Study on gene regulation mechanism of Qiliqiangxin capsule on myocardial fibrosis in myocardial infarction rats
JI Xiao-di, WU Ai-ming, LV Meng, YANG Ding, LOU Li-xia, NIE Bo, ZHAO Jiu-li, ZHAO Ming-jing
1.Dongzhimen Hospital, Beijing University of Chinese Medicine; Ministry of Education and Beijing Key Laboratory of Traditional Chinese Medicine Internal Medicine, Beijing 100700, China
Keywords:
ABSTRACT
1. Introduction
Myocardial infarction (MI) is one of the leading causes of hospitalization and death worldwide. There is a high incidence rate in all age groups, which seriously affects human life and health[1,2]. Heart failure is the end stage of myocardial infarction, and myocardial fibrosis plays an important role in the progression of heart failure after myocardial infarction [3]. After MI, the heart responds to stress and undergoes structural and functional remodeling, mainly manifested by cardiomyocyte hypertrophy and extracellular collagen deposition. Initially, cardiac remodeling is a compensatory adaptive process, however, as the disease progresses,the heart occurs significant structural changes and myocardial fibrosis is the key pathological link [4,5]. There is increasing evidence that micoRNA is closely related to the fibrotic process after MI and is potential therapeutic target [6-8]. miR-133a is involved in the occurrence and development of MI [9,10]. A number of bioinformatics softwares show that TGF-β1 is a target gene of miR-133a [11]. TGF-β1 is considered a major pro-fibrotic factor,which activates smad2, smad3 by phosphorylation for fibrotic signaling[12-14]. Research clues suggest that the miR-133a/TGF-β1/smads pathway may be a potential intervention pathway to inhibit the progression of myocardial fibrosis after MI[15,16].
Qiliqiangxin capsule, consisting of Radix Astragali, Ginseng,Radix Aconiti Lateralis Preparata, Salviae Miltiorrhizae Radix, Alismatis Rhizoma,Semen Lepidii, Rhizoma Polygonati Odorati, Ramulus Cinnamomi, Carthami Flos, Pericarpium,Citri TangerineCortex Periplocae,which can replenish qi, warm yang;activates blood, dredge the meridian and induce diuresis to alleviate edema, is used for the prevention and treatment of heart failure after myocardial infarction. Captopril, an angiotensin converting enzyme inhibitor(ACEI), is a classic drug in clinical treatment of heart failure which has been reported in several studies to improve myocardial fibrosis in rats with heart failure [17-19]. Therefore, our study used captopril as a positive control drug, aiming at explore the molecular mechanism of Qiliqiangxin capsule on improving myocardial fibrosis in MI rats from the perspective of miR-133a/TGF-β1/smads pathway, and provide more basic research evidence for its clinical application.
2. Materials and Methods
2.1 Materials
2.1.1 Animals
Specific-pathogen-free male Sprague Dawley rats were used at 6 weeks old and weighing 200±20 g. Rats were obtained from the Beijing Weitong Lihua Laboratory Animal TechnologyCo., Ltd.(approval number 2012-0001).The animal experiment was approved by the Laboratory Animal Welfare and Ethics Committee of Dongzhimen Hospital,Beijing University of Chinese Medicine.
2.1.2 Medicines and reagents
Qiliqiangxin capsule, 0.3 g per capsule, (SFDA approval number Z20040141, Shijiazhuang Yiling PharmaceuticalCo.,Ltd). Captopril,12.5 mg per table (product batch number AAN9869, Sino-American Shanghai Squibb Pharmaceuticals Ltd). Trizol regent (product batch number 15596026, Thermo Fisher Scientific); miRNA extraction kit (product batch number 217004,QIAGEN Company); miRNA reverse transcription kit (product batch number 4366596,Applied Biosystems); Real-time PCR amplification kit (product batch number 4472897, Applied Biosystems); Masson Trichrome Staining Kit (product batch number D026, Nanjing Jiancheng Technology Co., LTD); The primers sequences of TGF-β1、Smad2、Smad3、col-Ⅰ、col-Ⅲ were synthesized by SinoGenoMax Co.,Ltd.
2.1.3 Instruments
Electrocardiograph((Japan Futian,model number:FX-7202);small animal ventilator(Alcott, model number: ALC-V8S); Gene amplifier(Applied Biosystems, model number: GeneAmp PCR system 9700); Real-Time PCR instrument(agilent, model number:Mx3000P); Ultraviolet spectrophotometer(Pharmacia Biotech,model number: GeneQuant); Paraffin slicer(Leica, model number:RM2235); Optical microscope(LEICA, model number: DM300).
2.2 Methods
2.2.1 Animal model preparation
Rat MI model was established by referring to paper [20]. The specific operation method is as follows: Rats were anesthetized with 1% pentobarbital sodium intraperitoneally. A 12-lead electrocardiogram was performed before operatiton. The rats were then intubated and cut the skin between the third and fourth costals at the left margin of the sternum. After this blunt forceps were used to separate the muscle layer, the rib layer, connected to a ventilator, and the chest was opened to fully expose the surgical field. The anterior descending branch of the left coronary artery was ligated 2-3 mm below the edge of the left atrial appendage of the heart. During the operation, myocardial ischemia turned white at the ligation site in the precardiac regionand then the chest was immediately closed and the ribs, muscles and skin were sutured layer by layer. As a parallel control, the sham group only had threading but no ligature. Penicillin was injected intraperitoneally for 3 days to prevent infection.
2.2.2 Grouping and administration
According to electrocardiogram 24 h after operation, rats with 6-8 pathological Q waves were selected to be included in the group. Rats according to the number of pathological Q wave were randomly divided into model group, Qiliqiangxin capsule group and captopril group. The sham group serves as the normal control group. Intragastric treatment was started on the second day after operation. The dose of Qiliqiangxin capsule group and captopril group were established by the equivalent conversion according to paper [21], Qiliqiangxin capsule group dose is 0.32 g/(Kg/d),captopril group dose is 2.25 mg/(Kg/d),equivalent to a clinical adult dose.The medicine was thoroughly mixed with deionized water and administered intragastric for 4 weeks. The sham group and model group were given 10 mL/ Kg/d deionized water by intragastric administration. After treatment, the sham group, model group,captopril group remaining 10 animals, qiliqiangxin capsule group remaining 8 animals.
2.2.3 HE Staining
The myocardial tissue was fixed by 4% paraformaldehyde, and then lumped, embedded in paraffin, sliced, roasted, dewaxed, and hydrated with gradient alcohol, followed by HE staining. Dye with hematoxylin for 8-15 min, rinse excess dye with running water. 1%hydrochloric acid alcohol for 30 s , wash thoroughly with tap water for 10 min. Staining with 1% eosin solution for 10min , rinse with running water for 1min. Then gradient alcohol dehydration, xylene transparent. Finally, seal the tablet with neutral gum, dried and then observed under an optical microscope.
2.2.4 Masson Staining
The myocardial tissue was fixed with 4% paraformaldehyde,lumped, embedded in paraffin, sliced, baked, dewaxed, hydrated with gradient alcohol, and then rinsed twice with warm water. Follow the instructions of the kit: the cell nucleus was stained for 60 s and washed with rinse solution for 30 s; cytoplasm was stained for 60 s and washed with rinse solution for 30 s; separation was performed for 5-8 min and then the separation solution was discarded; aniline blue was stained for 5-8 min and washed with anhydrous ethanol.Finally, seal the tablet with neutral gum, dried and then observed under an optical microscope. Collagen content was analyzed using Image-Pro Plus and then calculate collagen volume fraction (CVF) .CVF= collagen area/(myocardial area + collagen area) ×100%.
2.2.5 Real-time PCR
Trizol method was used to extract total RNA from marginal area of heart infarction. The absorbance of OD260/OD280 was determined by Ultraviolet spectrophotometer.And then calculate the sample concentration. Reverse transcription is performed strictly in accordance with the kit instructions. We detect gene expression through Real-time PCR. The amplification conditions of miR-133a were as follows: Pre-denaturation 94℃*10 min, denaturation 94℃*15 s, annealing 60℃*60 s, extension 72℃*10 s, a total of 45 cycles. The amplification conditions of TGF-β1、Smad2、Smad3、col-Ⅰ、col-Ⅲ were as follows: Pre-denaturation 95℃*10 min,denaturation 95℃*30 s, annealing 55℃*30 s, extension 72℃*20 s, a total of 40 cycles. Using U6 and GAPDH as internal reference,the expression levels of miR-133a,TGF-β1、Smad2、Smad3、col-Ⅰ、col-Ⅲ mRNA in each group were calculated by 2-△△CT.Primer sequences are shown in Table 1.

Tab1 PCR primer sequences
2.2.6 Statistical Analysis
SPSS 25.0 software was used for statistical analysis. Measurement data were expressed as mean ± standard deviation(±s ). The variables consistent with normal distribution were analyzed by one-way ANOVA. Kruskal-Wallis is used for variables that do not conform to normal distribution. P<0.05 was considered statistically significant.
3. Results
3.1 Effect of Qiliqiangxin capsule on myocardial histopathology in rats
As shown in Figure 1, in the sham group, the myocardial cells were arranged neatly, the nuclei were clear, the cytoplasm was abundant,and the interstitium was normal. Compared with the sham group,hypertrophy and disorder of myocardial cells were observed, some nuclei were lost, muscle fibers were broken in the infarcted area,and the muscle space was significantly widened in the model group.Compared with the model group, Qiliqiangxin capsule group and captopril group myocardial tissue morphology are improved to a certain extent, reflected in the myocardial cells arranged relatively regular, damage to reduce, muscle space significantly reduced.
3.2 Effect of Qiliqiangxin capsule on myocardial fibrosis in rats
As shown in Figure 2, there was no collagen fiber deposition in sham group;but compared with the sham group, the deposition of myocardial collagen fiber in model group was significantly increased, which was characterized by myocardial fibrosis;Compared with the model group, Qiliqiangxin capsule group and captopril group myocardial collagen fiber deposition was significantly reduced, myocardial fibrosis was significantly improved.
As shown in Table 2, compared with the sham group, CVF increased significantly in model group(P<0.01). Compared with the model group, CVF decreased in Qiliqiangxin capsule group and captopril group(P<0.05).

Fig1 HE staining results of myocardial tissue of rats in each group (×200)
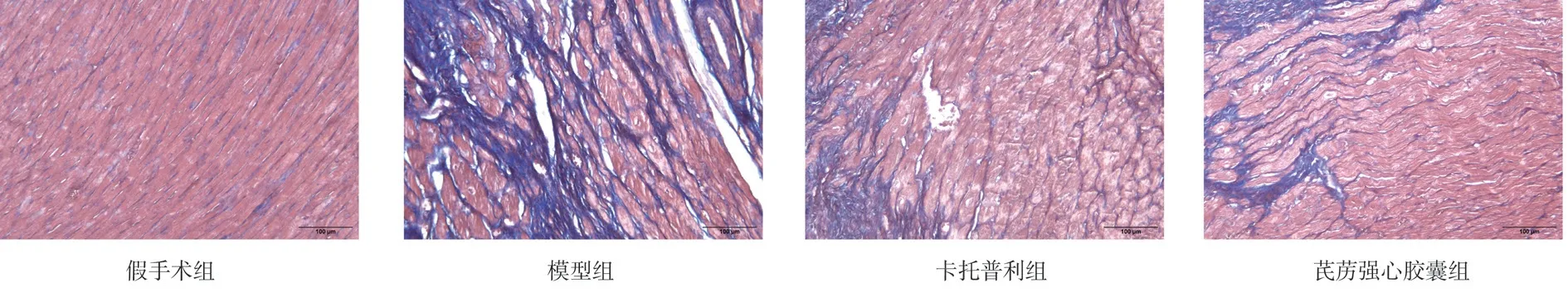
Fig2 Masson staining results of myocardial tissue of rats in each group(×200)

Tab2 CVF results of myocardial tissue in each group(x±s)
3.3 expression of col-Ⅰ、col-ⅢmRNA in myocardium of rats in each group
As shown in Table 3, compared with the sham group, the expression of col-Ⅰ、col-ⅢmRNA increased in model group(P<0.01).Compared with the model group, The expression of col-Ⅰ、col-Ⅲ mRNA expression in Qiliqiangxin capsule group and captopril group decreased, but the difference was not statistically significant(P>0.05).GroupNCol-Ⅰcol-Ⅲ

Tab3 Col-Ⅰ、col-Ⅲ mRNA expression in myocardial tissue of rats in each group(x± s)
3.4 Expression of miR-133a,TGF-β1、Smad2、Smad3 mRNA in myocardial tissue of rats in each group
As shown in Table 4, compared with the sham group, the expression of miR-133a was decreased in the model group, while the mRNA expression of TGF-β1, Smad2 and Smad3 was increased(P<0.05 or P<0.01). Compared with the model group, the expression of miR-133a in Qiliqiangxin capsule group and captopril group were increased, the expression of TGF-β1, Smad2, Smad3 mRNA were decreased(P<0.05 or P<0.01).

Tab4 miR-133a, TGF-β1、Smad2、Smad3 mRNA expression in myocardial tissue of rats in each group(x±s)
4. Discussion
MI is an adverse cardiovascular event, and its pathological changes are often accompanied by ventricular remodeling, affecting the normal systolic function of the heart, and eventually developing into heart failure. This is the main reason for the high hospitalization and mortality rates in MI [22,23]. In early MI, scarring is important to preserve the structural integrity of the infarcted ventricular wall,but continued excessive fibrosis leads to cardiac dysfunction [14].Therefore, improving tissue fibrosis after MI is of great significance for improving cardiac function and preventing heart failure after MI.Qiliqiangxin capsule, is a multi-center, large-scale clinical trial confirmed to be able to treat chronic heart failure of Chinese patent medicine [24]. Studies have shown that it is effective in regulating myocardial fibrosis and improving heart failure after MI [25,26].However, its gene regulation mechanism remains to be further studied.
Our results show that, Myocardial cell hypertrophy, local fracture and large area collagen hyperplasia were observed in the interstitium of rats in MI model group; compared with the sham group, CVF and the expression of col-Ⅰ、col-Ⅲ mRNA were significantly increased,indicating that the heart of the model group had obvious fibrosis changes. After 4 weeks of treatment with Qiliqiangxin capsule, myocardial fibrosis was significantly improved, which was manifested by relatively regular arrangement of myocardial cells,narrowing of cell space, and significantly decreased CVF. However,the expression of col-Ⅰ、col-Ⅲ mRNA in Qiliqiangxin capsule group only decreased, there was no statistical difference. After reviewing the relevant literature, we found that Qiliqiangxin capsule on collagen gene inhibition can be strengthened with the increase of treatment time, for example, Wang et al reported Qiliqiangxin capsule treatment of spontaneous hypertension rats for a year, can significantly reduce the expression of col-Ⅰ、col-Ⅲ mRNA in myocardial tissue [27], which provides a reference for Qiliqiangxin capsule prevention and treatment of myocardial fibrosis in the long and long-term application.
To reveal the molecular mechanism of Qiliqiangxin capsule to improve myocardial fibrosis, this study further observed the fibrosis pathway miR-133a/TGF-β1/Smads gene expression changes.The results show that, in the MI model group, the expression of miR-133a was decreased, and the expression levels of TGF-β1, Smad2 and Smad3 mRNA were significantly increased, confirming that the occurrence of myocardial fibrosis after MI was related to the activation of miR-133a/TGF-β1/Smads. miR-133a, one of the most abundant heart-specific miRNAs, is involved in early MI pathology and subsequent ventricular remodeling, and its overexpression can inhibit cardiac hypertrophy and reduce collagen deposition [28,29].TGF-β1, its downstream target gene, is a key proliferative factor of fibrosis. TGF-β1 activates Smad2 and Smad3 transcription factors by binding to its receptor, and the activated Smads are transferred to the nucleus to regulate the synthesis of promoting fibrogenic genes such as col-Ⅰ、col-Ⅲ[30,31]. Smad3 can also directly bind to the Smad binding element within the gene promoter, thereby enhancing the transcription of target genes and playing a key role in fibrosis signaling[32]. Our results show that, the expression of miR-133a in the heart of rats in the MI model group is down-regulated,which seems to lead to its reduced inhibition of the downstream profibrosis factor TGF-β1, which then activates Smad2、3, leading to the occurrence of myocardial fibrosis. After 4 weeks of treatment with Qiliqiangxin capsule, the expression of miR-133a in the heart was significantly increased, and the inhibition of TGF-β1 was restored, resulting in the significantly decreased expression of TGFβ1, Smad2、3 mRNA.This may be the molecular mechanism of its improvement of myocardial fibrosis.
All in all, this study showed that Qiliqiangxin capsule can improve myocardial fibrosis in MI rats to a certain extent, and its mechanism is related to the regulation of miR-133a/TGF-β1/Smad2、3 signaling pathway gene expression. This study only in the gene level preliminary discussion the regulation of qiliqiangxin capsule on MI rat myocardial fibrosis, next we will be respectively from the protein level to continue to study, and carry out cell experiments to further verify.
Author conflict of interest statement
There is no conflict of interest.
The author contributions: The experiment was designed by corresponding authors Aiming Wu and Mingjing Zhao, animal model preparation and experimental technical guidance were carried out by Lixia Lou, Nie Bo and Jiuli Zhao, administration intervention and experimental index detection were carried out by Xiaodi Ji, Lv Meng and Yang Ding. The article was written by ji Xiaodi, the first author.
 Journal of Hainan Medical College2022年21期
Journal of Hainan Medical College2022年21期
- Journal of Hainan Medical College的其它文章
- In vitro study on the antiviral activity of 9 extracts of traditional Chinese herbal medicine against the human respiratory syncytial virus
- Pharmacokinetics of Jiaotai pill self-microemulsion in insomnia rats
- Correlation between IL-33/sST2 signaling pathway and patients with essential hypertensive left ventricular hypertrophy
- Design and characterization of a bi-functional bybrid antibacterial peptide LLM against Pseudomonas aeruginosa
- Screening and comprehensive analysis of key genes in liver hepatocellular carcinoma based on bioinformatics
- Mechanism of Gan Dou Ling in improving liver fibrosis in Wilson disease based on network pharmacology and experimental verification
