presented standard deviation (SD) from three independent repeats and different lower letters indicated significant difference by using Ducan’s multiple range test (P<0.05).
3.3.VIGS of SlPti5 in tomato plants affected vegetative growth
Four weeks after VIGS infiltration,the pTRV2:SlPDSinfiltrated tomato plant leaves (positive control) showed a photo-bleaching phenotype. The silencing efficiency ofSlPti5in the leaves was determined by qRT-PCR in accordance with the standard VIGS procedure. As shown in Fig.2-A,SlPti5in the pTRV2:SlPti5-infiltrated tomato plants showed 36% of the expression level in the pTRV2:00-infiltrated tomato plants,with a silencing efficiency for the pTRV2:SlPti5-infiltrated tomato plants of >80%. During our three repeated and independent experiments,silencing ofSlPti5in tomato plants exerted no lethal effect on the plant growth,but considerably inhibited the growth of the pTRV2:SlPti5-infiltrated plants(Fig.2-B),whose height was about 64% of the pTRV2:00-infiltrated or wild-type tomato plants at 5 wk after VIGS infiltration (Fig.2-C). However,no significant differences in growth was observed in the tomato plants from the different groups seven weeks after VIGS infiltration.

Fig.2 Silencing of SlPti5 affected vegetative growth of VIGS-infiltrated tomato plants. A,the relative expression of SlPti5 in tomato leaves of SlPti5 silenced and negative control plants.* indicated significant difference at P<0.05. B and C,the height of the pTRV:SlPti5 infiltrated tomato plants recorded 4 wk after VIGS infiltration. Data are mean±SD for three independent experiments.Different lowercase letters indicate significant differences (P<0.05) according to Duncan’s multiple range test.
3.4.VIGS of SlPti5 decreased resistance to B.cinerea
We compared the phenotypes of the wild-type,pTRV2:00-infiltrated tomato plants,and pTRV2:SlPti5-infiltrated tomato plants afterB.cinereainfection to determine the effects of silencing ofSlPti5on disease resistance toB.cinereain tomato plants. The third to the 5th leaves from the top of the wild-type,pTRV2:00-infiltrated tomato plants,and pTRV2:SlPti5-infiltrated tomato plants were detached and inoculated with 2.5 μL ofB.cinereasuspension or were uniformly sprayed withB.cinereasuspensionin vivo. Symptoms of disease were noted and recorded at 2 days post-inoculation (dpi). On the one hand,the lesions in the detached leaves of theSlPti5-silenced tomato plants grew rapidly and were more extensive than those in the wild-type and pTRV2:00 groups (Fig.3-A). Meanwhile,the quantitative analyses of lesion areas in the detached leaves showed a dramatic increase in the pTRV2:SlPti5-infiltrated tomato plants compared with the two other groups (Fig.3-B). The lesion area in the leaves from the pTRV2:SlPti5-infiltrated tomato plants was measured as 42.1 mm2on average,equal to 61.9 and 76.9% increases over that in the wildtype and pTRV2:00-infiltrated plants,respectively (average of 26.0 and 23.8 mm2,respectively). On the other hand,in vivodata confirmed those from the detached leaves(Fig.3-C–G). As shown in Fig.3-E,more and larger lesions were observed in the pTRV2:SlPti5-infiltrated tomato plants than in the other samples. In addition,theBcActinAgene transcript levels were used to evaluate the growth rate ofB.cinerea. TheB.cinereabiomass(Fig.3-F),as estimated by the folds ofBcActinA/SlActin,was significantly higher in the pTRV2:SlPti5-infiltrated tomato plants than in the wild-type and pTRV2:00-infiltrated plants,leading to 2.54-and 2.32-fold of increases at 1 dpi,respectively. This result implied that the silencing ofSlPti5in tomato plants impairedB.cinereagrowth. Plant photosynthetic systems are usually irreversibly damaged by phytopathogen infection (Huet al.2018). We,therefore,conductedSlPti5functional analysis in relation to chlorophyll fluorescence of theΦPSIIat 2 dpi (Fig.3-C and G). Compared with the wildtype and pTRV2:00 group plants,silencing ofSlPti5did not influence theΦPSIIreading. TheΦPSIIof the tomato leaves decreased significantly afterB.cinereainfection,especially noticeable in the pTRV2:SlPti5-infiltrated tomato leaves.Trypan blue staining was used to assess leaf cell death(Fig.3-D). It was noticeable that the numbers of dead cells on the leaves of pTRV2:SlPti5-infiltrated tomato plant leaves were both markedly increased and more densely distributed compared with those of the wild-type and pTRV2:00 groups,Taken together,these findings indicated thatSlPti5silencing weakened the resistance to disease caused byB.cinerea,demonstrating thatSlPti5was required for the tomato immune response againstB.cinerea.

Fig.3 Silencing of SlPti5 impaired tomato defense against Botrytis cinerea. A,disease symptoms developed on the detached leaves of silenced and control tomato plants at 2 days post-inoculation (dpi). B,lesion area of the leaves inoculated with B.cinerea.C,representative chlorophyll fluorescence imaging of ΦPSII. D,image of trypan blue staining for cell death in tomato leaves at 2 dpi with B.cinerea. E,disease symptoms developed on the in vitro leaves of silenced and control tomato plants at 2 dpi. F,relative B.cinerea actin transcript abundance in infected leaves at 1 and 2 dpi in vivo. G,quantification of ΦPSII at 2 dpi. Bar=100 μm.Error bars represent the mean value±SD of three independent biological replicates. Different lowercase letters indicate significant differences between treatments using the Duncan’s multiple range test (P<0.05).
3.5.VIGS of SlPti5 accelerated ROS generation after B.cinerea infection
As ROS are related to the immune response of plants against necrotrophic fungal pathogens such asB.cinerea(Pietrowskaet al.2015),we investigated whetherSlPti5silencing modulates ROS levels in tomato plants withB.cinereainfection. In the mock-infiltrated tomato plants(24 hpi),we found no significant difference in ROS accumulation in the leaves of the wild-type,pTRV2:00-and pTRV2:SlPti5-infiltrated tomato plants,implying thatSlPti5silencing did not influence the ROS accumulation in tomato plants. However,24 h afterB.cinereainfection,levels of H2O2and superoxide dismutase (SOD) were dramatically increased in the leaves of the wild-type,pTRV2:00-and pTRV2:SlPti5-infiltrated tomato plants,compared with those in the mock-infiltrated tomato plants at 24 hpi. Moreover,ROS accumulation was more obvious in the pTRV2:SlPti5-infiltrated tomato plants than in the wild-type and pTRV2:00-infiltrated tomato plants(Fig.4-A and B). We further detected the expression difference in the ROS scavenging-related geneSlSOD1and in SOD enzyme activity in the leaves of the wildtype,pTRV2:00-and pTRV2:SlPti5-infiltrated tomato plants. At 12 hpi,SlSOD1expression was reduced by 2-fold in the pTRV2:SlPti5-infiltrated tomato plants compared with the wild-type and pTRV2:00-infiltrated tomato plants (Fig.4-C). Moreover,as shown in Fig.4-D,SOD enzyme activity continued to increase sharply and reached the maximum at 24 hpi in general,whereas the SOD enzyme activity in the pTRV2:SlPti5-infiltrated tomato plants was distinctly lower than that in the wild-type and pTRV2:00-infiltrated tomato plants. These data suggested thatSlPti5silencing could enhance ROS accumulation by influencing the expression of the ROS scavenging system after infection withB.cinerea.
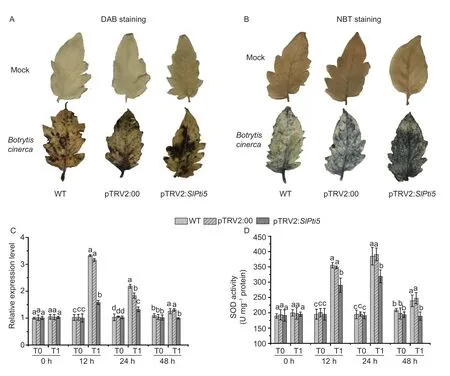
Fig.4 Silencing of SlPti5 prompted reactive oxygen species (ROS) accumulation,and affected the expression of ROS scavenging related superoxide dismutase gene (SOD) and SOD enzyme activity after Botrytis cinerea infection. Tomato plants were inoculated by foliar spraying with B.cinerea spore suspension or mock with similar volumn of solution at 4 wks after VIGS infiltration and leaf samples were taken 24 hours post-inoculation (hpi). A,detection of accumulation of H2O2 after B.cinerea infection. B,detection of accumulation of superoxide anion after B.cinerea infection. C,expression of ROS scavenging-related gene SlSOD1 analyzed by qRT-PCR after B.cinerea infection.The transcript data were normalized by the value of SlActin gene and relative expression levels were shown as folds of the SlActin gene transcript values. D,detection of SOD enzyme activity after B.cinerea infection. T0,mock;T1,B.cinerea. Data were presented as mean±SD from three independent experiments and different letters above the columns indicated significant differences at P<0.05 level.
3.6.VIGS of SlPti5 weakened ET/JA-mediated signaling and the defense response after infection with B.cinerea
To probe the signaling pathways involved in the functioning ofSlPti5in the immune response againstB.cinerea,we detected and analyzed expression level differences in the ET/JA-mediated signaling genes and their relevant defenserelated genes in the leaves of the pTRV2:00,and pTRV2:SlPti5-infiltrated tomato plants before and afterB.cinereainfection.SlACS2(GenBank ID:606304),SlACO1(GenBank ID:544052),SlETR4(GenBank ID:543588),SlERF1(GenBank ID:606712),andSlLOX1(GenBank ID:543994),SlCOI1(GenBank ID:543911),SlMYC2(GenBank ID:544165),SlJAZ1(GenBank ID:838501) are involved in or regulated by either the ET or JA-mediated signaling pathways.Of these genes,SlACS2,SlACO1,SlERF1andSlLOX1,SlCOI1,andSlMYC2in the pTRV2:00-infiltrated tomato plants were dramatically induced afterB.cinereainfection compared with those in the mockinfiltrated tomato plants,whereas their expression levels in the pTRV2:SlPti5-infiltrated tomato plants decreased by 3–7-fold compared with those in the pTRV2:00-infiltrated tomato plants at 12 hpi (Fig.5-A and B). The expression ofSlETR4andSlJAZ1in the pTRV2:SlPti5-infiltrated tomato plants,however,were higher than those in the pTRV2:00-infiltrated tomato plants at 12 hpi (Fig.5-A and B). Twelve hours after infection,the expression of all these genes decreased slightly until the end of the experiment. These results imply thatSlPti5silencing weakens ET/JA-mediated signaling and the plant defense response afterB.cinereainfection.
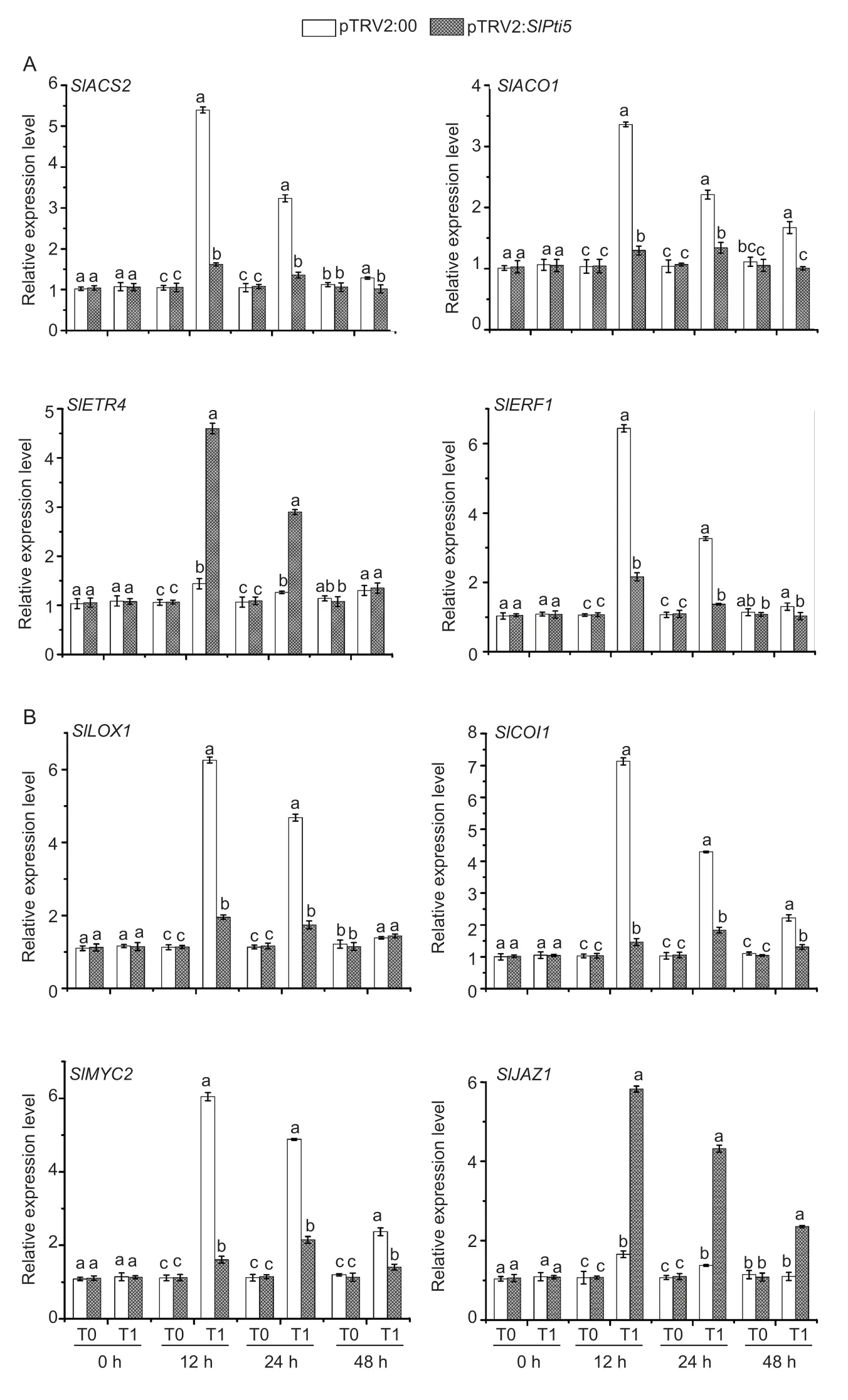
Fig.5 Silencing of SlPti5 influenced the expression of ethylene (ET;A)-and jasmonic acid (JA;B)-mediated signaling responsive genes after Botrytis cinerea infection. Tomato plants were inoculated by foliar spraying with B.cinerea spore suspension or mock with similar volume of solution at 4 wk after VIGS infiltration.At least six leaves from six individual tomato plants were collected at 0,12,24 and 48 hours post-inoculation (hpi) and used for analysis of gene expression.Relative expression levels were shown as folds of the SlActin gene transcript values. T0,mock;T1,B.cinerea. Data were presented as mean±SD from three independent experiments and different letters above the columns indicated significant differences at P<0.05 level.
3.7.VIGS of SlPti5 impaired the expression levels of PR genes upon B.cinerea infection
To further explore the mechanism involved in the functioning ofSlPti5in the immune response againstB.cinerea,we detected and analyzed the levels of expression of PR genes,specifically,SlPR1(GenBank ID:100191111),SlPR5 (GenBank ID:543878632),SlTSI-1(GenBank ID:544134),Chitinase(SlCHI,GenBank ID:101251136) andβ-1,3-glucanaseb(SlGlub,GenBank ID:543987) in the leaves of the wild-type,pTRV2:00-and pTRV2:SlPti5-infiltrated tomato plants before and afterB.cinereainfection. The expression levels ofSlPR1,SlCHI3,SlTSI-1,SlPR5 andSlGLUbin the pTRV2:00-infiltrated tomato plants were markedly increased compared with those in the pTRV2:SlPti5-infiltrated tomato plants and reached their maximum values at 12 or 24 hpi (Fig.6). The expression values of these five genes were notably impaired in the pTRV2:SlPti5-infiltrated tomato plants and were 0.26-(Fig.6-A),0.31-(Fig.6-B),0.38-(Fig.6-C),0.34-(Fig.6-D) and 0.29-fold(Fig.6-E) lower than those in the pTRV2:00-infiltrated tomato plants,respectively. These results suggested thatSlPti5silencing attenuated the immune response againstB.cinereainfection by downregulating several PR genes.

Fig.6 Silencing of SlPti5 impaired the expression levels of pathogenesis-related genes SlPR1 (A),SlPR5 (B),SlTSI-1(C),SlCHI3 (D) and SlGLUb (E) after Botrytis cinerea infection. Relative expression levels were shown as folds of the SlActin gene transcript values. T0,mock;T1,B.cinerea.Data were presented as mean±SD from three independent experiments and different letters above the columns indicated significant differences at P<0.05 level.
4.Discussion
The AP2/ERF family is a large transcription factor family that participates in the immune response to various stresses,both biotic and abiotic,as well as the modulation of plant growth and development.SlPti5,a B3 group member of the tomato ERF family,is known to be involved in plant defensive functions against the potato aphid,Macrosiphumeuphorbiae(Thomas) (Wuet al.2015) and biotrophic pathogens such asPseudomonassyringaepv.tomato(Guet al.2002). However its potential function in plant development and responses to necrotrophic pathogens such asB.cinereahas received relatively less attention and remains poorly researched. In addition,most reports studied the function ofPti5through the overexpression or exogenous induction approaches.Here,the VIGS-based analyses showed thatSlPti5may affect the vegetative growth of tomato in the earlier stage and can effectively promote disease resistance through ROS scavenging system and ET/JA signal pathways.These findings extended our knowledge of the functions of the B3 group of the ERF family in tomato. We used several lines of evidence to support these conclusions.
First,B.cinereainvaded the tomato plant mainly through the leaf and fruit,andSlPti5had a high expression in the leaf and fruit beforeB.cinereainoculation (Fig.1-A).Moreover,SlPti5expression was shown to be induced byB.cinereainoculation,demonstrating its responsiveness to this infection. Expression ofSlPti5has been found to be induced specifically by biotic stresses such asPst DC3000orErysipheorontii(Tharaet al.1999). AfterB.cinereainfection,SlPti5expression in the leaves continued to rise until 24 hpi and reached its peak value in the wild-type tomato plants (Fig.1-B). These results imply thatSlPti5participate in the interaction between the tomato plant andB.cinerea. Many members of the AP2/ERF family play vital roles in growth and development in plants. Recent studies have found thatSlERF.B3andSlERF.A2(SlERF1) are related to the ripening and softening of the tomato fruit (Liuet al.2013,2016). In addition,silencing ofSlERF.A3(Pti4) markedly restrains the growth of pTRV2:SlPti4-infiltrated tomato plants while silencing ofSlERF.B1orSlERF.C2causes the death of the pTRV2:SlERF.B1-and pTRV2:SlERF.C2-infiltrated plants within 7 d after VIGS infiltration (Ouyanget al.2016). Here,silencing ofSlPti5markedly suppressed the growth of the pTRV2:SlPti5-infiltrated tomato plants at an earlier stage (Fig.2-B and C),suggesting that appropriate expression level ofSlPti5is required for early development in tomato plants.
Second,SlPti5silencing increased the susceptibility of the tomato plants toB.cinereainfection,as shown by increased fungal growth and disease severity,and also expanded the lesion area in the pTRV2:SlPti5-infiltrated tomato plants (Fig.3-A,B,E,and F). Similarly,previous studies have reported that silencing ofSlERF.A1,SlERF.A3,SlERF.B4,SlERF.C3,SlHUB1,andSlHUB2significantly impaired leaf defense responses,rendering the plant more susceptible to infection withB.cinerea(Zhang Y Fet al.2015;Ouyanget al.2016). When subjected to exogenous biotic or abiotic stress,the photosynthetic system of plants suffers various degrees of damage which is reflected by the fluorescence parameters of the chloroplast (Townsendet al.2018). As shown in Fig.3-C and G,the photosynthetic system of the leaves from pTRV2:SlPti5-infiltrated tomato plants suffered a higher degree of damage compared with the leaves from the wild-type and pTRV2:00-infiltrated tomato plants. These results supported the assumption thatSlPti5is essential for tomato immunity againstB.cinerea.
Third,cell death was examined through trypan blue staining (Fig.3-D). Cell death is frequently related to ROS generation,so we examined ROS accumulation.ROS are induced by pathogen infection and influence the immune response. In general,ROS could be used and may benefit the establishment of infection by necrotrophic pathogens,such asB.cinerea,while pathogen-induced ROS act as signaling molecules in disease resistanceagainstPstDC3000(Mengiste 2012). In the present study,we observed accumulation of both the superoxide anion and H2O2in the pTRV2:SlPti5-infiltrated tomato plants afterB.cinereainfection. Nevertheless,no significant differences in ROS accumulation were seen between the uninfected pTRV2:SlPti5-infiltrated tomato plants and the wild-type and pTRV2:00-infiltrated tomato plants (Fig.4-A and B). This indicates thatSlPti5silencing may result in uncontrolled ROS scavenging. The hypothesis was verified by the changes in the expression of gene and activity of enzymes that participate in ROS scavenging in the pTRV2:SlPti5-infiltrated plants.The expression ofSlSOD1,which is involved in ROS scavenging,significantly decreased in the infected pTRV2:SlPti5-infiltrated tomato plants (Fig.4-C). This result was consistent with a previous report that plants in whichOsGRXS17had been downregulated showed elevated H2O2production within guard cells (Huet al.2017). Moreover,SOD activity showed a similar trend to that ofSlSOD1,although its maximum level was reached slightly later than that ofSlSOD1. Therefore,we hypothesized thatSlPti5silencing contributed to theB.cinerea-induced accumulation of ROS by influencing the expression of genes involved in ROS scavenging and attenuating their enzyme activity,thereby impairing disease resistance toB.cinerea.
In general,activation of both ET-and JA-mediated signaling pathways is required in tomato for resistance againstB.cinerea(El Oirdiet al.2011). Here,the expression of ET-and JA-mediated signaling and responsive genes was noticeably induced byB.cinereain the pTRV2:00-infiltrated tomato plants (Fig.5),indicating activation of these pathways uponB.cinereainfection.SlACS2andSlACO1are both necessary for ET biosynthesis and,likeSlERF1,are ERF proteins having strongly conserved DNA-binding domains that bind specifically to GCC motifs in the promoter regions of various PR genes (Akagiet al.2011). The ET receptorSlETR4is a negative regulatory factor required for ET responses to the ET signal (Mataet al.2018).SlLOX1is a key rate-limiting enzyme gene involved in JA biosynthesis andSlCOI1functions as a JA receptor,recognizing and binding to JA resulting in the degradation ofSlJAZ1.SlJAZ1is a repressor that prevents the transcription factorSlMYC2from activating JA-responsive genes and defense-related genes (Shigenaga and Argueso 2016;Laiet al.2018). We found that the expression levels ofSlACS2,SlACO1,SlERF1andSlLOX1,SlCOI1,andSlMYC2were significantly suppressed,whereas those ofSlETR4andSlJAZ1were activated in the pTRV2:SlPti5-infiltrated tomato plants afterB.cinereainfection (Fig.5).This result suggests thatSlPti5silencing led to weakened ET-and JA-mediated signaling and thereby impaired the immune response,which resulted in the attenuated resistance toB.cinereaand confirmed that the ET-and JA-mediated signaling pathways are involved inSlPti5-regulated immune response againstB.cinerea. We,therefore,hypothesized thatSlPti5played a role in resistance toB.cinereaby involving the ET-and JAmediated signaling pathways.
Finally,the expression of five PR genes was assayed.PR proteins are traditionally classified into 17 families and serve critical functions in seed germination,fruit ripening,and plant adaptation to environmental stress,such as disease resistance (Zhanget al.2016). SlPR1 proteins are widely considered to play important roles in the defense against a number of plant pathogenic fungi and are significantly induced in tomato plants showing increased tolerance toB.cinereainfection (Segarraet al.2013). Moreover,proteins from the SlPR5 and SlTSI-1 families possess antiviral,antibacterial,and antifungal activities. Chitinase (CHI) and β-1,3-glucanase (GLU)are two enzymatic PR proteins that break down chitin and β-1,3-linked glucans,respectively,in the fungal cell wall inhibiting pathogen growth (Souzaet al.2017). All of the five PR genes were significantly downregulated in the pTRV2:SlPti5-infiltrated tomato plants compared with the pTRV2:00-infiltrated tomato plants uponB.cinereainfection (Fig.6). This downregulation lasted for at least 48 h,implying thatSlPti5played a role in the immune response againstB.cinereaby modulating the expression of some key PR proteins.
5.Conclusion
Taken together,we have demonstrated thatSlPti5stimulateed the tomato plant’s immune response toB.cinereainfection by involving the ET-and JA-mediated pathways and modulating the expression of some key PR proteins genes. Further studies such as the analysis of physiological and biochemical indexes,gene expression,proteomics and metabolomics data between theSlPti5-overexpressed plants andSlPti5-silenced plants afterB.cinereainfection should be conducted to determine whether the ET-and JA-mediated signaling pathways act in combination or independently under the influence ofSlPti5and to elucidate the details of the mechanism ofSlPti5in tomato immune response againstB.cinerea.
Acknowledgements
This research was supported by the National Key Technology R&D Program of China (2016YFD0401201),the National Natural Science Foundation of China(31801602 and 31571897),the Project of Tianjin Education Commission Scientific Research Plan,China(2018KJ094) and the National Science and Technology Major Project of China (2018ZX10101003-002-004).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendixassociated with this paper is available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2022年3期
Journal of Integrative Agriculture2022年3期
- Journal of Integrative Agriculture的其它文章
- Cotton cultivation technology with Chinese characteristics has driven the 70-year development of cotton production in China
- Recent advances in plant immunity with cell death:A review
- Locus TUTOU2 determines the panicle apical abortion phenotype of rice (Oryza sativa L.) in tutou2 mutant
- The removal of nitrate reductase phosphorylation enhances tolerance to ammonium nitrogen deficiency in rice
- Fine mapping and genetic analysis of resistance genes,Rsc18,against soybean mosaic virus
- Improvement in winter wheat productivity through regulating PSII photochemistry,photosynthesis and chlorophyll fluorescence under deficit irrigation conditions
