轮型多金属氧酸盐复合物膜的制备及在检测亚硝酸盐中的应用
初明月,李峰博,高 宁,杨 昕,于婷婷,马慧媛,杨桂欣,庞海军
(1.哈尔滨理工大学材料科学与化学工程学院,哈尔滨 150040;2.哈尔滨石油学院化工学院,哈尔滨 150000)
1 Introduction
Nitrite is widely used in many fields such as food industry,textile industry and fertilizers.As we all know,it is usually used in cured meat products and commercially available vegetables because it can prevent bacterial growth and food oxidation[1].However,excessive consumption of nitrite by humans and animals can cause poisoning,and the lethal dose to humans is 33 mg per kilogram of body weight[2].According to the reports from the World Health Organization,the lethal dose of nitrite ingested by humans ranges from 8.7 to 28.3µmol/L[3].Nitrite has been found to be the precursor of many carcinogens,such as nitrosamines[4]and aromatic carbocations[5].Moreover,the excess level of nitrite in blood causes the irreversible oxidation of hemoglobin which reduce the concentration of oxygen in tissues.And also it produces highly carcinogenic N-nitrosamine compounds when reacts with amines and amides.
To avoid these problems,several effective methods for nitrite determination have been reported,such as liquid chromatography,ion chromatography[6,7],spectrophotometry[8]and the Griess reaction[9—12].However,those methods are time consuming and involve tedious procedures,compared to their electrochemical counterparts.So a simple electrochemical method is used in the present study.Nitrite has mainly been detected by using amperometry[13—17],cyclic voltammetry[18—22],and differential pulse voltammetry[23,24].Various modified electrodes have been used for the electrocatalytic detection of nitrite.
Polyoxometalates(POM),as a rich class of inorganic metal oxide clusters,have a variety of structures,sizes and compositions.The most important feature of POMs is that they can keep their structure unchanged after undergoing rapid,reversible and gradual multiple electron transfer reactions[25].Its unique redox characte-ristics make it widely concerned in electrochemical sensors,electrocatalysis[26—28]and electroanalysis.Impressively,coronal-shape phosphotungstate K28Li5H7P8W48O184·92H2O(P8W48)as a kind of polyoxometalate,possesses great stability in a large pH domain from 0 to 8 and abundant electrons are involved in its reversible redox processes,which permits it to be used as an electrochemical active component for fabricating sensor[29].Moreover,P8W48has not been applied to the detection of NO2-,therefore,we choose P8W48to prepare the electrochemical sensor for exploration.However,the low specific surface area and low electrical conductivity of POMs are also important shortcomings to be overcome.Therefore,a good carrier material is required for its composite.
Over the past few years,carbon materials have been widely used as fixed substrates for POM due to their excellent chemical stability and electron transfer rate.Among them,as the carbon nanotubes themselves have unique electronic properties,high surface area,high chemical stability and relatively high mechanical properties[30—33],they can accelerate the rate of electron transfer and endow excellent performance to chemical sensor or biosensor.Carbon nanotubes can effectively enhance the electrocatalytic activity and reduce the ability of surface fouling[34—36].Chitosan(CS)is one of the most potential candidates for drug delivery system with excellent biocompatible,biodegradable adhesion ability,excellent film forming and nontoxic properties.Moreover,CNTs-CS composites have been actively applied in materials science especially in electrochemical sensors[37,38],because CS can enhance the biocompatibility and hydrophilicity of CNTs,resulting in good dispersity and long-term stability of CNTs-CS.
In recent years,metal nanoparticles have been used in many fields such as photocatalysis,catalysis,magnetic materials and information storage due to their special properties[39—41].The chemical and physical properties of nanoparticles(NPs)are related to their shape and size.The combination of NPs and other materials can prevent the aggregation of materials.Nickel nanoparticles(Ni NPs)have been widely studied because they have potential application in magnetic devices[42,43],catalysts[44,45],batteries[46,47],electrochromic devices[48,49],fuel cells[50,51]and sensors[52—55].Due to good conductivity and the oxidation of Ni NPs to generate the redox pair Ni(OH)2/NiOOH in alkaline solution,it has been used in electrochemical sensors[56~58].Thus Ni NPs were used as an active component of as-prepared composite film in this work,which was synthesized by using poly(vinylpyrrolidone)(PVP)as protective agent to prevent the aggregation[58].
Considering all of these issues,we have chosen P8W48,CNTs-CS and Ni NPs as active components to construct a novel composite film by using layer-by-layer self-assembly and electrodeposition method together to highly sensitive detecting NO-2.The addition of CNTs-CS and Ni NPs not only retains the excellent catalytic properties of P8W48,but also solves the problem of poor conductivity and easy aggregation of P8W48.The proposed sensor integrated the unique redox property of the P8W48with the excellent conductivity of CNTs-CS and Ni NPs,showing low detection limit,wide linear response range,short response time,and good selectivity.Moreover,the established composite film was used to detect the NO2-in real seamples,demonstrating the applicability of the method in practical application.
2 Experimental
2.1 Materials
Poly(ethylenimine)(PEI,Mw=750000),PVP(Mw=1300000),NaBH4,L-cysteine,ascorbic acid,dopamine,uric acid and glucose were purchased from Aldrich.Na2HPO4,NaH2PO4,ethanol,ethylene glycol,hydrochloric acid and Ni(CH3COO)2·4H2O were obtained from Yongchang Chemical Company.All the reagents were analytically pure,no further purification was required when used.Ni NPs,CNT-CS,and P8W48was synthesized according to the literatures,respectively[59—61].Deionized water(DW)with a resistivity of 18 MΩ·cm was used as the solvent.
2.2 Apparatus
Transmission electron microscopy(TEM)images were obtained using an H-7650 TEM from Hitachi.FTIR analysis was performed on a BioRad FTS 3500 GX spectrophotometer.X-ray diffraction patterns were obtained using an XRD-6000 diffractometer.X-ray photoelectron spectra(XPS)was made by an ESCALABMKII spectrometer.Scanning electron microscopy(SEM)images were obtained using an S-4300 SEM from Hitachi.Electrochemical workstation(CHI 760D)was purchased from Shanghai Chenhua Instrument Co.,Ltd.A three-electrode structure was used,including an Ag/AgCl electrode as reference electrode,a composite film{PEI/P8W48/CNTs-CS/Ni/P8W48}-coated GCE as a working electrode and a platinum counter electrode.
2.3 Preparation of the Composite Film
GCE was polished with 1.0,0.3 and 0.05µm alumina powder on the polishing cloth,ultrasonically treated in ethanol and DW in ultrasonic bath and then dried with N2stream.For preparation of the electrochemical sensor,a PEI precursor film was deposited on a cleaned GCE substrate by immersing the substrate in PEI(10 mmol/L)solutions for 12 h,followed by rinsing with DW and drying under a nitrogen stream after each immersion.Then,the pre-coated substrate was sequentially immersed in the solutions of P8W48and CNTs-CS each for 1 h.For Ni NPs electrodeposition,a typical experiment was performed under the deposition potential of-0.2 V for 1000 s in 0.2 mol/L PBS(pH=7.0).The coated substrate was then immersed in the P8W48solution for 1 h,the composite film,denoted as{PEI/P8W48/CNTs-CS/Ni/P8W48}was obtained. The schematic illustration of the construction process of{PEI/P8W48/CNTs-CS/Ni/P8W48}is shown in Scheme 1.For comparison,a similar procedure was used to prepare the{PEI/P8W48/PEI/P8W48}and{PEI/P8W48/Ni/PEI/P8W48}films.

Scheme 1 Schematic illustration of the process of constructing the{PEI/P8W48/CNTs⁃CS/Ni/P8W48}composite film
3 Results and Discussion
3.1 TEM and SEM
The Ni NPs was characterized by using TEM to confirm the particle size and distribution.Fig.1 shows that the native particles are nonspherical,interconnected in shape,having an average size of approximately 75 nm.

Fig.1 TEM image of Ni NPs
The surface morphology and the homogeneity of{PEI/P8W48/CNTs-CS/Ni/P8W48}was investigated by scanning electron microscopy(SEM).It can be seen from Fig.2(A)that the composite film{PEI/P8W48/CNTs-CS/Ni/P8W48}displays an amount of small particles uniformly attached to the regular tubular structure,proving the existence of CNT,Ni and P8W48.And the diameter of the CNTs-CS bundle is observed to be about 47 nm.Fig.2(B)displays the cross-sectional morphology of the{PEI/P8W48/CNTs-CS/Ni/P8W48}composite film.From the cross-sectional observations,the surface of the composite film is flat and dense,its thickness was estimated to be about 257 nm.

Fig.2 SEM micrograph(A)and cross⁃sectional SEM image of the{PEI/P8W48/CNTs⁃CS/Ni/P8W48}(B)
3.2 X-Ray Photoelectron Spectra
The chemical state and composition of elements in the composite film of{PEI/P8W48/CNTs-CS/Ni/P8W48}have been analyzed by using X-rays photoelectron spectroscopy(XPS).From Fig.3,it can be seen that W shows two peaks at 35 eV(W4f7/2)and 37 eV(W4f5/2),P2pshows one peak at 132.78 eV,N1sshows one peak at 399 eV,C1sshows two peaks at 284.5 and 287 eV,Ni shows two peaks at 855 eV(Ni2p3/2)and 872 eV(Ni2p1/2)[62,63].Through XPS spectra analysis,it can be confirmed that N,P,W,Ni,O and C elements are present in the composite film.
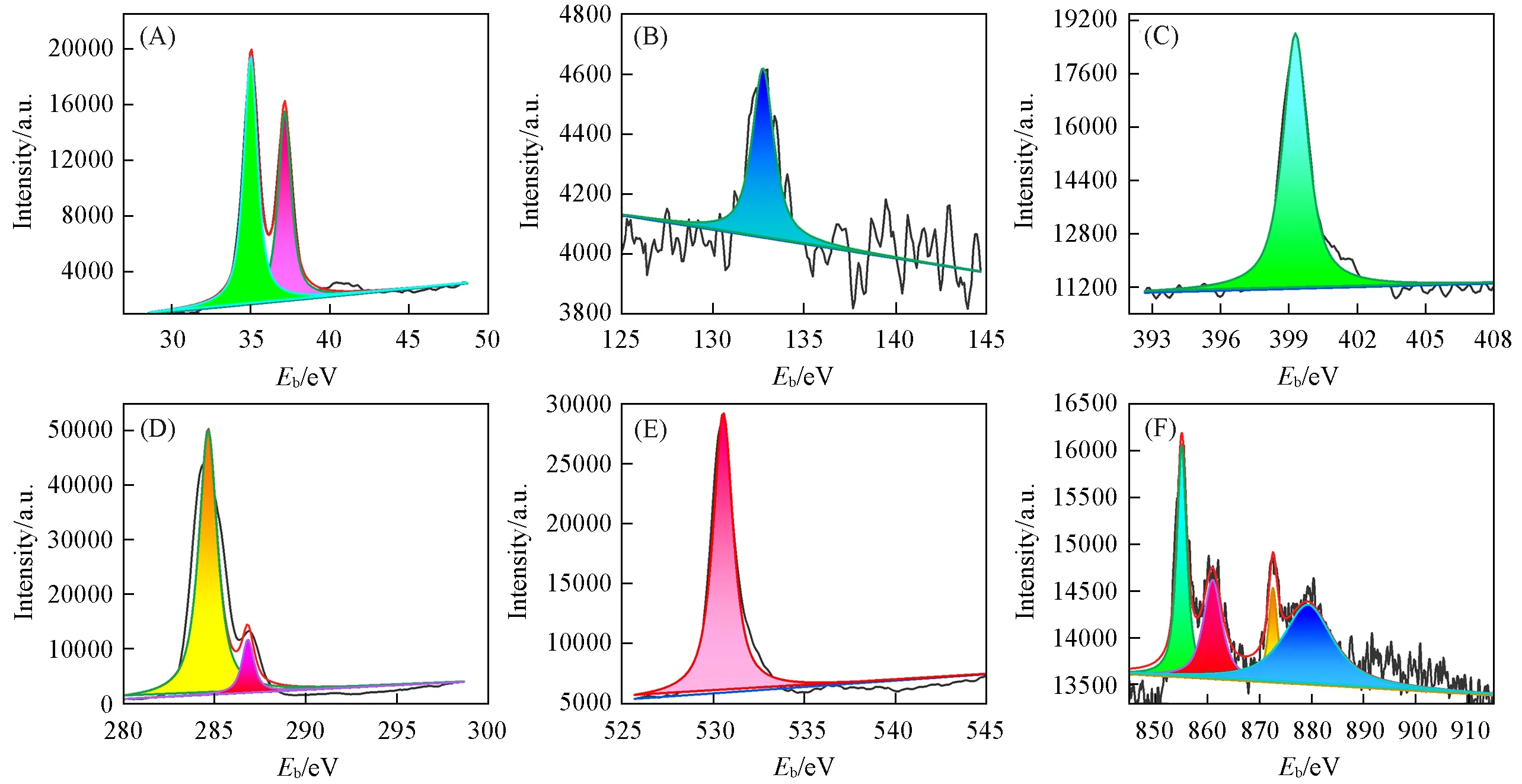
Fig.3 XPS spectra of W4f(A),P2p(B),N1s(C),C1s(D),O1s(E),Ni2p(F),and of the{PEI/P8W48/CNTs⁃CS/Ni/P8W 48}composite film
3.3 Cyclic Voltammetry Curves
The CV curves of{PEI/P8W48/CNTs-CS/Ni/P8W48}(in 0.2 mol/L PBS,pH=7.0)displays four potential peaks with mean peak potentials ofE1/2(I)=0.12 V,E1/2(II)=-0.035 V,E1/2(III)=-0.065 V,E1/2(IV)=-0.07 V,whereE1/2=(anodic peak potentials-cathodic peak potentials)/2(Fig.4).The two pairs of redox peaks I/I'and II/II'were ascribed to two one-electron tungsten redox process(WVI→WV)and the redox peaks III/III'and IV/IV'were due to two one-electron transfers centered on tungsten(WVI→WV)[26,27].Fig.4(B)shows the CV curve of the{PEI/P8W48/CNTs-CS/Ni/P8W48}composite film at different scan rates.The peak currents increased with the increase of the scan rate.The peak currents were proportional to the scan rate in the range of 50—400 mV/s in Fig.4(C),indicating that the electron transfer was controlled by a surfaceconfined process.
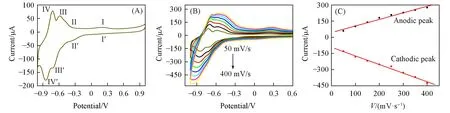
Fig.4 CVs of the{PEI/P8W48/CNTs⁃CS/Ni/P8W48}composite film in 0.2 mol/L PBS(p H=7.0)at 50 mV/s(A),and from 50 mV/s to 400 mV/s(step 50 mV/s)(B)and plots of the cathodic and anodic peak currents of waves against the scan rate(C)
The surface coverage(Г)of P8W48can be calculated according to the equation:Г=4ipRT/n2F2νA[64](ipis the peak current;nrepresents the number of electrons in the reaction;vis the scan rate;Ais the surface area of the working electrode;Ris the molar gas constant;Tis the thermodynamic temperature;Fis Faraday constant).Taking the forth couple of redox peaks as an example,it was calculated that the surface coverage of P8W48of the{PEI/P8W48/CNTs-CS/Ni/P8W48}is 0.62×10-8mol/cm2.
3.4 Electrochemical Test
Electrochemical impedance spectroscopy(EIS)is an effective method for testing the electron transfer ability of the composite film surface.The EIS plots of PEI/P8W48/CNT-CS/Ni/P8W48,PEI/P8W48/Ni/PEI/P8W48and PEI/P8W48/PEI/P8W48modified electrodes are shown in Fig.5(A).It was observed from Fig.5(A)that the semicircle diameter of the composite film{PEI/P8W48/CNTs-CS/Ni/P8W48}is the smallest,indicating that it has the lowest electron transfer resistance and the highest electron transfer rate.This showed that the effective combination of the three components accelerates the electron transfer rate in the composite film.A simple equivalent circuit was chosen to fit the impedance data of{PEI/P8W48/CNTs-CS/Ni/P8W48}composite film and is shown in inset of Fig.5(A).The circuit diagram includes four components,namely,the intrinsic resistance of electrolyte(Rs),the constant phase element(CPE),the resistance to charge transfer(Rct),and the Warburg impedance.
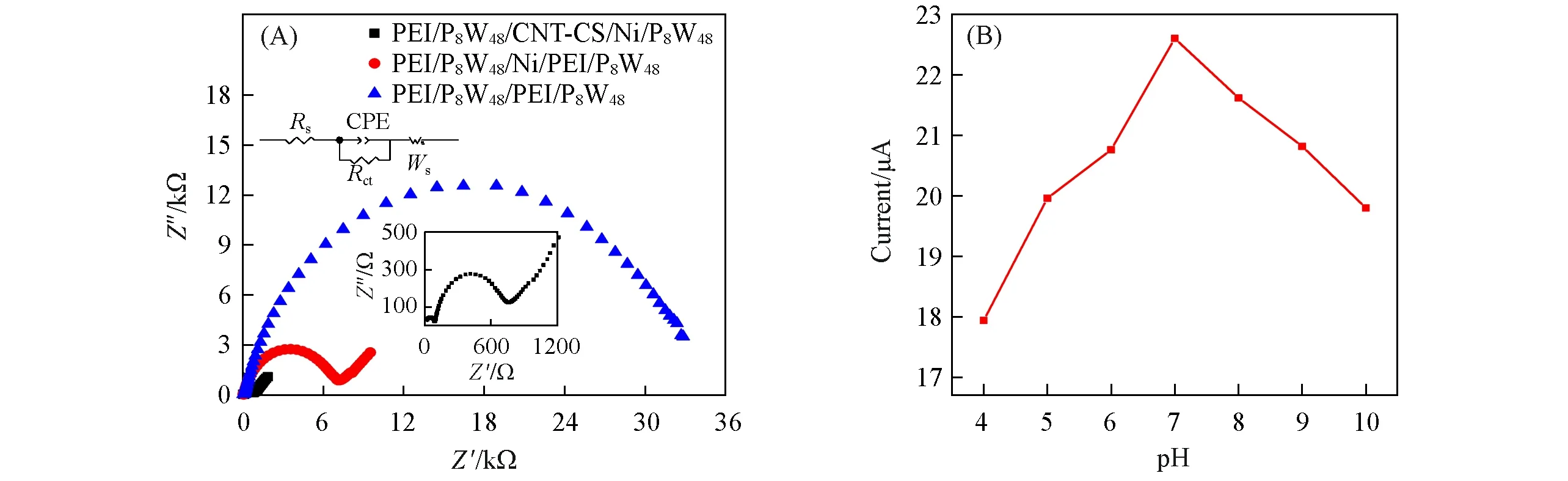
Fig.5 EIS plots of different films(A)and influence of p H on the peak current(I pa)of{PEI/P8W48/CNTs⁃CS/Ni/P8W 48}(B)
Generally,the pH value of the buffer system has a certain influence on the performance of the composite membrane electrode.Therefore,we studied the influence of pH on the peak current of NO-2oxidation on the prepared sensor in the pH range of 4.0 to 10.As shown in Fig.5(B),The oxidation peak current of NO-2increased with the increase of pH value,then reached the maximum at pH=7 and decreased with the increase of pH value.
3.5 Electrocatalytic Activity of PEI/P8W48/CNT-CS/Ni/P8W48
Fig.6 shows the differential pulse voltammograms(DPV)of the{PEI/P8W48/CNTs-CS/Ni/P8W48}composite film in 0.2 mol/L PBS(pH=7.0)for the detection of NO-2.It can be seen from Fig.6 that a clear oxidation peak appears around 0.75 V,and the oxidation current increases with the concentration of NO-2,which means that the{PEI/P8W48/CNTs-CS/Ni/P8W48}composite film can detect NO-2.The inset of Fig.6 shows the relationship between NO-2concentration and catalytic current.It can be seen from the inset that the concentration of NO-2has a linear relationship with the peak oxidation current,indicating that the prepared composite film can be applied to the actual detection of NO-2.
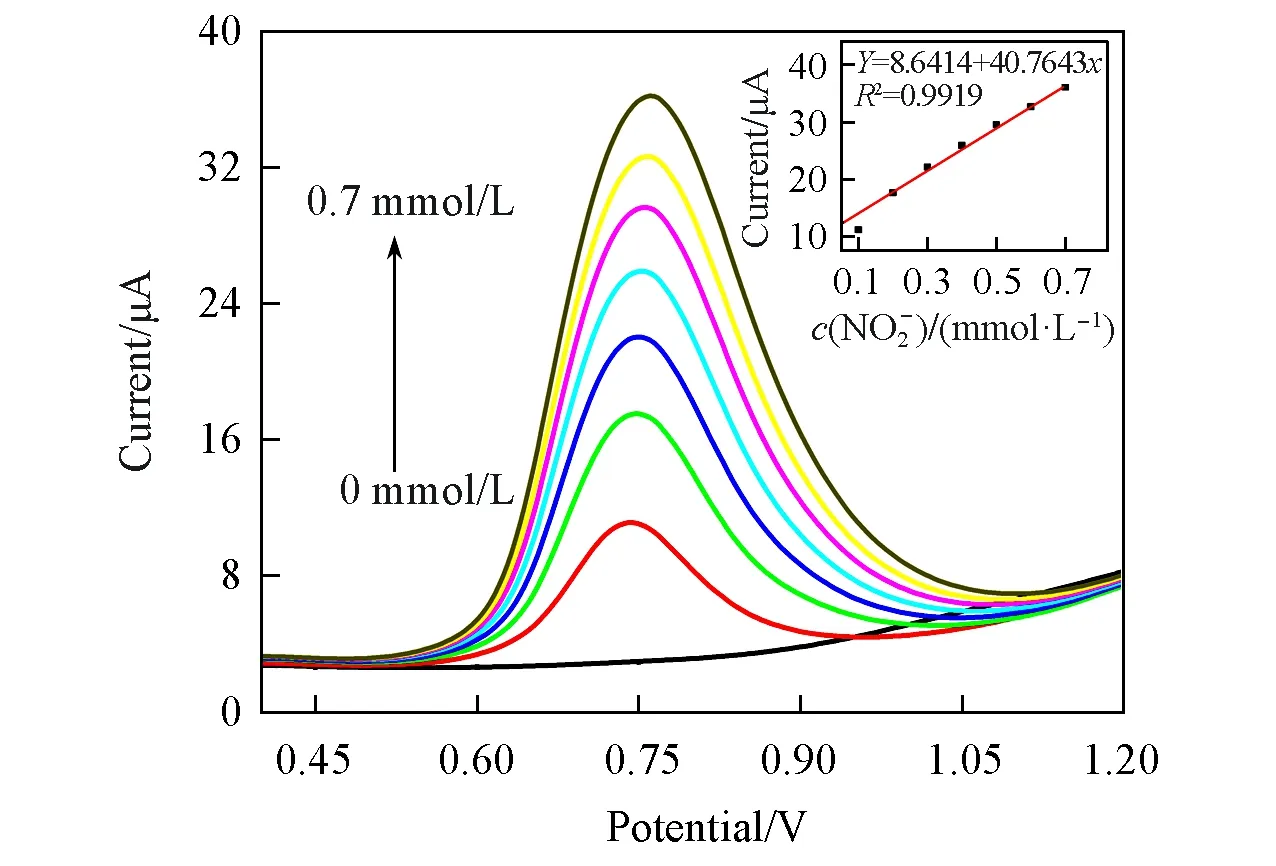
Fig.6 Differential pulse voltammograms of{PEI/P8W 48/CNTs⁃CS/Ni/P8W 48}in 0.2 mol/L PBS(pH=7.0)containing different concentrations of NO2-
The electrochemical oxidation responses of NO2-at the{PEI/P8W48/PEI/P8W48}and the{PEI/P8W48/Ni/PEI/P8W48}were also investigated using the DPV method,and the results are shown in Fig.7.Compared with{PEI/P8W48/CNTs-CS/Ni/P8W48},the redox peaks of{PEI/P8W48/PEI/P8W48}and{PEI/P8W48/Ni/PEI/P8W48}to oxidation of NO2-both are located at a higher potential,and the peak shape is blunter.And the response current of NO2-on{PEI/P8W48/CNTs-CS/Ni/P8W48}is much higher than those of{PEI/P8W48/PEI/P8W48}and{PEI/P8W48/Ni/PEI/P8W48}.This shows that{PEI/P8W48/CNTs-CS/Ni/P8W48}has excellent electrocatalytic activity to oxidation of NO-2due to synergistic effect of three active components.

Fig.7 DPVs of the{PEI//P8W 48/PEI//P8W48}(A)and{PEI//P8W 48/Ni/PEI//P8W 48}(B)composite films in 0.2 mol/L PBS(p H=7.0)containing different concentrations of NO-2
3.6 Selectivity Profile of the Composite Film
Selectivity is also an important consideration for electrochemical sensors,and it also depends on the applied potential.Fig.8 shows the current response of eight species of potential interferences(glucose,acetic acid,citric acid,KBrO3,KIO3,Na2CO3,KCl,and fructose)along with NO-2at the composite film under different applied potentials in 0.2 mol/L PBS(pH=7.0).From Fig.8,it can be seen that the prepared composite film reaches the maximum oxidation peak current for NO-2detection at 0.75 V.It can also be seen that there are almost no current response to be observed at these applied potentials for the eight interferences,proving that the compo-site film has a good selectivity for detecting NO-2,and 0.75 V is chosen as optimized applied potential for sensing NO-2.

Fig.8 Anti⁃interference performance of the{PEI//P8W 48/CNTs⁃CS/Ni/P8W48}composite film obtained with glucose,ace⁃tic acid,citric acid,KBr O3,KIO3,Na2CO3,KCl,fructose and NaNO2 at different applied potentials in 0.2 mol/L PBS(p H=7.0)
3.7 Linear Range,Detection Limit and Stability of the Composite Film
Fig.9(A)displays the amperometric responses of the{PEI/P8W48/CNTs-CS/Ni/P8W48}upon successive addition of NaNO2to 0.2 mol/L pH=7.0 PBS at an applied potential of 0.75 V under stirring.The prepared sensor reaches a steady-state current for detection of NO-2within 2 s,which means that the sensor has a very high sensitivity.A typical time-current curve was obtained after about 1600 s,the linear regression equation was:Y=1.51838+0.02382X,R2=0.9902.Under optimal test conditions,a calibration graph was constructed for NO-2[Fig.9(B)],with a linear range from 1.25µmol/L to 2500µmol/L.The limit of detection(LOD)was 0.02µmol/L of NO-2.Compared with other NO-2sensors listed in Table 1,the sensor based on{PEI/P8W48/CNTs-CS/Ni/P8W48}had wider linear range and lower detection limit.The prominent electrocatalytic ability of{PEI/P8W48/CNTs-CS/Ni/P8W48}should be attributed to the excellent electrocatalytic performance of P8W48in the redox process,the tunneling effect of small-sized Ni NPs and the excellent electrical conductivity of carbon nanotubes and Ni NPs.
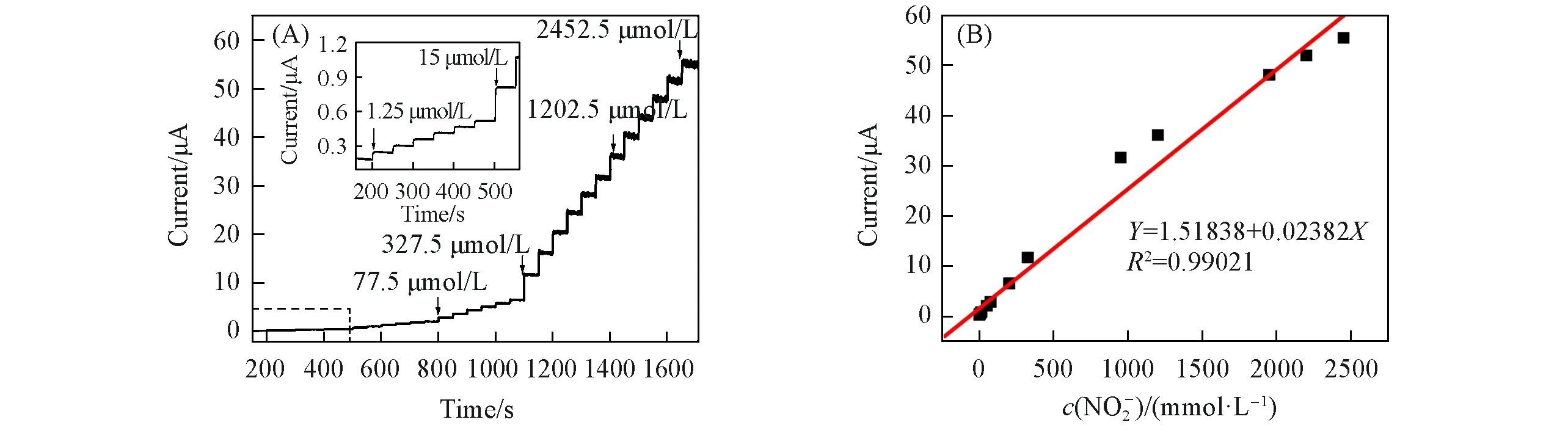
Fig.9 i⁃t curve of the{PEI/P8W48/CNTs⁃CS/Ni/P8W 48}composite film during successive additions of NO 2-at 0.75 V(A),and calibration plot of current and concentration of NO2-obtained at the{PEI/P8W48/CNTs⁃CS/Ni/P8W48}composite film(B)
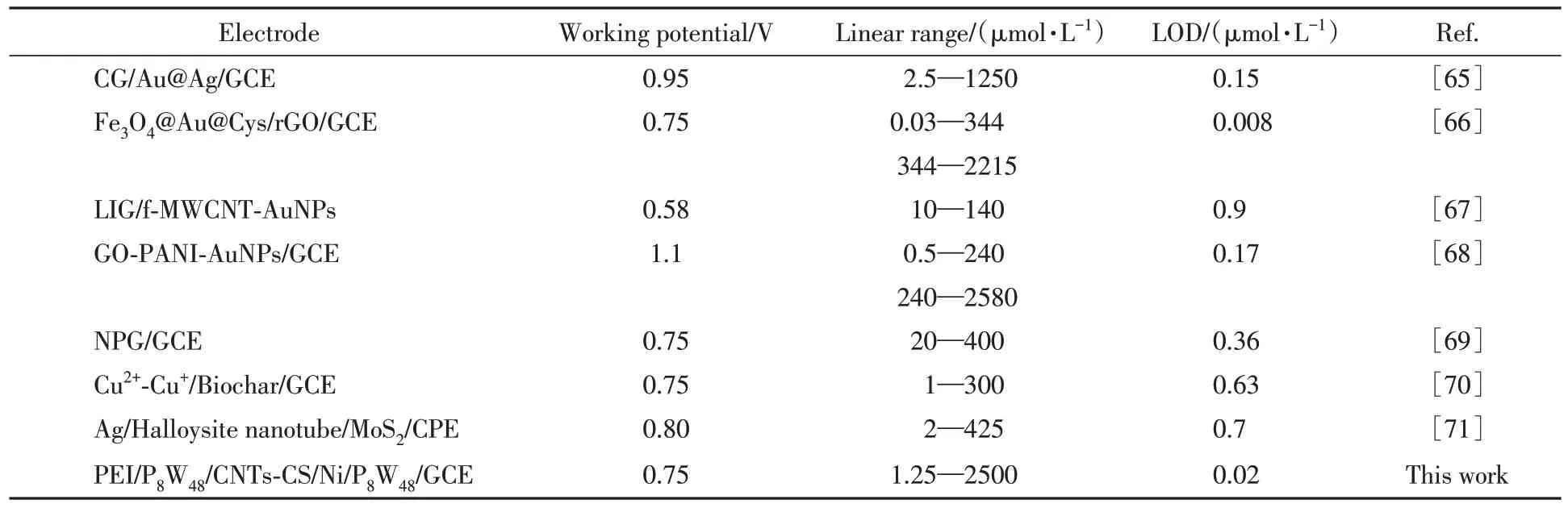
Table 1 Comparison of the sensor performance between the prepared sensor and other sensors for the determination of nitrite
Stability is a key factor to evaluate nitrite sensor performance based on{PEI/P8W48/CNTs-CS/Ni/P8W48}composite film.In order to evaluate the stability of the sensor,the sensor was used for cyclic voltammetry scanning for 100 circles and just very little loss of electrocatalytic current signal could be observed in Fig.10,indicating the good stability of the sensor.
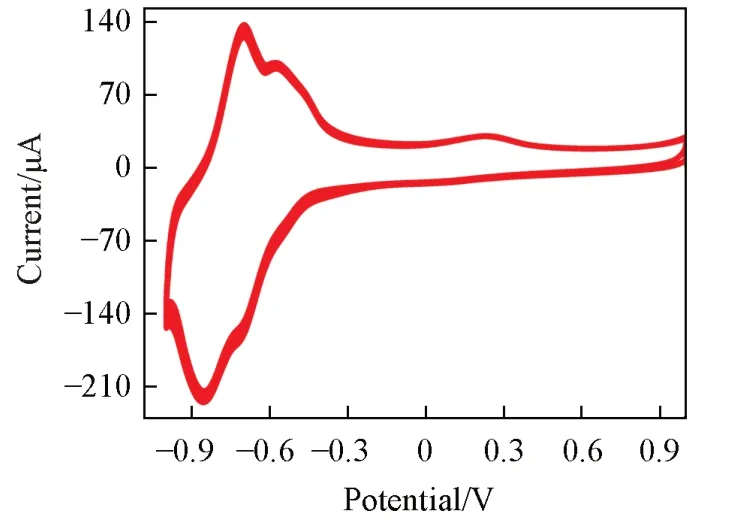
Fig.10 CVs of the{PEI/P8W48/CNTs⁃CS/Ni/P8W48}multilayer film for 100 circles in 0.2 mol/L PBS(p H=7.0)
3.8 Real Samples Analysis
To evaluate the feasibility of the{PEI/P8W48/CNTs-CS/Ni/P8W48/GCE}sensor for real samples analysis,the sensor was used to detect nitrite in fruit juice(Master Kong orange juice).The fruit juice samples were diluted with buffer solution using the standard addition method.Results showed that the recoveries ranging from 99.76%to 100.01%(Table 2),indicating that the present methods could be efficiently used for the determination of nitrite in fruit juice in practical applications.

Table 2 Results of the recovery tests obtained for determination of NO 2-in real sample
4 Conclusions
In this paper,a nitrite sensor based on the{PEI/P8W48/CNTs-CS/Ni/P8W48}composite film was successfully prepared by combination of layer-by-layer self-assembly and electrdeposition.After P8W48was combined with CNTs-CS and Ni NPs,the composite film maintains unique redox characteristics of P8W48,meawhile obtains quick electron transfer rate.Therefore,the prepared sensor exhibits excellent sensing performance for detecting NO2-,with a wide linear range of 1.25µmol/L to 2500µmol/L,a low detection limit of 0.02µmol/L(S/N=3).Further,the composite film sensor can selectively determine NO2-in the presence of common interferents,demonstrating that this as-prepared sensor has very superior selectivity.In addition,the prepared sensor has been tested in real samples,the results shows that{PEI/P8W48/CNTs-CS/Ni/P8W48}has the promising potential to be applied to the actual detection of nitrite.

