丝素蛋白再生医学材料对细胞功能调控的研究进展
赵瑞波 谢番 罗丹丹 孔祥东

摘要: 丝素蛋白是重要的天然生物高分子材料,具有出色的机械性能、生物相容性、生物降解性,易于化学修饰等特性,成为再生医学研究中重要的生物材料。近年来,基于丝素蛋白的再生医学材料在骨、皮肤、神经、胰岛等组织修复和再生医学中被广泛应用研究,絲素蛋白材料对细胞功能调控作用逐渐被阐明并成为其指导设计和构建医用丝素蛋白材料结构的重要参考,加速了丝素蛋白材料在临床医学上的应用。本文在对丝素蛋白性质与再生医学关系进行综述分析的基础上,总结丝素蛋白基生物材料在骨、皮肤、神经、胰岛等再生医学领域中对细胞及关联干细胞的功能调控作用,为丝素蛋白材料的生物医学设计和应用提供新的思路。
关键词: 丝素蛋白;骨细胞;皮肤细胞;神经细胞;胰岛细胞;再生医学
中图分类号: TS102.1;Q813 文献标志码: A 文章编号: 1001-7003(2022)01-0010-10
引用页码: 011102DOI: 10.3969/j.issn.1001-7003.2022.01.002
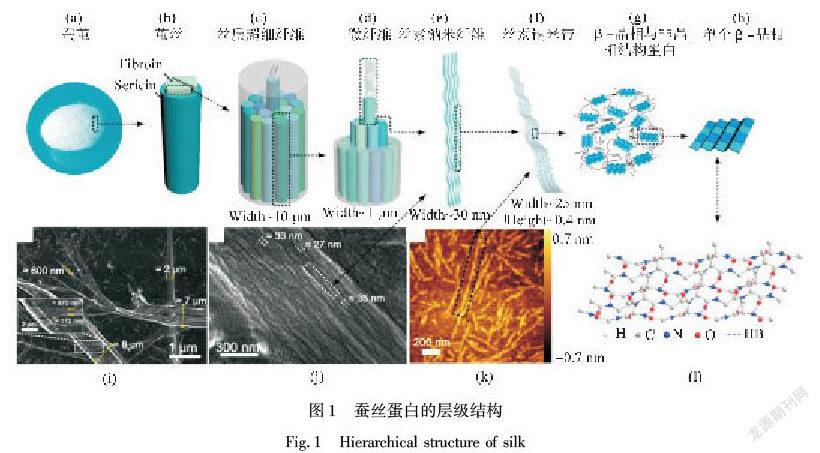
丝素蛋白(Silk fibroin,SF)是蚕丝、蜘蛛丝等丝蛋白的主要组成。如图1[1]所示,蚕茧中的蚕丝主要包含两根丝素蛋白纤维(图1(a)(b)),纤维外层被丝胶蛋白包裹,内层丝素蛋白纤维由一束丝质超细纤维组成(图1(c)),在溶解过程中,脱胶丝素蛋白发生剥离,变成直径几百纳米到1 μm的微纤维(图1(d))。丝素纤维由直径约30 nm的丝素纳米纤维组成(图1(e)),丝素纳米纤维由平均厚度约0.4 nm和宽度约为20~32 nm的丝素纳米带组成(图1(f)),丝素纳米带包含β-晶相和非晶相结构蛋白(图1(g)(h))。此外,SF一级结构包含两条肽链:一条重链,相对分子质量约为391 kDa,主要由甘氨酸和丙氨酸疏水重复序列(GAGAGS)(GAGAGY)组成(占总纤维蛋白50%),形成反向平行的β-折叠;另一条为相对分子质量26 kDa的轻链和糖蛋白P25组成[2]。SF二级结构以三种形式存在:Silk Ⅰ、Silk Ⅱ和Silk Ⅲ。其中,Silk Ⅰ为水溶性亚稳态溶液,存在α-螺旋结构及Ⅱ型β-折叠[3],另外含有无规卷曲结构;Silk Ⅱ主要由β-折叠组成,结构稳定且不溶于水;而Silk Ⅲ主要为三重螺旋链构象,主要存在于水/空气界面。在蚕丝形成过程中,SF蛋白构象从溶解态Silk Ⅰ转变为凝固的Silk Ⅱ。目前,通过有机溶剂(甲醇或乙醇)处理、物理剪切、电磁场等仿生策略可实现Silk Ⅰ向Silk Ⅱ的转化[4],并在骨组织、皮肤组织、神经组织、胰岛组织修复再生中具有广阔的应用前景。
本文主要基于丝素材料特征分析,综述丝素材料性质与细胞功能调控作用的关系,讨论丝素蛋白材料在组织再生医学中对骨细胞、皮肤细胞、神经细胞、胰岛细胞及其他关联细胞可能发挥的调控作用(图2)。
1 丝素生物材料的主要特征
生物医用材料在组织修复及再生医学中需要具备3个关键特征。1) 材料必须具备生物相容性或生物安全性,具有较低的宿主免疫反应,可支持或提高细胞生命活动促进组织修复再生[5-6]。2) 材料具有适当的结构和高的比表面积及良好的通透性,支持氧气/营养素运输,实现并维持细胞间的相互作用。3) 对再生修复材料需具有生物降解性或吸收性,降解速率应与组织再生速率相匹配。SF作为天然生物材料,具备优异的生物相容性,可通过交联或共混调节材料结晶度、存在形式等控制丝素蛋白材料的机械性能和降解速率,在组织工程和再生医学研究中具有良好的应用潜力。
SF是一种生物相容性优异且免疫原性较低的天然材料,降解产生的氨基酸和多肽可以被细胞吸收利用。丝素蛋白材料的生物相容性取决于蛋白的脱胶提取和纯化过程,常将碳酸钠、氢氧化钠溶液用于SF的脱胶[7]。脱胶的SF在体内外具有免疫惰性,丝素蛋白材料的体内生物相容性已开展广泛研究,研究显示与SF刺激有关的淋巴细胞活化因子IL-1β和炎性环氧合酶-2(COX-2)基因表达水平与胶原蛋白没有明显差异[8],可与骨细胞、胰岛细胞、成骨细胞、成纤维细胞、内皮细胞、间充质干细胞等细胞高度相容。目前,SF已获得美国食品药品监督管理局(FDA)批准用于生物医学应用,SF基外科手术网已通过了ISO 10993生物相容性和安全性测试并符合医学标准[9-10]。SF降解后产物为氨基酸,无毒且安全性良好,其二级结构和含量是影响其在体内降解速率的重要因素。SF中β-折叠的含量越高SF降解越慢,再生医学材料中SF的含量越高,孔径越小,SF材料在体内的降解速率越小。此外,SF在体内降解也与材料所处的组织微环境密切相关。SF材料皮下植入时,在组织细胞如巨噬细胞的吞噬作用下,抗张强度逐渐降低,并最终缓慢降解[11-12]。在组织修复过程中,较低的降解速率可保持材料长时间的稳定性与机械强度,有利于制备伤口敷料,另外可使得材料的降解与新组织再生/修复的进程协调,具有良好的应用潜力。
机械强度可调节是SF应用于生物医学领域的另一重要特征,在细胞调控中,材料刚性需与目标细胞刚性匹配,SF基材料刚性及强度变化对细胞分裂及功能分化具有重要作用。此外,丝素蛋白材料刚性会影响材料稳定性和降解性。研究显示SF二级结构与机械性能密切关联,可通过调控材料中β-折叠含量调节SF的机械性,形成不同刚性SF溶液、凝胶或支架等,该策略无需交联即可提供与细胞相匹配的机械性能[13]。此外,研究显示再生SF支架材料及丝素膜的机械性能与天然丝纤维相比较弱,可通过甲醇或乙醇诱导提高丝素内β-折叠含量增强材料机械性能[13-14],如将SF膜于甲醇中分别浸泡10 min和60 min,其弹性模量可分别增至40 MPa和80 MPa[15]。此外,不同来源SF的机械性能也有差异,与非桑树来源的SF相比,桑树来源的蚕丝丝素材料具有更高的机械性能[16]。
除以上性质外,丝素蛋白材料易于加工,可根据功能和应用领域加工成为可注射溶液、纤维材料、薄膜材料、水凝胶材料、支架材料等,并已在硬组织(骨骼)和软组织(皮肤、神经、胰岛)等再生医学研究中发挥细胞调控的重要作用。
2 丝素蛋白材料影响细胞行为的主要因素
細胞-胞外基质间的相互作用与细胞增殖、迁移、分化和功能调控密切相关。通过调节丝素蛋白材料的性质可模拟细胞外基质的主要功能,并可调控细胞黏附、生长和分化等行为。丝素蛋白材料影响细胞行为的主要因素可概括为三方面:丝素蛋白的氨基酸组成,SF材料的机械强度及材料的拓扑结构如材料尺寸、孔径、表面特征等[17]。
丝素蛋白由18种氨基酸组成,其中甘氨酸(Gly)、丙氨酸(Ala)和丝氨酸(Ser)含量最多,占总氨基酸量的75%左右,与壳聚糖等氨基聚糖类似材料联用,可模拟天然细胞外基质。不同蚕丝来源的丝素蛋白在氨基酸组成上有稍许差异,与家蚕丝素蛋白相比,天蚕等非家蚕丝素蛋白含有Arg-Gly-Asp(RGD)三肽序列[18],RGD可以与细胞膜上的整合素特异性结合,诱导整合素相关的信号通路,增强细胞的黏附[19],更好地促进细胞生长。
SF材料的生物力学性能对调节细胞生长、形态、分化、迁移和功能具有重要影响[20]。细胞可以通过感知基质力学性质,将机械刺激转化为化学信号,刺激/抑制因子的分泌,从而调控细胞分化。机械信号是干细胞迁移及分化的关键调节剂[21],如较低的剪应力(0.2 kPa)可以通过SDF-1/CXCR4、Jun N端激酶、p38丝裂原活化蛋白激酶途径诱导细胞迁移[22]。机体内不同组织刚度差异较大,如大脑的刚度约为01~1 kPa,而骨基质的刚度超过25 kPa[23-25]。研究证实,细胞外基质组成和结构及所产生的机械特性可以诱导干细胞增殖和分化为谱系细胞,如间充质干细胞(MSC)在体外经不同刚性如0.1~1 kPa、8~17 kPa和25~40 kPa材料诱导时会分别分化成神经细胞、肌肉细胞和成骨细胞[23];高的力学性能更利于成骨分化,当力学性能大于25 kPa时,骨髓间充质干细胞(BMSCs)倾向于成骨方向分化,力学性能低于20 kPa时,BMSCs易向神经方向分化[23,26]。此外,丝素材料在胚胎分化中同样发挥重要作用,Sun等[27]制备的1 kPa左右的丝素蛋白-明胶水凝胶可以诱导小鼠胚胎干细胞分化为外胚层。
SF基生物材料形貌、大小等因素也会影响细胞功能。Bondar等[28]研究显示170~250 nm丝素纤维可与内皮细胞整合素受体识别,促进细胞黏着斑形成,诱导细胞整合素分泌,显著提高细胞黏附及生长。在丝素蛋白的支架中,Bidgoli等[29]通过加入纳米级(<100 nm)和微米级(6 μm)生物玻璃微球,形成抗压强度分别为0.94 MPa和1.2 MPa的复合SF支架材料,结合支架内10~50 μm和500~600 μm的分级孔径可使骨髓间充质干细胞(BMSC)的黏附效率协同提高50%,并显著促进成骨细胞分化。此外,支架材料的孔径和表面粗糙度与细胞生长、分化等行为也密切相关,100~300 μm孔径的SF支架中培养的细胞比其他孔径中培养的细胞显示出更强的生长、分化和分泌胞外基质的能力。材料孔径为100~300 μm的SF支架能使BMSC具有更好的增殖能力,可提高胞外基质的密度,具有促进成骨分化和骨骼愈合的特性[30]。SF表面微结构的改变通过影响细胞黏着斑的形成进而调控细胞行为,Diener等[31]证实SF材料表面粗糙度对成骨细胞(MG63)黏附与生长具有重要作用,相对光滑表面更利于细胞黏附与生长。
3 再生医学中丝素蛋白调控细胞的应用
3.1 骨细胞调控
丝素蛋白材料在骨组织生物医用材料研究和转化潜力巨大。在骨修复中,SF修复材料可诱导骨缺损部位的组织再生,原位降解产物可被新生骨组织细胞吸收。骨组织工程材料中,SF支架材料可诱导成骨细胞增殖、黏附和分化,诱导新生骨血管生成,促进骨组织再生[32-34]。骨组织中皮质骨和松质骨的杨氏模量范围分别为15~20 GPa和0.1~2 GPa;皮质骨和松质骨的抗压强度分别为100~200 MPa和2~20 MPa[35],而新骨组织生长最佳支架孔径为200~350 μm[36-38]。SF支架具有适当的机械性能和空隙率可调性,可根据修复要求不同(如皮质骨和松质骨),合成不同力学性能、不同空隙、不同降解时间的梯度骨修复支架材料,实现体内可控降解并为新骨生长留出空间,为骨细胞生长分化提供仿生微环境,诱导骨细胞的增殖[36-38]。
在骨细胞调控中,丝素蛋白含有的Arg-Gly-Asp(RGD)多肽可有效与细胞外基质中整联蛋白结合,促进成骨细胞黏附和增殖,提高骨再生能力[39]。与桑树来源SF相比,非桑树源SF具有更高比例的RGD多肽[40]。研究证实,非桑树SF可显著促进Saos-2成骨细胞的细胞附着和增殖能力,且细胞活力随SF含量增加而明显提升[40]。在骨组织修复中,多种信号通路与成骨细胞分化有关,Jung等[41]研究表明丝素蛋白可抑制Notch激活的基因,上调碱性磷酸酶(ALP)的表达量,促进骨髓细胞向成骨分化。此外,机械刺激对成骨细胞信号通路活化发挥重要调控作用,研究显示2 000 μstrain 0.2 Hz力学刺激能够激活JNK和ERK 1/2信号通路,可上调成骨样细胞中护骨素(Osteoprotegerin,OPG)的表达量,促进成骨细胞分化和形成[42]。基于此,将丝素蛋白与磷酸钙、生物玻璃等无机材料支架可以制备成具有可控的力学性质复合支架材料,以此调控细胞丝裂原活化蛋白激酶通路中ERK和JNK信号通路,调控成骨细胞分化和形成。
此外,当丝素蛋白中甘氨酸和丙氨酸含量超过70%时,降解过程中会形成六肽(GAGAGA和GAGAGY)序列,推测此类六肽序列可能参与抑制Notch并激活MAPK信号通路等促成骨因子分泌和传递的重要分子机制,但其调控作用仍需进一步研究。丝素蛋白材料中蛋白相对分子质量与细胞成骨及骨矿化密切相关,研究显示低相对分子质量丝素蛋白(2~10 kDa),具有良好的亲水性并呈现负电性,具有促进生物矿化的作用。采用低相对分子质量丝素蛋白制备支架材料在骨再生过程中显示出良好的骨诱导特性,具有替代骨形态发生蛋白(BMP2)的应用潜力[43]。丝素蛋白材料硬度等性质对骨细胞进行矿化行为调控也密切相关,并对骨细胞胶原蛋白和骨钙素的分泌具有显著调控作用。
3.2 皮肤细胞调控
SF基材料已被广泛用于皮肤再生治疗中,如缝扎结扎丝线、丝素面膜、腹壁重建外科手术网,整形外科中的丝素海绵及声带填充物等[44-46]。丝素蛋白可在密封伤口腔辅助伤口愈合时缓慢降解,降解产物可被机体吸收利用。此外,SF还具有保水性和弹性,也利于皮肤组织的修复与再生。
成纤维细胞是皮肤修复调控的重要细胞,具有形成胶原纤维、弹力纤维构筑皮肤基质的功能。SF材料对皮肤损伤止血及修复效果显著[12],当SF材料与皮肤接触后,SF蛋白可与纤维蛋白原和血小板结合,诱发凝血级联反应,发挥止血作用。利用纤维蛋白原、凝血酶与丝素蛋白制备成多孔海绵状复合材料,将其用作止血基质并可有效协同纤维蛋白原和凝血酶的止血作用[47]。Park等[48]研究显示皮肤损伤修复时,SF材料可激活细胞MEK、JNK、PI3K信号通路刺激成纤维细胞迁移到伤口部位,同时抑NF-κB信号通路,可提高成纤维细胞内cyclin D1蛋白、纤维连接蛋白、波形蛋白和VEGF的表达量,诱导伤口愈合再生[45]。此外,将纤维细胞(如正常细胞L929、NIH/3T3)和癌细胞(Saos-2、CaSki)封装到SF水凝胶中,发现SF能显著抑制Saos-2、CaSki的生长,并维持L929、NIH/3T3的正常生长,推测原因可能与SF凝胶机械性能及微结构有关[49]。血管化是促进组织修复中的关键问题之一,设计和开发血管诱导系统对于维持血管生成、促进缺损组织的修复中发挥重要作用。在伤口修复中,基于SF纳米纤维负载血管生成因子在刺激缺损区域内的血管生成中具有良好作用,并显著加快伤口修复进程。在血管化内皮细胞的调控中,SF材料在内皮祖细胞募集激发血管形成中发挥显著作用,制备丝素蛋白材料时,调节蛋白溶液至pH值为4.0,可消除电荷排斥,以实现更强的亲水相互作用,促进蛋白相互组装,以此制备的丝素材料可显著提高细胞血管内壁黏附因子CD31分泌,促进伤口血管化[50]。进一步在丝素蛋白材料中添加胶原肽和S-亚硝基谷胱甘肽,可提高细胞外基质富集作用,激活内皮细胞一氧化氮信号通路,在体内可促进新血管形成[51-52],显示丝素蛋白与细胞外基质在促进血管化调控中具有协同作用。
在皮肤创伤修复调控中,丝素蛋白具有较低的免疫原性,在使用的初始阶段会引起轻度炎症,有利于破坏损伤部位存在的病原体,同时募集免疫细胞刺激其分泌趋化因子和生长因子(IL-1β、IL-6等)[15]。伤口修复过程中,丝素蛋白材料中富含氨基酸能促进伤口细胞活化并形成抗菌微环境;同时激活巨噬细胞JNK-STAT信号通路,介导巨噬细胞形成M2极化,进一步降低损伤组织部位炎症反应,分泌血管生长因子,并促进成纤维细胞增殖和胶原蛋白分泌组装,招募成纤维细胞和毛细血管等细胞,加速组织修复再生。在使用后期,SF材料缓慢降解,炎症反应减弱进一步促进组织修复[53-54]。在大面积皮肤修复中,基于SF材料的人造真皮可有效促進细胞浸润,血管形成和细胞外基质富集[55]。在Ⅲ级烧伤创面治疗研究中显示,与对照组相比,SF水凝胶材料治疗后创伤面血管密度提高约10倍[53],并显著促进组织中CK 10和CK 14表达,表明SF材料可显著促进新形成表皮中角质细胞的分化,并增强上皮再生和组织向内生长。此外,在顽固性皮肤损伤修复中,SF材料同样显示良好应用潜力,丝素材料在急性伤口和糖尿病伤口愈合初期可快速促进组织形成毛细血管[56]。
3.3 神经细胞调控
神经组织再生医学材料研究聚焦于材料对神经细胞行为(生长、细胞扩散、迁移和分化)的调控,介导神经细胞及神经干细胞对材料所处微环境的正向响应,实现基于材料调控的神经组织修复是当前面临的主要挑战。丝素纤维材料在神经组织修复中具有信号传导的作用,为神经细胞调控提供契机,并在脑组织工程修复中已显示良好前景[57-59]。将丝素蛋白微粒填充到脑损伤部位后,可显著减少脑损伤体积,并在修复14 d后诱导感觉功能的修复[57]。脑组织修复中,丝素蛋白可诱导P12神经细胞黏附因子(E-cadherin和N-cadherin)表达增加,使神经细胞能良好地黏附生长于丝素材料表面[58]。细胞黏附后NCAM可促进神经细胞形态伸长,L-CAM提高神经细胞的迁移,促进神经突出的形成,协同诱导神经细胞的分化成熟。此外,NCAM和L1-CAM与丝素蛋白结合后可加速与其他细胞的相助作用,原位调控神经细胞微环境,加快神经组织修复[59]。细胞外基质中的层黏连蛋白(Laminin,LN)是维持细胞外基质的关键结构蛋白,是脑基质的主要结构组成,基于LN蛋白及其衍生多肽已成为神经调控重要因子,如Arg-Gly-Asp(RGD)、Ile-Lys-Val-Ala-Val(IKVAV)、Tyr-Ile-Gly-Ser-Arg(YIGSR)、Arg-Tyr-Val-Val-Leu-Pro-Arg(YVVLPR)和Arg-Asn-Ile-Ala-Glu-Ile-Ile-Lys-Asp-Ile(RNIAEIIKDI)。IKVAV多肽是LN蛋白α链C末端的组成结构,可快速激活细胞MAPK/ERK1/2和PI3K/Akt信号通路[60],促进神经干细胞黏附、神经突生长、血管生成和Ⅳ胶原酶表达,可有效促进神经干细胞向神经元分化。基于IKVAV肽修饰的SF水凝胶多孔支架可显著提升神经干细胞中β-微管蛋白Ⅲ(神经元分化的标志物)和MAP-2(成熟神经元标记物)表达,有效提高神经元分化和成熟,并显著提升细胞活性[39]。再生丝素蛋白取向对神经细胞分化关系密切,将神经祖细胞(NPCs)与定向和随机再生丝素蛋白(RSF)支架共培养研究发现:定向RSF和随机RSF可显著促进神经祖细胞增殖增,细胞增殖效率分别为143.8%±13.3%和156.3%±14.7%;在神经元分化调控中,定向RSF和随机RSF介导神经元分化效率为93.2% ±6.4%和3 167.1%±4.8%,且RSF可为NPC提供功能性微环境,可为神经组织工程提供新策略[61]。
SF蛋白凝胶的机械性能对神经干细胞同样有显著调控作用,与高模量(1 028、1 735 Pa及19 700 Pa)SF水凝胶相比,183 Pa丝素蛋白水凝胶中的神经干细胞分化和成熟水平显著提高。IKVAV修饰的丝素蛋白水凝胶是脑组织工程的潜在3D支架。苏州大学吕强团队[62-65]研究证实,不同处理的丝素蛋白纳米纤维与神经干细胞的增殖、分化和迁移行为密切相关,其分别采用真空水处理、50%甲醇、80%甲醇处理制备了丝素蛋白纳米纤维。将不同处理与神经干细胞共培养时发现,与对照组相比,真空水处理、50%甲醇和80%甲醇处理的丝素蛋白纳米纤维均能显著降低神经干细胞的死亡,且真空水处理和50%甲醇处理组可促进神经干细胞向星形胶质细胞的分化,而80%甲醇处理组能抑制神经干细胞向星形胶质细胞的分化,實现了基于丝素蛋白材料对神经干细胞的直接调控。
脊髓损伤与修复中神经轴突的形成是实现脊髓损伤修复的关键环节。Qu等[66]将神经元和星形胶质细胞掺入400、800 nm和1 200 nm SF纤维中,与对照组相比,SF材料处理可显著提高星形胶质细胞迁移效率,诱导神经突排列和伸长,直径小于1 200 nm SF更有利于脑室下区域衍生神经元发育和成熟,促进神经胶质纤维酸性蛋白表达,400 nm SF处理的神经细胞伸展面积显著高于1 200 nm SF处理组。由此显示,SF纤维材料可为脊髓等中枢神经细胞突触生长提供良好微环境,且有望通过调控纤维尺度实现神经轴突的再生能力。
3.4 胰岛细胞调控
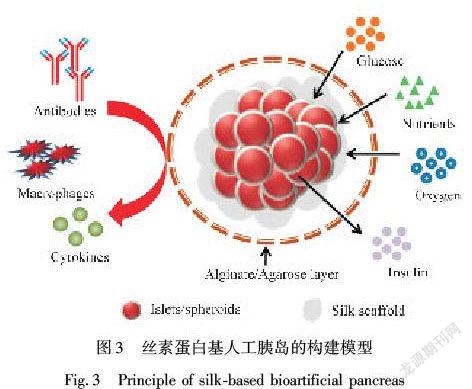
胰岛细胞是机体重要的分泌细胞,胰岛B细胞的移植是治疗I型糖尿病重要策略之一。然而,移植胰岛中细胞基质网络和脉管系统会由于手术、机体免疫等因素被破坏,造成胰岛细胞功能丧失,仿生构建组织/细胞生长微环境在胰岛B细胞功能维持方面显示良好的应用潜力[5]。SF水凝胶及其多孔支架材料具有与细胞相匹配的弹性模量,在加工过程中,可通过调节丝素蛋白结构类型及氨基酸组成比例等方式形成可注射水凝胶或蛋白支架,该SF水凝胶或蛋白支架可在移植中形成免疫屏障为胰岛细胞提供良好的生长空间,SF蛋白材料形成的包裹层具有多孔结构,利于营养物质和气体运输(图3)[67]。此外,丝素蛋白中的RGD序列可以与胰岛细胞表面的整合素相互作用,以促进胰岛细胞的黏附、增殖,并调节改善胰岛微环境和内皮细胞活性促进移植胰岛的血管化,对维持胰岛素的分泌有积极影响。
目前,基于SF包裹胰岛细胞或间充质干细胞用于Ⅰ型糖尿病治疗已成为当前研究的热点,有研究证实丝素蛋白用于构建胰岛的ECM[68-69],可增加胰岛细胞的存活率及对胰岛素的敏感性[70]。研究证实:SF水凝胶包裹小鼠胰岛可为胰岛细胞提供天然微环境,在体外包封7 d后胰岛细胞仍然存活,并维持胰岛素分泌以应对葡萄糖刺激。与未包裹细胞相比,包封胰岛细胞中胰岛素、胰高血糖素等基因表达量增加,而细胞角蛋白19和波形蛋白表达下降,表明经SF凝胶包裹的胰岛细胞分化程度降低,可维持更长时间的生理活性和功能。此外,将胰岛细胞与MSCs共同包裹时,胰岛素分泌可协同提高3.2倍[71],可以改善移植胰岛的活性,且包裹胰岛与MSCs的小鼠可快速恢复到正常血糖,而仅包裹胰岛细胞的处理组小鼠在4 d恢复到正常血糖水平,仅包裹MSCs的治疗时间为15 d[72],表明丝素水凝胶包裹可显著调促进间充质干细胞与胰岛细胞的协同作用,并有利于胰岛素的分泌。此外,SF水凝胶材料中加入肝素、白介素4、地塞米松等[40,73]促进周围组织的血管形成,诱发M2巨噬细胞极化作用,可进一步延长移植胰岛细胞的生理活性,具有良好的临床应用潜力。
3.5 其他细胞调控
除骨组织、皮肤组织、神经组织和胰岛组织相关的细胞调控,丝素再生医学材料研究已延伸于多种组织细胞的调控中,如将丝素蛋白膜与人牙周膜成纤维细胞共同培养发现,丝素蛋白膜可显著提高细胞的黏附力和生存时间[74]。肝细胞经SF胶囊化构筑的活性再生医学复合材料,可显著提升肝细胞中葡萄糖、尿素和白蛋白代谢速率[75],为急性肝衰竭肝细胞移植提供具有功能活性的细胞群体。研究显示,将骨髓间充质干细胞(BMSCs)种植在再生丝素蛋白(RSF)支架上,形成SF支架-干细胞复合体(RSF-MSC),该复合体可使MSCs分化为肝细胞样细胞,并在小鼠急性肝脏损伤模型中稳定存活3个月,同时促进损伤部位的血管、胆小管样结构和肝细胞样细胞的形成,加速肝脏修复,由此显示在急性肝衰竭或慢性肝损伤的情况下,MSCs复合地RSF载细胞复合材料在组织再生中具有巨大的潜力[76]。此外,SF材料中富含羧基和氨基活性基团,易于对生物分子或配体的功能化修饰,可与抗生素、生长因子和其他生物活性分子交联,在多功能SF材料研制中具有重要应用潜力[77-79]。
4 结 语
丝素蛋白材料已被广泛用于组织修复与再生医学研究中,丝素蛋白具有良好的生物相容性,可发挥积极的细胞调控作用,显示出良好的修复医学应用前景,已成为备受关注的医学生物材料。当前,丝素蛋白生物材料研究已经取得重要的进展,并可以根据需求加工形成颗粒、薄膜、纤维(管状)、支架、水凝胶、海绵状等,在组织工程和再生医学相关细胞的功能调控中发挥重要作用,在未来骨组织、皮肤组织、神经组织、胰岛组织等再生医学中具有广泛的应用预期。
当前,构筑载细胞的活性医用材料已成为再生医学研究的焦点,而丝素蛋白的广泛研究及其对细胞调控作用,使其成为最具有应用前景的再生医学材料之一。丝素蛋白材料影响细胞行为的主要因素可概括为以下三方面:丝素蛋白的氨基酸组成,丝素蛋白的生物力学性能,丝素蛋白材料的拓扑结构如尺寸、孔径、表面特征等。尽管丝素蛋白材料的物理、化学制备和加工方面及相关理化学性质调控研究已积累了很多有价值的成果,然而阐明丝素蛋白及其降解或衍生材料与细胞的相互作用等生物学调控功能,仍然是当前丝素蛋白活性生物材料研究和实用化面临的挑战。为此需要对丝素蛋白材料在活体中的形态特征及其细胞功能调控机制开展更深入的研究,加快推动丝素蛋白生物材料实用化及相关医疗器械的开发研究。
参考文献:
[1]NIU Q Q, PENG Q F, LU L, et al. Single molecular layer of silk nanoribbon as potential basic building block of silk materials[J]. ACS Nano, 2018, 12(12): 11860-11870.
[2]INOUE S, TANAKA K, ARISAKA F, et al. Silk fibroin of bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6 ︰ 6 ︰ 1 molar ratio[J]. Journal of Biological Chemistry, 2000, 275(51): 40517-40528.
[3]ASAKURA T. Structure of silk Ⅰ (bombyx mori silk fibroin before spinning) -type Ⅱ beta-turn, not alpha-helix[J]. Molecules, 2021, 26(12): 3706-3725.
[4]ZHAO M H, QI Z Z, TAO X S, et al. Chemical, thermal, time, and enzymatic stability of silk materials with silk Ⅰ structure[J]. International Journal of Molecular Medicine, 2021, 22(8): 4136-4151.
[5]明津法, 黃晓卫, 宁新, 等. 丝素蛋白材料制备及应用进展[J]. 丝绸, 2021, 58(2): 20-26.
MING Jinfa, HUANG Xiaowei, NING Xin, et al. Preparation and application of silk fibroin materials[J]. Journal of Silk, 2021, 58(2): 20-26.
[6]ZHAO R B, CAO J P, YANG X Y, et al. Inorganic material based macrophage regulation for cancer therapy: Basic concepts and recent advances[J]. Biomaterials Science, 2021, 9(13): 4568-4590.
[7]SAHOO J K, CHOI J, HASTURK O, et al. Silk degumming time controls horseradish peroxidase-catalyzed hydrogel properties[J]. Biomaterials Science, 2020, 8(15): 4176-4185.
[8]EOM S J, LEE N H, KANG M C, et al. Silk peptide production from whole silkworm cocoon using ultrasound and enzymatic treatment and its suppression of solar ultraviolet-induced skin inflammation[J]. Ultrasonics Sonochemistry, 2020, 61: 104803-104810.
[9]TOMEH M A, HADIANAMREI R, ZHAN X. Silk fibroin as a functional biomaterial for drug and gene delivery[J]. Pharmaceutics, 2019, 11(10): 494-515.
[10]KIJANSKA M, MARMARAS A, HEGGLIN A, et al. In vivo characterization of the integration and vascularization of a silk-derived surgical scaffold[J]. Journal of Plastic, Reconstructive and Aesthetic Surgery, 2016, 69(8): 1141-1150.
[11]LI Y W, LIU Z M, TANG Y P, et al. Three-dimensional silk fibroin scaffolds enhance the bone formation and angiogenic differentiation of human amniotic mesenchymal stem cells: A biocompatibility analysis[J]. Biochimica et Biophysica Acta, 2020, 52(6): 590-602.
[12]CHOUHAN D, MANDAL B B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside[J]. Acta Biomaterialia, 2020, 103: 24-51.
[13]张媚, 王富平, 魏如男, 等. 丝素蛋白β-折叠含量影响细胞生长的研究[J]. 丝绸, 2019, 56(5): 14-19.
ZHANG Mei, WANG Fuping, WEI Ru’nan, et al. Study on effect of -sheet content of silk fibroin on cell growth[J]. Journal of Silk, 2019, 56(5): 14-19.
[14]CHOI M, CHOI D, HONG J. Multilayered controlled drug release silk fibroin nanofilm by manipulating secondary structure[J]. Biomacromolecules, 2018, 19(7): 3096-3103.
[15]GHOLIPOURMALEKABADI M, SAPRU S, SAMADKUCHAKSARAEI A, et al. Silk fibroin for skin injury repair: Where do things stand[J]. Advanced Drug Delivery Reviews, 2020, 153: 28-53.
[16]GUAN J, ZHU W, LIU B, et al. Comparing the microstructure and mechanical properties of bombyx mori and antheraea pernyi cocoon composites[J]. Acta Biomaterialia, 2017, 47: 60-70.
[17]胡豆豆, 楊明英, 朱良均. 丝素蛋白生物材料对细胞行为的影响[J]. 蚕桑通报, 2016, 47(1): 6-10.
HU Doudou, YANG Mingying, ZHU Liangjun. Influence of silk fibroin-based biomaterials on cell behaviors[J]. Bulletin of Sericulture, 2016, 47(1): 6-10.
[18]ASAKURA T, TANAKA C, YANG M, et al. Production and characterization of a silk-like hybrid protein, based on the polyalanine region of samia cynthia ricini silk fibroin and a cell adhesive region derived from fibronectin[J]. Biomaterials, 2004, 25(4): 617-624.
[19]PATRA C, TALUKAR S, NOVOYATLEVA T, et al. Silk protein fibroin from antheraea mylitta for cardiac tissue engineering[J]. Biomaterials, 2012, 33(9): 2673-2680.
[20]杨亚, 闫凤祎, 王卉, 等. 丝素蛋白/磷酸八钙复合材料生物界面的蛋白质吸附和细胞响应[J]. 纺织学报, 2021, 42(2): 41-46.
YANG Ya, YAN Fengyi, WANG Hui, et al. Protein adsorption and cell response on bio-interfaces of silk fibroin/octacalcium phosphate composites[J]. Journal of Textile Research, 2021, 42(2): 41-46.
[21]CHEN L J, HUANG T, QIAO Y N, et al. Perspective into the regulation of cell-generated forces toward stem cell migration and differentiation[J]. Journal of Cellular Biochemistry, 2019, 120(6): 8884-8890.
[22]YUAN L, SAKAMOTO N, SONG G B, et al. Low-level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways[J]. Stem Cells and Development, 2013, 22(17): 2384-2393.
[23]ENGLER A J, SEN S, SWEENEY H L, et al. Matrix elasticity directs stem cell lineage specification[J]. Cell, 2006, 126(4): 677-689.
[24]COX T R, ERLER J T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer[J]. Disease Models & Mechanisms, 2011, 4(2): 165-178.
[25]BUITRAGO J O, PATEL K D, El-FIQI A, et al. Silk fibroin/collagen protein hybrid cell-encapsulating hydrogels with tunable gelation and improved physical and biological properties[J]. Acta Biomaterialia, 2018, 69: 218-233.
[26]OH S H, AN D B, KIM T H, et al. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior[J]. Acta Biomaterialia, 2016, 35: 23-31.
[27]SUN W, INCITTI T, MIGLIARESI C, et al. Genipin-crosslinked gelatin-silk fibroin hydrogels for modulating the behaviour of pluripotent cells[J]. Journal of Tissue Engineering and Regenerative Medicine, 2016, 10(10): 876-887.
[28]BONDAR B, FUCHS S, MOTTA A, et al. Functionality of endothelial cells on silk fibroin nets: Comparative study of micro-and nanometric fibre size[J]. Biomaterial, 2008, 29(5): 561-572.
[29]BIDGOLI M R, ALEMZADEH I, TAMJIA E, et al. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: Effect of particle size on physico-mechanical properties and in vitro cellular behavior[J]. Materials Science and Engineering C, 2019, 103: 109688-109715.
[30]ZHANG Y F, FAN W, MA Z C, et al. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7[J]. Acta Biomaterialia, 2010, 6(8): 3021-3028.
[31]DIENER A, NEBE B, LUTHEN F, et al. Control of focal adhesion dynamics by material surface characteristics[J]. Biomaterials, 2005, 26(4): 383-392.
[32]CAI Y R, GUO J M, CHEN C, et al. Silk fibroin membrane used for guided bone tissue regeneration[J]. Materials Science and Engineering C, 2017, 70: 148-154.
[33]DU C L, JIN J, LI Y C, et al. Novel silk fibroin/hydroxyapatite composite films: Structure and properties[J]. Materials Science & Engineering C, 2009, 29(1): 62-68.
[34]JIN Y S, KUNDU B, CAI Y R, et al. Bio-inspired mineralization of hydroxyapatite in 3D silk fibroin hydrogel for bone tissue engineering[J]. Colloids and Surfaces B: Biointerfaces, 2015, 134: 339-345.
[35]BHARADWAZ A, JAYASURIYA A C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration[J]. Materials Science and Engineering C, 2020, 110: 110698-110716.
[36]BOSE S, ROY M, BANDYOPADHYAY A. Recent advances in bone tissue engineering scaffolds[J]. Trends Biotechnology, 2012, 30(10): 546-554.
[37]WANG C, HUANG W, ZHOU Y, et al. 3D printing of bone tissue engineering scaffolds[J]. Bioactive Materials, 2020, 5(1): 82-91.
[38]MURPHY C M, HAUGH M G, O’BRIEN F J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering[J]. Biomaterials, 2010, 31(3): 461-466.
[39]SUN W, INCITTI T, MIGLIARESI C, et al. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide[J]. Journal of Tissue Engineering and Regenerative Medicine, 2017, 11(5): 1532-1541.
[40]KUMAR M, GUPTA P, BHATTACHARJEE S, et al. Immunomodulatory injectable silk hydrogels maintaining functional islets and promoting anti-inflammatory M2 macrophage polarization[J]. Biomaterials, 2018, 187: 1-17.
[41]JUNG S R, SONG N J, YANG D K, et al. Silk proteins stimulate osteoblast differentiation by suppressing the Notch signaling pathway in mesenchymal stem cells[J]. Journal of Food and Nutrition Research, 2013, 33(2): 162-170.
[42]楊敏, 黄凌云, 吕泽平, 等. MAPK信号通路在力学刺激对MG-63成骨样细胞护骨素表达中的作用[J]. 中华骨质疏松和骨矿盐疾病杂志, 2019, 12(1): 58-64.
YANG Min, HUANG Lingyun, L Zeping, et al. Effects of MAPK signaling pathway on mechanical stimulation-induced osteoprotegrin expression of MG-63 osteoblast-like cell[J]. Chinese Journal of Osteoporosis and Bone Mineral Research, 2019, 12(1): 58-64.
[43]MIDHA S, MURAB S, GHOSH S. Osteogenic signaling on silk-based matrices[J]. Biomaterials, 2016, 97: 133-153.
[44]HORAN R L, BRAMONO D S, STANLEY J R, et al. Biological and biomechanical assessment of a long-term bioresorbable silk-derived surgical mesh in an abdominal body wall defect model[J]. Hernia, 2009, 13(2): 189-199.
[45]CHOUHAN D, DEY N, BHARDWAJ N, et al. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances[J]. Biomaterials, 2019, 216: 119267.
[46]BROWN J E, GULKA C P, GIORDANO J, et al. Injectable silk protein microparticle-based fillers: A novel material for potential use in glottic insufficiency[J]. Journal of Voice, 2019, 33(5): 773-780.
[47]TEUSCHL A H, ZIPPERLE J, HUBER G C, et al. Silk fibroin based carrier system for delivery of fibrinogen and thrombin as coagulant supplements[J]. Journal of Biomedical Materials Research, 2017, 105(3): 687-696.
[48]PARK Y R, SULTAN M T, PARK H J, et al. NF-κB signaling is key in the wound healing processes of silk fibroin[J]. Acta Biomaterialia, 2018, 67: 183-195.
[49]LAOMEEPHOL C, GUEDES M, FERREIRA H, et al. Phospholipid-induced silk fibroin hydrogels and their potential as cell carriers for tissue regeneration[J]. Journal of Tissue Engineering and Regenerative Medicine, 2020, 14(1): 160-172.
[50]HAN H Y, NING H Y, LIU S S, et al. Silk biomaterials with vascularization capacity[J]. Advanced Functional Materials, 2016, 26(3): 421-436.
[51]FAROKHI M, MOTTAGHITALAB F, REIS R L, et al. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges[J]. Journal of Controlled Release, 2020, 321: 324-347.
[52]RAMADASS S K, NAZIR L S, THANGAM R, et al. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: A multifunctional approach for the treatment of ischemic chronic wounds[J]. Colloids and Surfaces B, 2019, 175: 636-643.
[53]CHOUHAN D, LOHE T U, SAMUDRALA P K, et al. In situ forming injectable silk fibroin hydrogel promotes skin regeneration in full thickness burn wounds[J]. Advanced Healthcare Materials, 2018, 7(24): 1801092-1801109.
[54]THURBER A E, OMENETTO F G, KAPLAN D L. In vivo bioresponses to silk proteins[J]. Biomaterials, 2015, 71: 145-157.
[55]GUAN G, BAI L, ZUO B, et al. Promoted dermis healing from full-thickness skin defect by porous silk fibroin scaffolds (PSFSs)[J]. Bio-medical Materials and Engineering, 2010, 20(5): 295-308.
[56]CHOUHAN D, JANANI G, CHAKRABORTY B, et al. Functionalized PVA-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling[J]. Journal of Tissue Engineering and Regenerative Medicine, 2018, 12(3): 1559-1570.
[57]MOISENOVICH M M, PLOTNIKOV E Y, MOYSENOVICH A M, et al. Effect of silk fibroin on neuroregeneration after traumatic brain injury[J]. Neurochemical Research, 2019, 44(10): 2261-2272.
[58]WEI G J, WANG L P, DONG D M, et al. Promotion of cell growth and adhesion of a peptide hydrogel scaffold via mTOR/cadherin signaling[J]. Journal of Cellular Physiology, 2018, 233(2): 822-829.
[59]AN B, TANG-SCHOMER M, HUANG W, et al. Physical and biological regulation of neuron regenerative growth and network formation on recombinant dragline silks[J]. Biomaterials, 2015, 48: 137-146.
[60]LI X W, LIU X Y, JOSEY B, et al. Short laminin peptide for improved neural stem cell growth[J]. Stem Cells Translational Medicine, 2014, 3(5): 662-670.
[61]LI G F, CHEN K, DAN Y, et al. Laminin-coated electrospun regenerated silk fibroin mats promote neural progenitor cell proliferation, differentiation, and survival in vitro[J]. Frontiers in Bioengineering and Biotechnology, 2019, 7: 190-202.
[62]DING Z Z, HAN H Y, FAN Z H, et al. Nanoscale silk-hydroxyapatite hydrogels for injectable bone biomaterials[J]. ACS Applied Materials & Interfaces, 2017, 9(20): 16913-16921.
[63]SANG Y H, LI M R, LIU J J, et al. Biomimetic silk scaffolds with an amorphous structure for soft tissue engineering[J]. ACS Applied Materials & Interfaces, 2018, 10(11): 9290-9300.
[64]WANG L L, LU G Z, LU Q, et al. Controlling cell behavior on silk nanofiber hydrogels with tunable anisotropic structures[J]. ACS Biomaterials Science & Engineering, 2018, 4(3): 933-941.
[65]LU Q, HU X, WANG X Q, et al. Water-insoluble silk films with Silk Ⅰ structure[J]. Acta Biomaterialia, 2010, 6(4): 1380-1387.
[66]QU J, WANG D, WANG H H, et al. Electrospun silk fibroin nanofibers in different diameters support neurite outgrowth and promote astrocyte migration[J]. Journal of Biomedical Materials Research, 2013, 101(9): 2667-2678.
[67]KUMAR M, NANDI S K, KAPLAN D L, et al. Localized immunomodulatory silk macrocapsules for islet-like spheroid formation and sustained insulin production[J]. ACS Biomaterials Science & Engineering, 2017, 3(10): 2443-2456.
[68]ZHU Y, WANG D Z, YAO X H, et al. Biomimetic hybrid scaffold of electrospun silk fibroin and pancreatic decellularized extracellular matrix for islet survival[J]. Journal of Biomaterials Science, 2021, 32(2): 151-165.
[69]PARK S Y, KIM B, LEE Y K, et al. Silk fibroin promotes the regeneration of pancreatic beta-cells in the C57BL/KsJ-Lepr(db/db) mouse[J]. Molecules, 2020, 25(14): 3259-3266.
[70]CHEN S, MATSUMOTO H, MOROOKA Y, et al. Smart microneedle fabricated with silk fibroin combined semi-interpenetrating network hydrogel for glucose-responsive insulin delivery[J]. ACS Biomaterials Science & Engineering, 2019, 5(11): 5781-5789.
[71]DAVIS N E, BEENKEN-ROTHKOPF L N, MIRSOIAN A, et al. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel[J]. Biomaterials, 2012, 33(28): 6691-6697.
[72]HAMILTON D C, SHIH H H, SCHUBERT R A, et al. A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo[J]. Journal of Tissue Engineering and Regenerative Medicine, 2017, 11(3): 887-895.
[73]MAO D, ZHU M F, ZHANG X Y, et al. A macroporous heparin-releasing silk fibroin scaffold improves islet transplantation outcome by promoting islet revascularisation and survival[J]. Acta Biomaterialia, 2017, 59: 210-220.
[74]GEAO C, COSTA-PINTO A R, CUNHA-REIS C, et al. Thermal annealed silk fibroin membranes for periodontal guided tissue regeneration[J]. Journal of Materials Research, 2019, 30(2): 27-45.
[75]NAYAK S, DEY S, KUNDU S C. Silk sericin-alginate-chitosan microcapsules: Hepatocytes encapsulation for enhanced cellular functions[J]. International Journal of Biological Macromolecules, 2014, 65: 258-266.
[76]XU L J, WANG S F, SUI X, et al. Mesenchymal stem cell-seeded regenerated silk fibroin complex matrices for liver regeneration in an animal model of acute liver failure[J]. ACS Applied Materials & Interfaces, 2017, 9: 14716-14723.
[77]NGUYEN T P, NGUYEN Q V, NGUYEN V H, et al. Silk fibroin-based biomaterials for biomedical applications: A review[J]. Polymers (Basel), 2019, 11(12): 1933-1957.
[78]GRABSKA-ZIELINSKA S, SIONKOWAKA A. How to improve physico-chemical properties of silk fibroin materials for biomedical applications? Blending and cross-linking of silk fibroin: A review[J]. Materials, 2021, 14(6): 1510-1540.
[79]RIBEIRO V P, SILVACORREIA J, GONCALVES C, et al. Rapidly responsive silk fibroin hydrogels as an artificial matrix for the programmed tumor cells death[J]. PLos One, 2018, 13(4): 194441-194461.
Abstract: Silk fibroin is an important natural biopolymer material with excellent mechanical properties, biocompatibility, biodegradability, and easy chemical modification of functional groups. It has received extensive attention in biomedical materials and regenerative medicine research. In recent years, silk fibroin-based regenerative medicine biomaterials have shown good application potential in repair and regenerative medicine concerning tissues such as bone, skin, nerves and pancreatic islets. The interactions between these materials and cells are gradually elucidated, which further provides positive feedback to the silk biomedical materials design and preparation, significantly accelerating the clinical translation of silk fibroin-based biomedical materials.
Currently, silk fibroin materials have been manufactured into injectable solution, fiber material, film material, hydrogel material and scaffold material that can mimic the functions of extracellular matrix in the regulation of cell adhesion, proliferation and differentiation. Generally, the biomedical materials in tissue repair and regenerative medicine have several characterizations, such as biocompatibility, low host immune response and good permeability, which could support or enhance cellular life activities to promote tissue repair and regeneration. As an important natural material, silk fibroin has displayed excellent biocompatibility, biodegradability, low immunogenicity, mechanical properties and easy chemical modification of functional groups, presenting high potential in cell regulation for regenerative medicine. The adjustable mechanical strength is another important feature of silk fibroin, which could make its rigidity match thatof cells, revealing an important role in cell division and functional differentiation. In the recent decade, the regulation of silk fibroin material on cell function has been gradually clarified and has become the important cue for design and construction of silk fibroin biomaterials, which accelerates the clinical medicine application of silk fibroin, showing great potential in cell regulation during bone, skin, nerve, pancreatic islets tissue repair and regenerative medicine.
In terms of bone cells regulation, silk fibroin scaffold material can induce osteoblast proliferation, adhesion and differentiation, induce new bone angiogenesis and promote bone tissue regeneration. More importantly, silk fibroin biomaterials could be degraded and absorbed in situ during new bone generations. As for skin cells regulation, silk fibroin-based materials are widely used in skin regeneration treatment, and they have been produced into suture and ligation silk thread, silk fibroin mask, abdominal wall reconstruction surgical net, silk fibroin sponge in plastic surgery and vocal cord filler. Furthermore, silk fibroin could influence the NF-κB associated signaling, and the treatment of fibroblasts with silk fibroin could increase the expression of cyclin D1, vimentin, fibronectin, and vascular endothelial growth factor, which benefits skin regeneration. In the recent decade, silk fibroin materials have further been investigated widely and deeply for nerve tissues engineering. During the process of nerve cell regulation, silk fibroin-based biomedical materials could induce the expression of neural cell adhesion molecules, which could enhance cell adhesion and proliferation. Besides, the regulations for neural cells are always concerned with the silk orientation, which is demonstrated in neural progenitor cells, and the migration efficiency of neurons and astrocytes on different diameters of silk fiber scaffolds has further been verified.
Materials-based encapsulation is an important strategy for cell behavior regulation. Due to the regulatory function and biosafety of silk fibroin material, it is regarded as a feasible cell coating material and is used for islet cells encapsulation in type I diabetes therapy research. Currently, the silk fibroin wrapping pancreatic islet cells or mesenchymal stem cells have become a hot research topic, and it has been confirmed that silk fibroin can be used to construct the ECM-similar structure to support long-term survival and insulin-secretion function of islet cells or islet microtissue in vitro and in vivo, which presents great potential for the islet implant.
At present, silk fibroin is synthesized into many types of biomedical materials according to clinical research demand, which reflectsits great potentials in cell regulations in regenerative medicine. The preparation of cell-carrying active medical materials has become the focus of regenerative medicine research, and the extensive research on silk fibroin and its cell regulation make it one of the most promising regenerative medicine materials. Although much valuable experience has been accumulated in physical and chemical preparation and processing of silk protein materials and in related physiochemical property regulation studies, the elucidation of the biological regulation functions of silk protein and its degraded or derived materials such as the interaction with cells is still a challenge for the research and practicalization of silk protein active biomaterials. Therefore, it is necessary to carry out in-depth biomedical research on the morphological and functional changes over the cellular effect of silk fibroin materials in vivo, which may accelerate the clinical application of silk fibroin-based biomedical materials and development of related medical devices.
Key words: silk fibroin; bone cells; skin cells; nerve cells; pancreatic islet cells; regenerative medicine

