Optimizing the carbon coating to eliminate electrochemical interface polarization in a high performance silicon anode for use in a lithium-ion battery
QI Zhi-yan, DAI Li-qin, WANG Zhe-fan, XIE Li-jing, CHEN Jing-peng, CHENG Jia-yao,SONG Ge, LI Xiao-ming, SUN Guo-hua, CHEN Cheng-meng,3,
(1.CAS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2.University of Chinese Academy of Sciences, Beijing 100049, China;3.Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract: Ordered and disordered carbons have been commonly used as coating materials for silicon (Si) anodes, however the effect of carbons with different crystallinities and pore structures on their electrochemical performance remains controversial.We used pitch and phenolic resin (PR) as the precursors of ordered and disordered carbon, respectively, to prepare carbon-coated silicon(Si@C) with strictly controlled carbon contents and surface functional groups.Their electrochemical behavior was investigated.An ordered crystalline structure is favorable for electron transport, and mesopores and macropores are conducive to the diffusion of lithium ions.Such a coating with a small pore volume is an excellent buffer for the expansion of Si, and the electrode maintains structural integrity for 50 cycles.A disordered porous structure is less robust and produces a large polarization, which produces continuous volume expansion with cycling and leads to inferior electrochemical performance.As a result, the capacity and capacity retention after 100 cycles at 0.5 A g−1 of Si@C-Pitch are respectively 8 times and 1.9 times those of Si@C-PR.This study provides theoretical guidance for the selection of carbon materials used in Si@C anodes.
Key words: Silicon-carbon composites;Crystalline structure;Pore structure;Failure mechanism;Polarization
1 Introduction
Silicon (Si) has attracted human’s wide interests as the next-generation anode materials of lithium-ion batteries (LIBs) owing to its theoretical specific capacity up to 3 579 mAh g−1, low working voltage(~0.3 V), and abundant natural reserves[1-3].But the tremendous volume expansion (>300%) during lithiation and low intrinsic electric conductivity of Si lead to inferior electrochemical stability and low utilization of active materials, which limit the Si anode in practical application[4,5].Combining with the metal oxide[6,7], conductive polymer[8]and carbon[9,10]etc., the Si based composite anode could achieve an ideal electrochemical performance.Among these, the siliconcarbon (Si@C) composite is a rational and effective strategy taking the high electrical conductivity and robustness of carbon materials into consideration.
Generally, previous works have devoted to optimize the structural design, for instance, yolk-shell,dual-shell, pomegranate-like structureetc.to build conductive network and create extra space for accommodating the volume change of Si.Yet the intrinsic properties of carbon, especially the crystalline structure and pore structure, also act an essential role in the performance of Si@C composites, such as conductivity, ion transportation, structural robustness andetc.Although some studies have played attention to the structure of carbon, the relationship among crystalline/pore structure of carbon and electrochemical performance is still not clear.Luo et al.[11]prepared the Si-disordered carbon composites using phenolic resin(PR) as carbon precursor, while Choi et al.[12]fabricated Si-ordered carbon composites using pitch as carbon precursor.Interestingly, both of these achieved high capacity, long cycle life, and improved rate performance.To explore the different effects of dis-ordered carbon and ordered carbon layer on Si-based anode, Fang et al.[13]prepared the Si particles coated with ordered and disordered carbon.They found that the Si coated with disordered carbon presented high initial discharge capacity owing to its high conductivity, while Si coated with ordered carbon presented enhanced cycling stability due to its good volume variation tolerance.Nevertheless, He et al.[14]presented opposite views.They prepared Si@C composites with carbon of different order degree and pore structure,and found that the capacity of Si@C composites increased as the crystallinity of carbon coating.Moreover, they believed that the pore preferred ion diffusion while decreased robustness.
Evidently, the effects of ordered and disordered carbon coating on electrochemical performance of Si@C composites are still controversial.More importantly, it is of significance to understand the structural changes and lithium storage mechanism of Si particles coated with ordered and disordered carbon during charging/discharging, and the corresponding failure process needs to be fundamentally explored.However, few reports have focused on this study.In fact, the carbon content and surface functional groups are also the influencing factors on the electrochemical performance of Si@C anodes[15,16].To objectively estimate the effect of crystalline structure and pore structure of carbon coating on Si@C anodes, the carbon content and surface functional group should be controlled in accordance with each other.Unfortunately, the previous research neglects the control of these parameters and this is may be the main reasons for the inconsistency of relevant research conclusions.
Herein, the pitch and phenolic resin (PR) are chosen as the precursor of ordered carbon and disordered carbon, due to their universality, to prepare the Si@C-Pitch and Si@C-PR with same carbon content and surface chemical configuration.We systematically investigate the relationship between the crystalline/pore structure of carbon coating and electrochemical properties of Si@C composites.Moreover, the structural evolution and failure cause of Si@C-Pitch and Si@C-PR during charging/discharging are further discussed by characterizing the fresh and cycled electrode.Ultimately, we propose a possible mechanism of ordered/disordered carbon coating in inhibiting volume expansion of Si@C composites, which will provide theoretical guidance for the selection of carbon materials in Si@C anode for LIBs.
2 Experimental
2.1 Materials synthesis
The sample of Si@C-Pitch were obtained through three simple steps, solution immersion, drying and carbonization.The 0.1 g Si nanoparticles(Aladdin) were firstly mixed with 10 mL tetrahydrofuran (THF, Macklin) and treated with ultrasound for 20 min in order to make Si sufficiently disperse in THF.0.9 g pitch (Yongdong Chemical Industry Co.LTD) was dissolved in 60 mL THF and stirred for 30 min.Then, the former solution was added to the latter and continued stirring for 24 h to obtain the Si@Pitch.Next, the precursor solution was placed on a heating plate and heated at 80 °C until the solution evaporated into a gelatinous substance.Finally, the target product was obtained after the precursor was carbonized in tubular furnace at 900 °C for 3 h under nitrogen atmosphere with the heating rate of 2 °C min−1,which was named as Si@C-Pitch.Si@C-PR was prepared by the same method, but the difference was that the mass ratio of Si to PR (Huai‘an Hongtai Bakelite Co., Ltd.) was 1∶7.
2.2 Materials characterization
The scanning electron microscopy (SEM, JSM-7001F, Japan) and high-resolution transmission electron microscopy (HR-TEM, JEM-2100F, Japan) were adopted to observe the morphologies.Thermogravimetric (TG, STA 449F3) was carried out to examine the carbon content at the heating rate of 10 °C min−1under flowing air from ambient temperature to 900 °C.The Fourier-transform infrared spectroscopy(FT-IR, Bruker Tensor 27, Germany) and X-ray photoelectron spectroscopy (XPS, Thermo Fischer, ESCALAB 250Xi, American) were employed to research the surficial chemical configuration.The crys-talline degree of the pure Si and Si@C composites samples was measured with X-ray diffraction analyzer (XRD, Bruker, Germany), Raman System (Renishaw plc, Wotton-Underedge, UK) and the Lambda 35 UV-vis spectrophotometer (Perkin-Elmer) in the wavelength range of 250-325 nm.The specific surface area and pore properties were analyzed on a TriStar II 3020 analyzer.
2.3 Electrochemical measurements
The assembled cells consisted the reference electrode (Li foil), separator (Celgard 2400 membrane)and working electrode.The working electrode included 75% active materials (Si or Si@C composites),10% carbon black (Super P), 10% styrene-butadiene rubber (SBR) and 5% carboxymethylcellulose sodium (CMC) with deionized water as solvent.The electrolyte was composed of ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC)(3∶4∶3 mass ratio with 1 mol L−1LiPF6) and 5%fluoroethylene carbonate (FEC) as additive.Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were implemented by Land CT2001A battery tester.
3 Results and discussion
3.1 Carbon content and surface functional groups in the composites
Fig.1a describes the preparation process from the pure Si particles to composites.The size of pure nano Si particles is 80-150 nm (Fig.1b).After Si particles are composited with pitch-derived carbon and PR-derived carbon, both of them present as micron-scale chunks (1-2 μm, Fig.1b and c).The carbon and Si content in these samples is investigated by TG(Fig.1d).The weight losses of Si@C-Pitch andSi@C-PR are 75% and 77%, respectively.It indicates that the carbon content surrounded on the Si particles surface is similar, which is consist with the requirements of model carbon materials.Meanwhile, Si is surrounded by the carbon layer, and almost no exposed Si particles are observed from the TEM images.The carbon layer thickness of Si@C-PR and Si@CPitch is 90-180 nm and 60-170 nm, respectively(Fig.1e, f).The same carbon content and the similar carbon coating thickness, ensure that the structural design of carbon material is consistent and provides a basis for the characterization of the carbon layer structure.
The functional groups of Si@C-Pitch and Si@CPR are studied with FT-IR and XPS.In Fig.2a,Si@C-Pitch and Si@C-PR display very consistent FT-IR spectra.The characteristic peaks at ~1 095 and 1 388 cm−1in both spectra are assigned to the stretch vibration and in-plane bending vibration of C―O/C―O―C, while the presented characteristic peaks centered at 1 635 and 3 440 cm−1belong to stretch vibration of C=O and stretching vibration of the O―H bond (Fig.2a)[17,18].It qualitatively reveals that the type of functional group of Si@C-Pitch and Si@C-PR is highly consist with each other.The surface configuration and content of Si@C composites is further confirmed by XPS spectra.Three characteristic peaks are observed in Fig.2b, which represent the existence of O, C and Si.In addition, the intensity of the peaks indicates that the elements in these complexes are almost identical (Fig.2b).The deconvoluted C 1s spectrum (Fig.2c) contains three peaks at 284.7, 285.6 and 286.8 eV, which belong to C=C(284.7 eV), C―O (285.6 eV), and C=O (286.8 eV)band, respectively.And for the O 1s spectrum(Fig.2d), the peaks centered at 532.4, 533.5, and 534.5 eV correspond to C=O, C―O and O―H band.The relative content of function groups is shown in Table 1 and 2.Evidently, the configuration and content of functional group on the surface of the two Si@C composites have little difference.Therefore,this implies that the influence of functional groups onperformance has been eliminated, so as to ensure the accuracy of study for carbon layer structure.
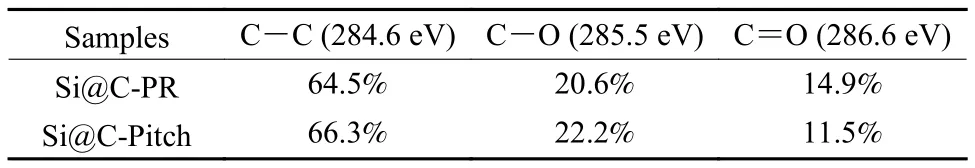
Table 1 The relative content of function groups according to C 1s spectrogram.
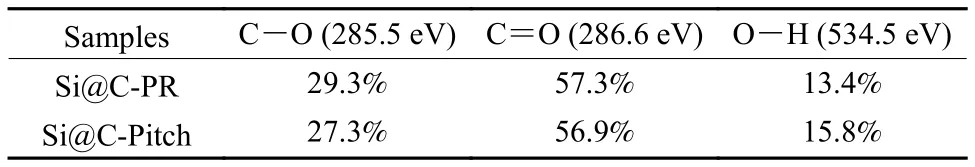
Table 2 The relative content of function groups according to O 1s spectrogram.
3.2 Characteristic of microcrystalline and pore structure
To describe the discrepancy of the carbon layer coated on Si, the micromorphology is observed by HR-TEM (Fig.3a, b).The pitch-derived carbon layer exhibits obvious turbostratic short-range ordered structure.The size of graphene layers and the number of parallel stacked layers is relatively large.While thePR-derived carbon presents disordered structure and the size of curved graphene layers is small.Meanwhile, the selected area electron diffraction of carbon coating presents an almost invisible diffused ring in the Si@C-PR, indicating an amorphous structure[19].In addition, some amorphous oxidate (SiOx) between the carbon phase and Si phase in the Si@C-Pitch and Si@C-PR is observed, which usually exists on the surface of pure Si (Fig.S1).
Furthermore, the XRD and Raman are utilized to investigate structure characteristics of Si@C composites, and Table 3 presents the calculated parameters.XRD patterns of Si@C-Pitch and Si@C-PR both present typical characteristic peaks of Si at 28.4°,47.3°, 56.1°, 69.1°, 76.4° and 88°[20](Fig.3c).Moreover, two broad weak peaks at 22°-25° and 43°are ascribed to the (002) and (100) planes of carbon phases[21].Notably, the 2θvalue of Si@C-Pitch is larger than that of Si@C-PR (Fig.S2).The calculated interlayer spacing (d(002)) of Si@C-Pitch (0.352 5 nm) is lower than Si@C-PR (0.401 0 nm).In addition, the larger thickness of the graphene layers (Lc) and corresponding larger number of parallel stacked layers (N)prove the higher graphitization degree of Si@CPitch[22,23].
Raman spectra (Fig.3d) show two characteristic peaks located at 1 352 cm−1and 1 598 cm−1, which are assigned to theD-band (disordered carbon vibration peak) and theG-band (lattice vibration of graphite characteristic carbon), respectively[24].The sample of Si@C-Pitch exhibits relatively smallerID/IG(1.09)than Si@C-PR (1.15) (Fig.3d and Table 1), and the Si@C-Pitch sample has higherLavalue (in-plane graphene domine size)[25], indicating that Si@C-Pitch has fewer defects into the graphite layers[26].Furthermore, the UV-vis spectra are also employed to detect the difference of carbon layer structure (Fig.3e).The wide peak at 260-290 nm corresponds to the electrontransfer from the bonding orbital (π) to the antibonding orbital (π*) on the hexagonal structure[27,28].The peak of Si@C-PR shifts to the left compared with Si@C-Pitch, indicating that the π conjugate region of PR-derived carbon is smaller than that of Pitch-derived carbon.Based on above structure properties, the carbon layer of Si@C-Pitch demonstrates higher graphitization degree and better crystallization, and thus possess larger in-plane graphene domine size and higher number of parallel stacked layers, which is conducive to the electrical conductivity[29].The result of powder electric conductivity in Fig.3f confirms the higher conductivity of Si@C-Pitch, which maybe reduce the electrochemical impedance and improve rate performance.Besides, the two carbon layers with different crystal structure may have a great impact on the inhibition of volume expansion during cycling, and we’ll discuss it later.
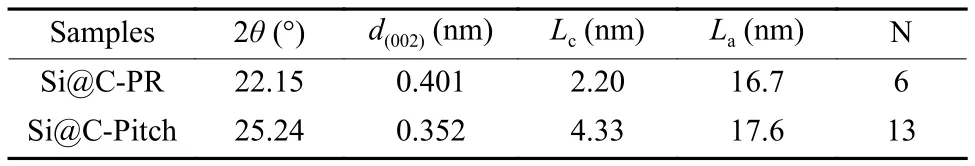
Table 3 The crystalline structure parameters of Si@C-PR and Si@C-Pitch.
As shown in Fig.4a and b, the specific surfaceareas of Si@C-PR and Si@C-Pitch are 363.8 m2g−1and 4.1 m2g−1, respectively.According to the pore size distribution model (Fig.4a inset, b inset), the Si@C-PR sample is identified as micropores mainly with total pore volume of 0.189 cm³ g−1(Table S1).While the Si@C-Pitch sample is mainly constructed by mesopores and macropores with only a small amount of micropores, and its total pore volume is only 0.006 cm³ g−1(Table S1).Mesoporous and macropores can provide rich transmission pathways for Li+and store electrolytes efficiently, especially ensuring the enough supply for high current density[30].Micropores can store Li+and increase lithium storage capacity[31].However, too much micropores will block the transportation of Li+, and the high specific surface area will lead to serious side effects and thick SEI,which may be detrimental to the electrochemical performance[32,33].The microstructure difference of carbon layer is determined by the carbon precursor.The pitch with large polycyclic aromatic hydrocarbons is easy to be graphitized during carbonization process,finally obtaining ordered carbon structure[34].While the PR with cross-linked structure is difficult to be graphitized and easily forms vacancies during the carbonization process, which result in disordered crystalline structure and large specific surface area[35].
3.3 Electrochemical measurement
To ordered/disordered carbon coating would have an impact on the electrochemical performance of Si@C composites.Fig.5a compares the first discharge/charge profiles at current density of 0.1 A g−1.The discharge capacity of pure Si is 3 740 mAh g−1with an initial Coulombic efficiency (ICE) of 74.5%.The Si@C-Pitch and Si@C-PR samples exhibit nearly the same initial discharge specific capacity, with the value of 1 082 and 1 035 mAh g−1, which is the result of the same Si/carbon mass ratio in both samples.However, the ICE values of the two composites are very different, which are 81.9% for Si@C-Pitch and 68.4% for Si@C-PR.The poor ICE of Si@C-PR is attributed to its high specific surface area, which causes serious side reactions and irreversible consumption of Li+to form SEI.Fig.5b, that is the enlarged area marked with green line in Fig.5a, explores the lithium storage behavior of silicon and carbon in the composites.The charge/discharge profiles present the slope region corresponding to the SEI formation and adsorption/desorption of the Li+in carbon material,and plateau region corresponding to the alloying reaction between Li+and Si[36].The capacity of PR-based carbon material is 43 mAh g−1higher than that of Pitch-based carbon materials, which is only about 3.9% of the total capacity of the Si@C composite.The difference is so slight that it can be neglected compared with the total capacity.Thus, the pore structure has little effect on first discharge capacity, but the influence of pore structure on the structural changes of Si@C during cycling needs to be further verified.
Cyclic voltammograms (CV) is employed to understand the lithium storage mechanism of the as synthesized composites.In Fig.5c, d, the broad cathodic peak around 0.7 V is assigned to the formation of SEI,which is observed in the first cycling curve and disappears in the second cycles[10].The much obvious peak of Si@C-PR than Si@C-Pitch indicates the lager irreversible capacity of Si@C-PR, which is due to its rich micropores and high specific surface area.The anodic peaks at ~ 0.35 and ~ 0.52 V represent the de-lithiation of Li+from LixSi alloy[36,37].The peak current in the second cycles is higher than that in the first cycle for both composites, which is because that more Si is activated during the scanning process.Additionally,the peak current of Si@C-Pitch electrode is significantly larger than that of the Si@C-PR electrode,which can be attributed to the higher electrical conductivity of the Si@C-Pitch electrode[38].
The rate performance is evaluated under the current densities from 0.1 to 5 A g−1.The Si@C composites electrodes both deliver improved rate performance compared with the pure Si electrodes (Fig.5e).It‘s worth noting that the rate performance of Si@CPitch is superior to Si@C-PR at any current density.Especially the Si@C-Pitch presents about 8 times specific capacity (315.6 mAh g−1) than that of Si@C-PR(31.3 mAh g−1) at 5 A g−1.The rate performance is mainly related to the electrical conductivity and thediffusion rate of Li+in the electrode[39].The EIS spectra after first cycle are conducted to further identify the influence of electron conductivity on rate performance (Fig.5f).All the plots contain two semicircles and a slope line, of which the middle frequency semicircles represents charge transfer resistance (Rct)[40].The smallerRctof Si@C-Pitch than that of Si@C-PR(Table S2), indicates that Si@C-Pitch has lowerRct,which is related to its ordered crystalline structure.The larger graphene layers thickness and parallel stacked layers of Si@C-Pitch benefit to electron conductivity, thus leading to lower charge transfer resistance.Furthermore, the diffusion coefficient of lithium ion () is obtained based on Randles-Sevcik equation[41]:
WhereIpis the peak current,nis the transfer number of charge,Ais the area of active electrode,Dis the diffusion coefficient of lithium ions,Cis the molar concentration of Li+in the anode,ωis the scan rate.As shown in Fig.S5,is 1.4 × 10−11cm2S−1for Si@C-PR and 2.7 × 10−11cm2S−1for Si@C-Pitch.Compared with PR-derived carbon, pitch-derived carbon shows rich mesopores and macropores, which lead to rapid electron and ion transportation.
Fig.6a illustrates the cycling stability at 0.5 A g−1.The specific capacity of pure Si decays rapidly with cycling (Fig.6a).In contrast, the Si@C composites display significantly improved cycling stability.The Si@C-Pitch and Si@C-PR samples retain a capacity of 629 and 330 mAh g−1after 100 cycles, corresponding to capacity retention of 74.8% and 59.9%, respectively.Obviously, the Si@C-Pitch sample exhibits superior cycling stability than Si@C-PR, due to thestructural integrity of pitch-derived carbon layer.To survey the structure evolution of Si@C composites during cycling, EIS, cross section SEM and charge/discharge curves on high-rate are investigated.Fig.6b, c and Table S2-S4 present the impedance after 1st, 10th, 50thcycles.The same impedance changing law of the two Si@C composites is observed.TheRctdecreases after the first cycle and then increases with cycling.The initial reduction ofRctis mainly due to the volume expansion of Si.This expansion renders an accumulation of stress inside the electrode, and consequently causes the formation of well-developed pores in the electrode and better contact between active materials and current collector[42].Evidently, the impedance of Si@C-Pitch increases slightly with the cycling from 10thto 50th, while the impedance of Si@C-PR increases dramatically during the whole cycle process.The possible reason is that Si@C-PR with disordered and porous carbon structure delivers huge volume expansion after multiple charging/discharging cycles, which leads to the active material lose electrical contact with the current collector, and thus the electron transportation is hindered.
To illustrate the reason that the capacity decreases with the cycling, the charge-discharge curves(Fig.6d) and corresponding dQ/dvplots (Fig.6e) are analyzed.Taking charging process as an example,Si@C-Pitch presents a long quasi plateau region (0.5-0.9 V), which represents the lithiation of Si.Accordingly, there is a prominent anodic peak around ~0.7 V in dQ/dvplots of Si@C-Pitch.The discharge quasi plateau region (0-0.15 V) is consisted with the cathodic peak around ~0.1 V in the dQ/dvplots of Si@CPitch, corresponding to delithiation process.The pure pitch-derived carbon only shows a capacity of 4.7 mAh g−1(Fig.S7) at 5 A g−1, which confirms that Si plays the main role for the high capacity of Si@CPitch.The increased delithiation voltage is owing to the increase of polarization, which is caused by the loose contact between active material[43].In contrast,Si@C-PR shows a sharp slope region both for charge and discharge profiles and shows negligible plateau region.Besides, there isn’t cathodic/anodic peak in the dQ/dvplots of Si@C-PR (Fig.6e), implying the nearly no lithiation/delithiation reaction with Si.That is, the severe detachment between active material and current collector causes significant polarization in the sample of Si@C-PR, which seriously hinders the alloy reaction of Li+and result in significant capacity fading.As discussed above, the significant capacity difference at 5 A g−1is determined by the microstructure of carbon layer, the high electron conductivity and rapid Li+diffusion coefficients ensure the excellent rate performance at high current density up to 5 A g−1.
3.4 The analysis of failure mechanism
We further observe the change of electrode thickness with cycling to confirm the above views.The thickness of the pure Si electrode increased by 1 641% after 50 cycles (Fig.S6).After Si is composited with carbon, the thickness expansion is significantly reduced, indicating that both the pitch-derived carbon and PR-derived carbon perform some effects in inhibiting the expansion of Si.The thickness of Si@C-Pitch electrode is increased by only 88% from fresh electrode to 50thcycle (Fig.7a1, 7a50), and it tends to be stable from the 10thcycles (49 μm) to the 50thcycles (51 μm) (Fig.7a10, 7a50).Even after 50 cycles, the Si@C-Pitch electrode still remains intact and dense.Whereas the thickness of Si@C-PF electrode is increased by 141% and cracks can be seen clearly (Fig.7b1, 7b50).The variation of the electrodes thickness is consistent with the variation of the EIS impedance.The less volume expansion of Si@CPitch leads to smaller impedance changes, while the severe volume expansion of Si@C-PR causes rapid increase of impedance.The schematic diagram (Fig.7c)describes the structural evolution of Si@C composites with ordered and disordered carbon layer with cycling and its mechanisms in buffering volume expansion.The excellent inhibition of pitch-derived carbon on the volume expansion is attributed to its turbostratic short-range ordered structure.The large in-plane graphene domine size and high number of parallel stacked layers guarantee the good flexibilityof carbon layer.In the process of volume expansion and contraction, the carbon layer can maintain its layered stacking state by compression and sliding.However, for Si@C-PR, its disordered and porous structure easily makes the carbon layer crush during the volume expansion/contraction process, resulting in weak inhibition of volume expansion and maybe spalling of active materials from the current collector.Besides, large amounts of pores will lead to the formation of thick SEI film, which will keep breaking and growing during the process of volume expansion/contraction.And finally, it results in continuous consumption of electrolyte and increase of irreversible capacity, thus leading to the poor cycle performance.
It can be seen that carbon precursors have an important effect on the structure of Si@C materials, especially on crystalline structure and pore structure,and ultimately have great impact on electrochemical performance.Thus, it is important to select appropriate precursor to composite with Si for achieving excellent performance.Besides, for some precursors,such as easy formation of porous structureetc., it’s better to regulate and control the crystalline structure or pore structure by deeply optimizing the process technology or surface modification.
4 Conclusion
In a word, we deliberately choose the pitch and PR as the carbon precursors of ordered carbon and disordered carbon and prepare the Si@C-Pitch and Si@C-PR composites with the same carbon content and surface chemical configuration.The pitch-based carbon exhibits short-range ordered crystalline structure and low specific surface area mainly composed of mesopores as well as macropores.While PR-derived carbon shows a disordered structure and its surface is mainly composed of micropores.The ordered crystal-line structure is favorable for electron transportation,furthermore, mesopores and macropores are conducive to ion transport.Besides, the carbon layer with ordered structure and small pore volume provides great buffer to the expansion of Si and guarantees the rapid transportation of electrons and Li+between the active material and the current collector after multiple cycles, which eventually contributes to a better cycle and rate performance.However, for Si@C-PR, its disordered and porous structure makes the carbon layer have poor structural robustness and low utilization of active Si, which leads to weak inhibition of volume expansion and poor electrochemical performance.The capacity of Si@C-Pitch is 8 times that of Si@C-PR at a high rate of 5 A g−1, and the capacity retention of Si@C-Pitch is 1.9 times that of Si@C-PR after 100 cycles at 0.5 A g−1.This study provides theoretical guidance for the selection of carbon materials in Si@C anode for LIBs.
Acknowledgements
All authors appreciate the financial support of Research and Development Project of Key Core and Common Technology of Shanxi Province(2020XXX014), National Key Research and Development (R&D) Program of China (2020YFB1505800).

