Recent progress in understanding the role of genes in the pathogenesis of cutaneous squamous cell carcinoma*
Yong He,Yilin Wu (Co-first author),Yueyue Zhang,Qun Lv,Liming Li (✉),Mingjun Jiang (✉)
1 Hospital of Dermatology,Chinese Academy of Medical Sciences and Peking Union Medical College,Nanjing 210042,China
2 Outpatient Department,Affiliated Jinling Hospital,Medical School of Nanjing University,Nanjing 210002,China
Abstract Cutaneous squamous cell carcinoma (cSCC) is the second most common skin tumor in humans.Ultraviolet (UV) radiation is an important environmental risk factor for cSCC;other risk factors include human papilloma virus (HPV) infection,chronic inflammation,and chronic wounds.A large proportion of patients present with an aggressive form of cSCC at the time of diagnosis,which is often accompanied by regional lymph node involvement and distant metastases.The long-term prognosis for these highly metastatic diseases is extremely poor,with a 10-year survival rate of less than 10%.Therefore,clarifying the pathogenesis of this tumor is of great significance and may contribute to the identification of novel biomarkers and development of new therapeutic strategies.In this review,we focus on the recent progress in genes related to the development of this tumor,intending to aid future investigations into the genetic alterations related to cSCC.
Key words:cutaneous squamous cell carcinoma (cSCC);genetics;pathogenesis;carcinogenesis
Skin cancers are classified into melanoma and nonmelanoma types.Most skin cancers are of the nonmelanoma type,which originate from epidermal keratinocytes and are further classified into cutaneous basal cell carcinoma (cBCC) and cutaneous squamous cell carcinoma (cSCC).cSCC is the second most common skin tumor in humans,adversely affecting the quality of life[1].It most frequently develops in the skin due to long-term exposure to the sun,which results in ultraviolet (UV)-induced DNA damage in the epidermal keratinocytes[2].cSCC carcinogenesis includes premalignant lesions [actinic keratosis (AK) andin situsquamous carcinoma/Bowen’s disease] and invasive and metastatic cSCCs,however,a multistep process is not always detected[3].Although multiple AKs are clearly strong risk factors of developing invasive cSCC,the rate of progression of an AK to invasive cSCC is not precisely known[4].Most patients with localized cSCC usually have an excellent outcome if the lesion is completely excised by surgery[5].However,a large number of patients have developed an aggressive form of cSCC with distant metastases by the time of diagnosis,leading to both severe morbidity and mortality rates[6].Furthermore,although radiotherapy and chemotherapy have been utilized in the treatment of advanced cSCC,their effect is very limited[7].Besides,at present,the knowledge on the molecular basis of cSCC progression from premalignant lesions to cSCCin situand eventually to invasive cSCC is limited[8].Therefore,clarifying the pathogenesis of this tumor is of great significance and may contribute to the identification of novel biomarkers and new therapeutic strategies[7].Increasing evidence has demonstrated that tumorigenesis,progression,invasion and metastasis of cSCC involve several genes such asTP53,NOTCH1/2,CDKN2A,TGFBR1,andRAS[9].Notwithstanding these advances,the genetic mechanisms of tumor development are far from clarified.With this in mind,we sought to review the latest advances regarding the role of mutated genes in carcinogenesis (Table 1).This review intends to aid future investigations into the genetic alterations related to cSCC.To simplify the analysis,the genetic alterations are presented according to different mechanisms associated with the development of cSCC.
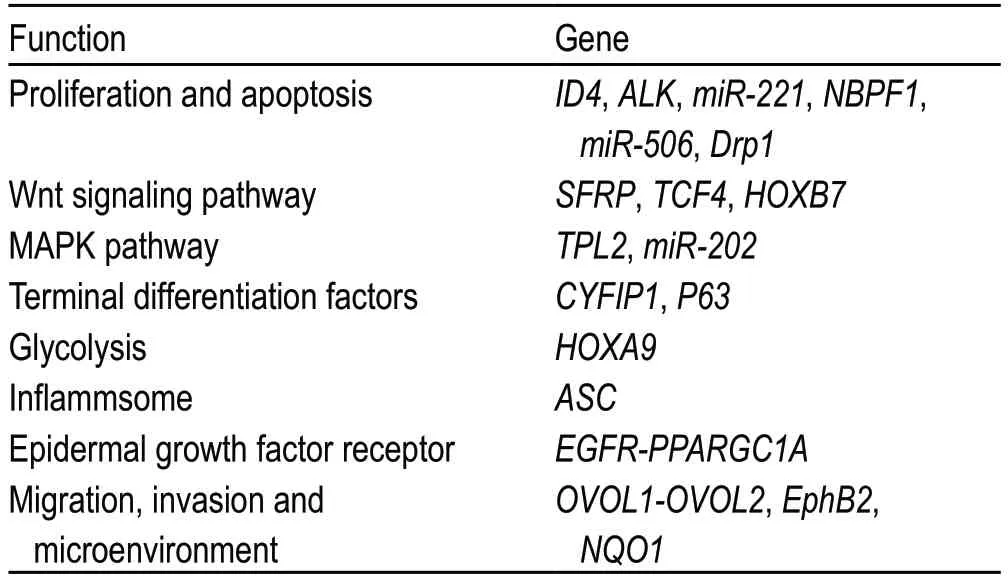
Table 1 The role of mutated genes in carcinogenesis
Proliferation and apoptosis
In previous studies,many genes have been shown to play crucial roles in regulating the proliferation and apoptosis of tumor cells.For example,p53,the gene most commonly and earliest mutated in cSCC,prevents tumor cell apoptosis and allows clonal expansion of tumor cells.TheCDKN2Agene encodes two alternatively spliced proteins,p16 and p14,which regulate cell cycle progression and proliferation through the retinoblastoma and p53 pathways,respectively[10].In recent years,more genes such asNBPF1,miR-221,andID4have been found to be involved in the development of cSCC by regulating the proliferation and apoptosis of tumor cells.
Inhibitor of DNA binding/differentiation 4 (ID4)
ID4 is a downstream mediator of the TGF-β/BMP/SMAD signaling pathway and regulates the growth and differentiation of embryonic tissues[11].Our research group found that UVB exposure could downregulate ID4 expression via DNA methylation to initiate cutaneous tumorigenesis[12].Silencing ofDNMT1and overexpression of TET1 and TET2 can increase ID4 expression,leading to reduced cell proliferation,migration,and invasion,and increased apoptosis in cSCC cell lines[12].Based on the results presented above,ID4acts as a tumor suppressor gene in cSCC carcinogenesis[12].
miR-221
miR-221is a member of themiR-221/222cluster,which is located on the X chromosome[13].It is significantly upregulated in cSCC tissues and cell lines.It can regulate several hallmarks of cSCC,including cell growth and colony formation.In addition,miR-221may act as anoncogene,and its aberrant expression may be linked to the progression of human cSCC.By targeting and repressing the expression of PTEN,miR-221 can regulate the expression of numerous genes related to cell proliferation,apoptosis,and invasion and is implicated in the progression of several tumors.These results suggest that miR-221 may be a potential target for cSCC diagnosis and treatment[14].
Anaplastic lymphoma kinase (ALK)
ALK,a receptor tyrosine kinase of the insulin receptor superfamily,plays a pivotal role in the pathogenesis of cSCC.The overexpression of the mutated ALK,ALKF1174L,is able to initiate the development of cSCC.ALKF1174Lcooperates with oncogenicKrasG12Dand loss ofp53,resulting in a more aggressive cSCC type associated with a higher histological grade.As mentioned above,inactivation ofp53induces cell cycle arrest,apoptosis,senescence,DNA repair,or changes in metabolism.As a key player in the pathogenesis of cSCC,oncogenic ALK signaling may serve as a target for future clinical trials[15].
Neuroblastoma breakpoint family member 1 (NBPF1)
NBPF1is located on chromosome 1p36 where many tumor suppressor genes have been identified.TheNBPF1gene is expressed at low levels in cSCC tissues and shows the lowest expression in the A431 cell line.In the A431 cell line,increased expression of NBPF1 leads to a significant decrease in cell viability and cell cycle arrest in the G1 phase.Meanwhile,overexpression of NBPF1 promotes apoptosis by promoting Bax and inhibiting Bcl-2 and survivin.Bax directly regulates apoptosis-related proteins and promotes apoptosis.Bcl-2 can inhibit apoptosis,and its overexpression is common in cSCC.Survivin is associated with cell viability[16-17].In addition,NBPF1can inhibit the activation of the Akt-p53-Cyclin signaling pathway.Akt regulates a variety of signaling pathways and is involved in tumor proliferation and cell apoptosis[18].Cyclin D1 and p53 are important Akt downstream factors that directly regulate the cell cycle[19].By inhibiting the phosphorylation of Akt protein,NBPF1can inhibit the activation of p53 and cyclin D1,thereby promoting apoptosis and arresting the cell cycle in the G1 phase[20].
miR-506
MicroRNAs (miRNAs) are non-coding RNAs that have a regulatory effect on protein expression at the posttranscription level[21].They are involved in the regulation of many biological processes such as proliferation,differentiation,migration,and invasion of cells.miR-506 expression is upregulated in cSCC tissues.Decreased miR-506 levels result in decreased proliferation of cSCC cells.Furthermore,miR-506 inhibition can also induce apoptosis and autophagy in cSCC cells.In addition,miR-506 decreases cSCC cell migration and invasionin vitro.miR-506functions as an oncogene in cSCC by targetingp65andLAMC1genes.P65 is a member of the NF-κB family,which can regulate many genes associated with apoptosis,proliferation,and differentiation of cells.The silencing ofmiR-506increases p65 expression,and consequently increases cellular apoptosis and impairs cell viability.LAMC1,an oncogene that belongs to the laminin family,is associated with the metastasis,signaling,differentiation,and adhesion of tumor cells.Silencing ofLAMC1,which can be directly targeted by miR-506 in cSCC cells,restores the migration and invasion properties of cSCC cells.Thus,it plays an important role in the activation and progression of cSCC.In conclusion,reduced miR-506 expression is highly associated with impaired tumor cell growth.Therefore,miR-506 can be further developed as a diagnostic and prognostic biomarker for cSCC[22].
Dynamin-related protein 1 (Drp1)
Drp1,a cytosolic protein,can mediate mitochondrial fission[23]and is involved in the process of cell proliferation or cell remodeling that facilitates the development of malignant neoplasms.Drp1 is more highly expressed in SCC than in the normal epidermis.Drp1 knockdown causes ATM-dependent G2/M cell cycle arrest and apoptosis.Morphologically,the depletion of Drp1 results in an elongated,hyper-fused mitochondrial network[24].Disrupted mitochondrial networks promote cell cycle arrest and apoptosis.In addition,Drp1 can also be regarded as a prognostic factor in several malignancies,and the expression levels of Drp1 positively correlate with advanced clinical stages.In conclusion,Drp1 plays an important role in cell proliferation,apoptosis,and cell cycle in cSCC and may serve as a novel target for skin tumor therapies[25].
Wnt signaling pathway
The Wnt/β-catenin signaling pathway is involved in cell growth,and its inhibition may lead to abnormal cell growth and differentiation.The abnormal expression of the Wnt pathway activates growth-and mitosis-related genes such asc-mycandcyclin D1,thus leading to the proliferation of tumor cells.
Secreted frizzled-related protein (SFRP)
SFRPs have been identified as negative regulators of Wnt signaling and play an important role in oncogenic activation of the Wnt pathway and tumor progression.
In hepatocellular carcinoma,SFRP1 can attenuate Wnt signaling,decrease the abnormal accumulation of β-catenin in the nucleus,and suppress cell growth.However,the precise role of the Wnt pathway in cSCC is unclear.Moreover,SFRP promoter hypermethylation has been identified in human cancers.Hypermethylation of these SFRPs,particularly SFRP1,is associated with the development of cSCC.The SFRP1 CpG site can be a possible biomarker of cSCC[26].Besides,SFRP1 loss results in early tumor initiation and cancer stem cell regulation.In anin vivomouse skin carcinogenesis model of multiple human epithelial cancers,SFRP1 downregulation was found to be associated with poor survival[27].Nevertheless,further studies are necessary to clarify the roles of SFRPs in Wnt signaling and tumor pathogenesis.
T-cell factor 4 (TCF4)
TCF4 is a high-mobility group (HMG) box-containing transcription factor that activates the Wnt/β-catenin signaling pathway in many cancers.Silencing of TCF4 can effectively inhibit tumor cell growth and invasion,indicating that TCF4 plays an oncogenic role in carcinogenesis and the development of cSCC.It may also be a novel therapeutic target for cancer treatment.In addition,TCF4 knockdown can also regulate the MAPK,insulin,and Rap1 signaling pathways.The MAPK pathway could antagonize the activity of Wnt/β-catenin,whereas insulin and Rap 1 can affect downstream targets of the Wnt/β-catenin pathway.Additionally,in cSCC cells,aberrant activation of TCF4 may result in a Wnt/βcatenin-independent regulation of gene transcription.In conclusion,TCF4 plays an important role in the development of cSCC via activation of different signaling pathways and may be a new therapeutic target for cSCC[28].
Homeobox B7 (HOXB7)
HOXB7gene,a member of the HOX family,serves as a transcriptional factor that regulates cell viability,growth,morphogenesis,and differentiation.Overexpression of HOXB7 is common in various cancers and is associated with tumorigenesis and tumor proliferation.Cancer patients with a higher expression of HOXB7 are more susceptible to distant metastasis and have a lower survival rate.The knockdown ofHOXB7can inhibit the expression of Wnt/β-catenin signaling pathway-related downstream genes,includingc-myc,cyclin D1,andLEF1.Through inactivation of the Wnt/β-catenin signaling pathway,silencing ofHOXB7can promote cell apoptosis and suppress cell migration and invasion in cSCC.Further studies are needed to assess whether HOXB7 can serve as a therapeutic target and prognostic biomarker[6].
MAPK pathway
The MAPK/ERK pathway is the most important cell survival pathway in non-tumorigenic keratinocytes and is triggered by EGF.Negative MAPK regulation and EGFRinduced STAT3 activation can increase the expression of anti-apoptotic molecules and thus lead to malignant progression of keratinocytes towards cSCC[1].
Tumor progression locus 2 (TPL2)
TPL2 is a serine/threonine MAP kinase kinase kinase 8 (MAP3K8) that regulates various signaling pathways associated with inflammation and cell growth.TPL2 overexpression has been found in cutaneous metastatic SCC and plays an important role in the tumorigenesis of cSCC.The overexpression of TPL2 in immortalized human keratinocytes promotes cell proliferation,inhibits apoptotic cell death,and induces cell transformation by activating its downstream signaling pathways,MEK/ERK MAPK,mTOR,NF-κB,and p38 MAPK.Rapamycin,an mTOR inhibitor,is routinely used for the treatment of SCC.In addition,TPL2 overexpression is necessary for maintaining the iTPL2 TG-driven SCC.The data presented above show that TPL2 is an oncogenic driver in cSCC,and further studies are needed to assess its potential as a new therapeutic target for cSCC treatment[29].
miR-204
miR-204 is an intronic miRNA located at theTRPM3gene,and its aberrant expression can affect several biological processes,including proliferation,apoptosis,and invasiveness.cSCC shows low expression of miR-204 compared to AK,a type of precancerous lesion.miR-204 may act as a“rheostat”that controls signaling towards the MAPK pathway or the STAT3 pathway in the progression from AK to cSCC.DNA methylation of the miR-204 promoter can lead to miR-204 silencing,which results in STAT3 activation and translocation to the nucleus.miR-204 activates the MAPK pathway,which is the most important cell survival pathway in non-tumorigenic keratinocytes.Both the MAPK and STAT3 pathways can drive the expression of multiple anti-apoptotic molecules and transform AK to cSCC[30].
Terminal differentiation factors
Previous studies have shown that NOTCH is involved in terminal differentiation of cSCC.Notch signaling has been associated with cellular development,progression,and differentiation[10].Besides,CYFIP1 and p63 can also regulate differentiation through different mechanisms.
CYFIP1
CYFIP1 functions as a novel invasion inhibitor in a variety of epithelial cancers.It is downregulated in cSCC at both mRNA and protein levels and is associated with differentiation and metastatic properties of tumors.CYFIP1 is a direct Notch1 target in keratinocytes.Notch signaling plays an important role in cell fate determination,and it induces differentiation and suppresses development of cSCC[27].Moreover,Notch activation is involved in the control of the cell cycle of keratinocytes via p21WAF1/Cip1.NOTCH 1 can also function as a promoter of differentiation and an inhibitor of invasion by inducing CYFIP1 expression.CYFIP1 may be a link between the loss of differentiation and invasive properties of cSCC[31].
P63
P63gene is a member of the p53/p63/p73 family of transcription factors and plays a critical role in the development and homeostasis of squamous epithelium,such as the epidermis.Dysregulated expression of p63 has been found in many squamous cancers and may contribute to cancer development through disruption of many cellular processes.ΔNP63α,the predominant p63 isoform in stratified squamous epithelium,is a key regulator of epidermal morphogenesis and epithelial tissue homeostasis.It influences keratinocyte lineage commitment,proliferation,and survival and blocks terminal differentiation,apoptosis,and senescence;additionally,it modulates the tissue microenvironment through remodeling of the extracellular matrix and vasculature and potentially influences the tumor immune microenvironment[32].Besides,p63 may be a strong predictor of poor differentiation in non-melanoma skin cancer[33].The clarification of the molecular mechanism of p63 holds promise for novel interventions in cancer prevention and treatment.
Glycolysis -HOXA9
HOXA9,a direct target of onco-miR-365,functions as a tumor suppressor in cSCC and is significantly downregulated in cSCC.The hypoxia-inducible factor (HIF)-1 pathway is involved in cancer-related biological processes,including hypoxic response,angiogenesis,glycolysis,and proliferation of cSCC stem-like cells.Loss of HOXA9 upregulates HIF-1α and the downstream glycolytic genes of the HIF-1 pathway,which contributes to glycolytic reprogramming,a key pro-survival mechanism of cancer that helps tumor cells to meet their oxygen demand.Besides,HOXA9 interacts with CRIP2 and epigenetically represses HIF-1α expression and inhibits the expression of glycolytic genes,such asHK2,GLUT1,andPDK1,which is critical for the inhibition of tumor cell growth.Future studies should focus on the newly identified miR-365-HOXA9-HIF-1α axis that may provide novel intervention targets for cSCC therapy[34].
Inflammasome -Apoptosis-associated speck-like protein (ASC)
The inflammasome adaptor ASC is essential for the secretion of pro-tumorigenic innate cytokines.ASC not only regulates caspase-1 activation and IL-1 expression but also controls cell proliferation in cSCC.ASCfunctions as a tumor suppressor gene,and downregulation of ASC expression by aberrant methylation has been found in numerous cancers.In addition,ASC might regulate the epithelial-mesenchymal transition (EMT)-like dedifferentiation of keratinocytes through activation of p53.Moreover,ASC expression does not correlate with metastatic potential but with the degree of dedifferentiation and can serve as an indicator for highly differentiated tumors.ASC is silenced in cSCC by promoter-specific methylation and impairs inflammasome function.This could be of therapeutic relevance as some treatment options for early skin cancers demand immune system activation[35].
Epidermal growth factor receptor (EGFR) -EGFR-PPARGC1A
Wild-type full-length EGFR is a transmembrane glycoprotein that binds EGF.EGFR activation or overexpression leads to upregulation of both MAPK and PI3K signaling pathways and is involved in the proliferation and pathogenesis of SCC,including cSCC.EGFR-PPARGC1A may induce tumor formation via phosphorylation,probably through conformational changes or through interaction with wild-type endogenous EGFR.EGFR-PPARGC1Ais a fusion gene that is associated with chronic sun exposure.Detection of EGFR-PPARGC1A by RT-PCR may be useful for the early diagnosis of cSCC,because this fusion can be detectedin situ.EGFR inhibitors (erlotinib and getinib) and EGFR antibodies (cetuximab and panitumumab) are widely used for lung SCC[36],and cetuximab has been reported to have therapeutic effects against cSCC.Further studies are needed to explore how the fusion geneEGFRPPARGC1Aregulates tumor formation in cSCC,which may lead to a better understanding of the pathogenesis of cSCC and the development of EGFR-targeted cancer therapies[7].
Migration,invasion,and microenvironment
Metastasis begins with the invasion of tumor cells into the stroma and migration toward the bloodstream.Multiple genes are involved in the regulation of tumor cell migration and invasion through different signaling pathways.
OVOL1-OVOL2
OVOL1andOVOL2are ubiquitous and conserved genes that encode C2H2 zinc-finger transcription factors in mammals.OVOL1andOVOL2act as guardians against epithelial-to-mesenchymal transition (EMT)[37]and govern the development,maintenance,and proliferation of epithelial cells via the Wnt signaling pathway.OVOL1,an upstream suppressor of c-myc in squamous cell carcinoma cells,is markedly downregulated in cSCC,and the downregulation of OVOL1 may be responsible for the aberrant expression of c-myc and is related to poor tumor prognosis.OVOL2 is typically expressed in the cytoplasm,but only sporadically in the nucleus.OVOL2 negatively affects the EMT process,and the downregulation of OVOL2 activity in SCC might be involved in the invasiveness of this tumor.OVOL1 can suppress OVOL2 expression,and the OVOL1-OVOL2 axis coordinately regulates the EMT transition process and invasiveness of cSCC[38].
EPHB2
Erythropoietin-producing hepatocellular (Eph) receptors and their ligand ephrins are membrane-bound molecules that are highly expressed in cSCC.EPHB2 functions as a biomarker for cSCC and plays an important role in the early stages of tumor progression to invasive cSCC.EPHB2knockdown suppresses the expression of genes involved in cell viability,migration,and invasion.Among the genes most downregulated byEphB2knockdown areMMP1andMMP13,two important proteinases that promote cSCC cell invasion[8].Besides,silencing ofEPHB2induces EMT-like morphological changes,which reduce cell-cell adhesion and expression of E-cadherin on the cell surface.EPHB2 plays a crucial role in promoting anchorage-independent cell growth through the suppression of EMT[39].Therefore,EphB2 may serve as an effective therapeutic target in this invasive skin cancer.
NAD(P)H dehydrogenase 1 (NQO1)
NQO1 is a ubiquitous flavoenzyme that plays a role in the mechanism of cellular defense against oxidative stress.Knockdown ofNQO1promotes colony forming activity and the proliferation,invasion,and migration of SCC cells,which may promote cancer development.By contrast,the overexpression of NQO1 can suppress the cell proliferation and colony forming activity.Besides,the expression of NQO1 can regulate the levels of phosphorylated AKT,JNK,and p38 MAPK.This may be one possible mechanism underlying the suppressive function ofNQO1.Further studies are needed to clarify the precise link between NQO1 and intracellular signaling pathways[40].
Conclusion and future perspectives
In this review,we summarized the latest advances in genes involved in the pathogenesis of cSCC and analyze their role in the development of this cancer.As mentioned before,these genes can regulate many biological processes,such as proliferation,apoptosis,terminal differentiation,glycolysis,and many signaling pathways.Specifically,HOXB7,TCF4,andSFRPcan target the Wnt pathway.TPL2and many other genes participate in the MAPK and PI3K/Akt/mTOR pathways (Fig.1).Among these pathways,we identified three pathways that deserve further investigation.The first is the Wnt pathway.It has been long investigated but the mechanism was still not fully elucidated.Recently,our research group (unpublished data) specifically focused on the the Wnt/calcineurin/NFAT pathway,which functions in keratinocyte differentiation,migration,and DNA repair.Furthermore,dysregulation of this signaling pathway contributes to squamous cell carcinoma formation,abnormal growth,and tumorigenic microenvironment.Our research group found that Wnt5a,FZD4,PLC,and NFATc4 are downregulated in cancer tissue.Wnt5a/Ca2+suppresses the development of cSCC,and FZD4 and NFATc4 interact with each other.However,further research is needed to clarify the specific mechanisms.The second pathway is the p63 pathway.P63 can directly target gene transcription and function as a key driver of critical networks linked to cellular identity and cell fate determination.Besides influencing keratinocyte lineage commitment,proliferation,and survival,p63 can modulate the tissue microenvironment and regulate the immune system.As p63 is involved in these coordinated pathways and plays an important role in cSCC development;it may serve as a promising target for cancer treatment.The last pathway involves Drp1,which regulates mitochondrial fission and plays an important role in cell proliferation,apoptosis,and cell cycle in cSCC.Therefore,this pathway may serve as a novel target for skin tumor therapies.

Fig.1 The figure depicts all the genes that are reviewed in this review.The arrows indicate facilitation of the pathway,and the black T-shaped line indicates inhibition of the pathway.Our research group mainly studied the pathway marked in blue
Many new therapeutics targeting these specific pathways are available.For example,cetuximab,an EGFR inhibitor,is administered to patients with cSCC.Patients with locally advanced SCC show good responses to cetuximab.However,it is ineffective in treating distant metastatic diseases.In addition,EGFR inhibitors are used in advanced cSCCs as a second-line treatment after chemotherapy failure and disease progression[10].The MEK inhibitor,PD325901,can inhibit cell proliferation,as well as the phosphorylation of ERK1/2 and Drp1[41].Good clinical results have been achieved with PD1-inhibitors in the treatment of cSCC,and Cemiplimab is currently the only immune checkpoint inhibitor approved by the FDA and EMA to treat patients with locally advanced or metastatic cSCC.Although many new drugs with various molecular targets have been developed and significant improvements in surgery,chemotherapy,and radiotherapy have been achieved,overall survival of patients with advanced cSCC has not markedly improved[36].Thus,further studies for a comprehensive understanding of the molecular basis of cSCC are of outstanding importance,especially for patients with metastatic disease in which prognosis is poor and effective therapies are lacking.Considering the complex molecular network,combined therapies targeting different genetic alterations and signaling pathways might provide more effective and personalized therapies for patients with cSCC.More accurate prognostic biomarkers make early intervention possible.In the next few years,scientists will be able to develop effective drugs or prognostic biomarkers that target these genetic alterations and improve the survival rate of patients with cSCC.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年6期
Oncology and Translational Medicine2021年6期
- Oncology and Translational Medicine的其它文章
- Autophagy-related lncRNA and its related mechanism in colon adenocarcinoma
- Effect of UBR5 on the tumor microenvironment and its related mechanisms in cancer*
- GFPT2 pan-cancer analysis and its prognostic and tumor microenvironment associations*
- Malnutrition as a predictor of prolonged length of hospital stay in patients with gynecologic malignancy:A comparative analysis*
- Effect of radiotherapy on tumor markers and serum immune-associated cells in patients with esophageal cancer*
- Correlation analysis of breast fibroadenoma and the intestinal flora based on 16S rRNA sequencing*
