一种具有发光传感、安培传感和染料吸附性能的多功能Zn(II)配位聚合物
马鉴新,刘晓东,徐 娜,刘国成,王秀丽
(1.渤海大学化学与材料工程学院,辽宁省太阳能电池转化材料专业技术创新中心,锦州121013;2.长春理工大学材料科学与工程学院,长春130022)
1 Introduction
During recent years,water pollution has turned into a worldwide concern and a menace to human health[1—7].As the basic components of protein,amino acids play a crucial role in maintaining the regeneration and repair functions in biological materials[8,9].Asparticacid(Asp),as one of the most common amino acids,deserves more attention due to its important excitatory neurotransmitter[10,11].Ascorbic acid(AA)is a basic biomolecule,which also has a momentous influence on the physiological health of the human bodies[12,13].Hence,it is significantly necessary to explore the detection method of trace Asp and AA in environment.Traditional methods are usually supported by large instruments,such as Raman spectroscopy,highperformance liquid chromatography,or even gas chromatography coupled with mass spectrometry[14,15].The large-scale instruments are not widely used because of their high cost,the need for professional testers,and the time-consuming analysis.Therefore,the rapid detection of Asp and AA in the daily environment is a hot research topic.
In the meantime,organic dyes that cause environmental pollution are more and more widely used in laboratory research and industries drainage,becoming one of the primary sources of water pollution[16—20].Most dye molecules are harmful to humans and animals,and even have carcinogenic effects[21—26].As is well known,methylene blue(MB),gentian purple(GV),and neutral red(NR)as water-soluble dyes are highly recognizable even at the trace level of exposure[26].As the growing global concern,there is an imperative need to remove MB,GV,and NR residual from the industrial wastewater.However,traditional adsorbents(such as active carbon)exhibit weak adsorption capacity for many dye molecules.Therefore,it is necessary to develop adsorbents with high performance and simple operation[25,27].
Coordination polymers(CPs)have aroused people’s close attention in the fields of sensing and dye adsorption due to their fascinating structures[28—33].Among them,photoelectric sensors are one of the research hotspots at present,and there are some reports on CP-based sensors towards luminescence and electrochemical sensing[34].Meanwhile,some literatures have reported the studies on dye adsorption of CPs[19,26].However,most of CPs possess single-or bi-functional application prospect,reports on the multi-functional CPs are still limited.Therefore,designing and synthesizing novel CPs as multi-functional material,which can not only detect amino acid but also adsorbe dyes,is an interesting and challenging task,and would broaden the practical usage of CPs-based materials.
Inspired by the above considerations,we have constructed a novel 2D CP[Zn(4-bpft)(1,4-bdc)](CP 1)using theN,N′-bis(4-pyridine formamide)-3,4-thiophene(4-bpft)as the main ligand and terephthalic acid(1,4-H2bdc)as the co-ligand.The S-containing bis-pyridine-bis-amide ligands have many advantages,such as:(1)due to the existence of multiple functional groups,they have a sufficient number of coordination sites to make it easier to coordinate to metal ions;(2)they have abundant hydrogen bonding sites and can be synthesized to obtain a framework of ideal size and channel.To our knowledge,the complexes constructed by S-containing bis-pyridine-bis-amide ligands are very limited.Based on IR and PXRD characterization and discussion,the luminescence sensing properties,amperometric sensing properties,and dye adsorption properties of CP 1 were studied in detail.
2 Experimental
2.1 Materials and Instruments
Materials and instruments are detailed in the Supporting Information of this paper.
2.2 Synthesis of[Zn(4-bpft)(1,4-bdc)](CP 1)
Zn(NO3)2·6H2O(0.060 g,0.2 mmol),4-bpft(0.017 g,0.1 mmol),and 1,4-H2bdc(0.016 g,0.1 mmol)were placed in 0.1 mol/L NaOH aq.solution(10.0 mL).The mixture was put in a Teflon autoclave reactor,heated to 120℃for 4 d,and then cooled to room temperature,thereby forming the colorlessbulk crystals with 44%yield(based on Zn).Elemental analysis(%),calcd.:C 53.66,H 3.46,N 9.62;found:C 53.61,H 3.49,N 9.55.IR(KBr),ν͂/cm-1:3436(m),2360(m),1611(m),1579(m),1493(s),1380(s),1305(w),1141(w),1007(m),846(s),737(s),660(w),574(w).
3 Results and Discussion
3.1 Structure Description of CP 1
The CP 1 crystallizes inspace group of triclinic crystal system.One Zn(II)ion,one 4-bpft ligand,and one1,4-bdc anion constitute the asymmetric unit.The five-coordinated Zn center is bonded to three carboxyl O atoms of 1,4-bdc and two N atoms from two 4-bpft ligands,respectively[Fig.1(A)].The Zn—O bond lengths vary from 0.20104(15)to 0.2482(2)nm,while the Zn—N bond lengths are from 0.20454(17)to 0.20688(18)nm,respectively(Table S2,see the Supporting Information of this paper).In CP 1,two Zn2+are connected by two 4-bpft ligands to form a closed loop[Zn2(4-bpft)2]4+,and adjacent loops are connected by the 1,4-bdc ligand to form a linear structure alongaaxis[Fig.1(B)].Meanwhile,the 1,4-bdc anions are connected by Zn2+to construct a zigzag chain[Zn(1,4-bdc)]nalongcaxis.The adjacent one-dimensional[Zn(1,4-bdc)]nchains are connected by the[Zn2(4-bpft)2]4+loops to generate a 2D network[Fig.1(C)].The topology of CP 1 shows a 3-connected(3-c)network with the point symbol of{63}[Fig.1(D)].Calculated by Platon,the solvent accessible void space is 17.3%.The crystal data and detailed structural refinement results are summarized in Table S1(see the Supporting Information of this paper).
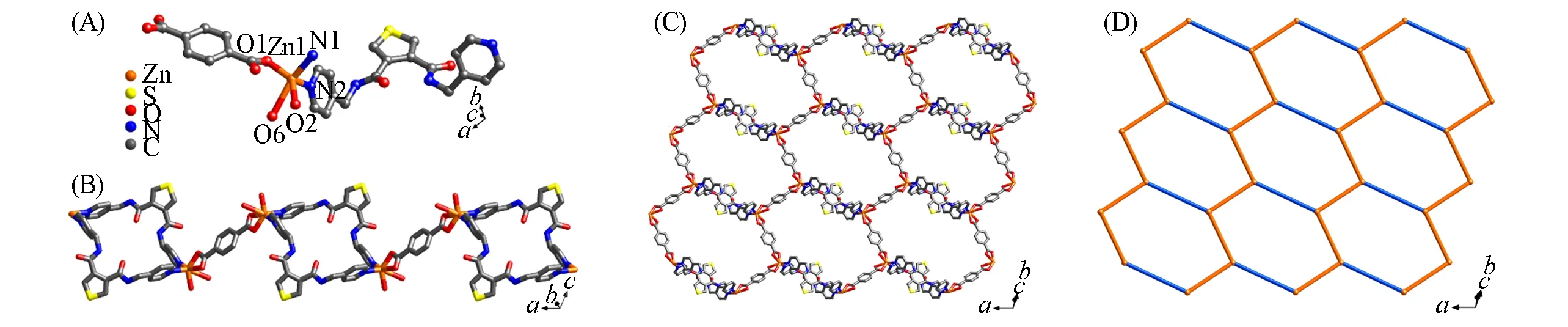
Fig.1 Coordination environment of Zn2+in CP 1(A)and[Zn2(4⁃bpft)2]loop and 1D chain along a axis(B),2D network(C)and 2D{63}topology(D)of CP 1
3.2 PXRD pattern and IR spectrum of CP 1
The powder X-ray diffraction pattern of CP 1 is in good agreement with the that simulated by the CIF file,proving that the experimentally synthesized CP 1 has high phase purity(Fig.S1,see the Supporting Information of this paper).
Fig.S2(see the Supporting Information of this paper)shows the IR spectrum of CP 1.TheνN—Hstretching bands of the 4-bpft ligand could be found at 3298 to 3028 cm-1[35].The bands at 1611 and 1380 cm-1may be belonged to the COO-stretching vibrations[36,37].The peaks at 737 and 658 cm-1indicate theνC—Nstretching vibration of the bis-pyridyl-bis-amide ligand[38].4-bpft shows the weak absorption peaks of the—CH2group from 1000 to 1310 cm-1[39].The IR spectrum of CP 1 accords,with the results of single crystal X-ray diffraction analysis.
3.3 Luminescence Properties
The solid-state luminescence properties of CP 1 have been studied at normal pressure and temperature.As illustrated in Fig.S3(see the Supporting Information of this paper),CP 1 reveals a maximum emission peak at 420 nm(λex=310 nm).The good luminescence performance of CP 1 promoted us to carry out fluorescence sensing experiments on it.Taking into account the principle of green chemistry,deionized water was chosen as a dispersant to research the capacity of CP 1 for fluorescence sensing of amino acids.As demonstrated in Fig.2(A),nine amino acid solutions of the same concentration have different effects on the luminescence intensity of CP 1.Among them,Asp can significantly enhance the luminescence intensity of CP 1,followed by Tyr,while the other seven amino acids have little effect on the luminescence intensity of CP 1.The experimental phenomena showed that CP 1 had high sensitivity towards Asp.Therefore,the“turn on”ability of the Asp for CP 1 was investigated.As can be seen from Fig.2(B),the luminescence intensity is gradually enhanced with the increase of Asp amount and shows a good linear relationship at low concentration,which reveals the turn-on fluorescence sensing of Asp.The relationship between(I0-I)/I0and Asp concentration can be expressed by a linear equation[40],(I0-I)/I0=0.0363×[Asp]-0.02043(R2=0.9964),in whichI0andIare the fluorescent intensities of CP 1 before and after the addition of Asp,respectively.The limit of detection(LOD)value of CP 1 for Asp was calculated through 3σ/k(k:the slope,σ:the standard error)[41],and the LOD value was about 2.48×10-8mol/L[Fig.2(C)].

Fig.2 Luminescence intensities of CP 1 scattered in amino acid solutions(A),changes in the luminescence spectra of CP 1 with different amounts of Asp(B),relationship between(I0-I)/I0 and the concentration of Asp(C),luminescence intensities for CP 1 without and with the addition of eight amino acids and Asp(D)
To further study the selectivity and anti-interference of CP 1 to Asp detection,the other amino acids(including Tyr,Leu,His,Thr,Val,Gly,Met,and AA)were selected as interfering substances.As demonstrated in Fig.2(D),the CP 1 fluorescence intensities in the presence of other eight amino acids are slightly lower than the original one,and after Asp aqueous solution was added into the suspensions containing CP 1 and eight amino acids,the fluorescence intensity of the mixture was significantly higher than that of CP 1 aqueous suspension.This demonstrats that CP 1 as a new fluorescence sensor exhibits good selectivity for detecting Asp.
Due to the unparalleled importance of cyclability in practical applications,cyclicity tests of CP 1 for sensing Asp were carried out under normal pressure and temperature.The results showed that the fluorescence intensity of CP 1 used after four cycles still retained 89%of the initial fluorescence intensity of CP 1(Fig.S4,see the Supporting Information of this paper),which indicated CP 1 can be used as a fluorescence sensor to detect trace Asp.
To explore the fluorescence sensing mechanism of CP 1,the PXRD pattern of CP 1 after soaking in Asp solution were tested,the result indicated that the fluorescence enhancement phenomena was not caused by the collapse of the framework(Fig.S5,see the Supporting Information of this paper).To further insight into the fluorescence enhancement mechanism,the emission lifetime of CP 1 was studied.As shown in Fig.S6(see the Supporting Information of this paper),the lifetime decay curves of CP 1 varied greatly before and after soaking in Asp,which indicated that the sensing mechanism was more inclined to dynamic enhancement.
3.4 Electrochemistry and Electrocatalytic Properties
The electrochemical properties of CP 1 bulk-modified CPE(1-CPE)was studied in 0.1 mol/L H2SO4and 0.5 mol/L Na2SO4aqueous solution as an electrolyte solution.As shown in Fig.3(A),the CV curves of CP 1 displayed reversible redox peaks within the measurement range from 0 to 0.6 V.The mean peak potential(E1/2)=(Epa+Epc)/2 was 0.303 V.Moreover,the peak shapes of the CV curves do not change with the scan rates,indicating that the amperometric characteristics of 1-CPE was stable.Further,the electrocatalytic oxidation behaviors of 1-CPE for AA were investigated.With the addtion of 8.0 mmol/L AA,the fully reversible redox peaks became irreversible and the enhancement of the oxidation peak currents was observed[Fig.3(B)].The experimental results indicated that 1-CPE possessed admirable electrocatalytic activity for AA,and can be selected as the potential electrocatalytic material.

Fig.3 CV response of 1⁃CPE at different scan rates(A),CVs of 1⁃CPE in testing solution containing different concentrations of AA(B),current response after continuously adding AA to 1⁃CPE(inset:the relation⁃ship between current and concentration of AA)(C)and stability of CP 1 to AA for working 25000 s(D)
Since 1-CPE had the ability of electrocatalytic oxidation of AA,it was considered as an amperometric sensor byi-tcurves to detect AA.Further,it is indispensable to analyze the optimal conditions for detecting AA through the electrochemical sensing platform.Considering the influence of different potentials on the system,we explored the optimal potential of the system by gradually adding 10µmol/L AA under different potentials.It is worth noting that the strongest potential for AA to the current appears at 0.42 V(Fig.S7,see the Supporting Information of this paper),indicating that 0.42 V was the best potential.Therefore,0.42 V was set as the optimal potential of CP 1 for electrochemical sensing.As illustrated in Fig.3(C),the corresponding current increases with the continuous addition of AA.The calibration curve[inset of Fig.3(C)]showed that the linear response relationship of correlation coefficient was 0.9994.Moreover,the linear regression equation wasI(µA)=0.00416c(µmol/L)+0.03694,and the limit of detection(LOD)of AA was calculated to be 2.46×10-4mol/L.
Selectivity and stability are important factors to appraise the nature of electrochemical sensors.When adding 0.1 mmol/L AA solution to the electrolyte solution,1-CPE can produce obvious current response,and keep the current response unchanged when containing Thr,Met,His,and Asp,indicating 1-CPE has eyecatching selectivity and anti-interference ability for AA(Fig.S8,see the Supporting Information of this paper).The stability is another vital element for the electrocatalytic material that can be used in practical applications.To investigate the stability,firstly,1-CPE was put into 0.1 mol/L H2SO4+0.5 mol/L Na2SO4aqueous solution for 30 min,then AA(10µmol/L)was added into above mentioned working system.With the AA concentrations gradually increasing,the currents showed a significant change.After 5 h,AA was added into the mixture again,and the currents of 1-CPE can still respond[Fig.3(D)],indicating that CP 1 had amazing electrochemical stability.Overall,the title complex exhibited low LOD,excellent selectivity,and high stability for AA detection,which could be an AA sensor with practical application prospects.
3.5 Adsorption Capability for Organic Dyes
Methylene blue(MB),gentian purple(GV),and neutral red(NR)were chosen as dye contaminants to estimate the adsorption activities of CP 1 in purifying wastewater.Typically,50 mg of CP 1 was added into 80 mL of MB,GV,and NR solution magnetically stirred in the dark,respectively.Then,5 mL of sample solution were taken out every 30 min,centrifuged,and analyzed by UV-Vis spectrophotometry to evaluate the adsorption abilities of CP 1.As shown in Fig.4,the color of the three dye solutions became lighter in the presence of CP 1 with increasing time,which is also consistent with the UV-Vis spectroscopy results.After 120 min,the adsorption efficiency of CP 1 was 69.6%for MB,72.1%for GV,and 75.0%for NR,respectively.These results indicated that CP 1 can be used as adsorbent of MB,GV,and NR.
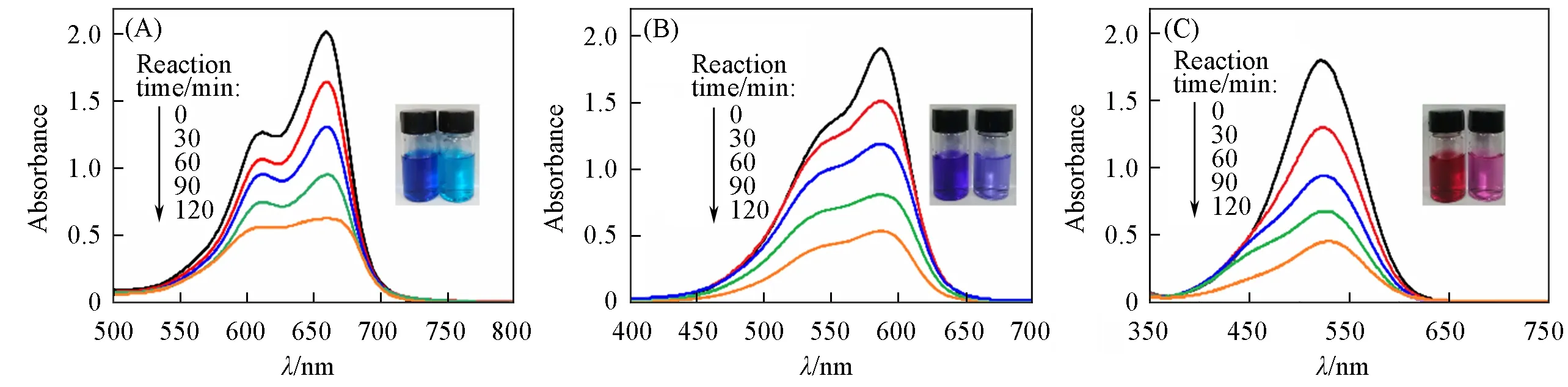
Fig.4 UV⁃Vis spectra at different time in the adsorption experiments and the corresponding adsorption pictures of MB(A),GV(B)and NR(C)
4 Conclusions
In summary,a new 2D porous Zn coordination polymer(CP 1)with excellent luminescence sensing properties,amperometric sensing properties,and dye adsorption properties was obtained under hydrothermal condition.CP 1 displays a distinguished luminescence turning on effect toward Asp,exhibiting high sensitivity,selectivity and reusability.In addition,CP 1 is a high efficiency amperometric sensor to probe AA.Thirdly,CP 1 displays a favorable dye adsorption performance for MB,GV,and NR as an adsorbent.Therefore,CP 1 can hold the post of a multifunctional and reliable material for detecting various substances.This work demonstrates the potential of Zn-CP in practical applications and has important significance for environmental protection.
The supporting information of this paper see http://www.jlu.edu.cn/CN/10.7503/cjcu20210585.

