五条早期生长发育特征及胚胎发育的温度适应特性*
方 璐 徐永江 柳学周 崔爱君 王开杰 王 滨 姜 燕 李文升
方 璐1,2徐永江1①柳学周1崔爱君1王开杰1王 滨1姜 燕1李文升3
(1. 中国水产科学研究院黄海水产研究所 农业农村部海洋渔业可持续发展重点实验室 青岛海洋科学与技术试点国家实验室深蓝渔业联合实验室 山东 青岛 266071; 2. 上海海洋大学水产与生命学院 上海 201306;3. 莱州明波水产有限公司 山东 烟台 261400)



1 材料与方法
1.1 亲鱼培育和受精卵获取

1.2 胚胎发育观察
每天检测亲鱼产卵情况,当发现亲鱼在培育池内产卵时,立即取样观察。收集受精卵置于方形白色PVC材质的塑料箱(容积为60 L)内,连续充气,流水孵化,控制水温为22℃~23℃。孵化期间,胚胎发育至原肠期前每15~20 min取样1次,胚体形成后每20~ 40 min取样1次。利用NIKON(MSZ800,日本)解剖镜进行胚胎发育观察,记录胚胎发育时序及其形态特征,并使用NIKON (Coolpix 4500,日本)数码相机进行显微拍照。
1.3 仔稚幼鱼生长发育观察
仔鱼孵化出膜后,利用室内圆形水泥池(容积为7 m3)开展苗种培育,初孵仔鱼布池密度为10,000尾/m2。培育条件:水温为22℃~24℃,盐度为27~30,pH为7.6~8.0,光照周期为12L : 12D。饵料系列:褶皱臂尾轮虫()→卤虫无节幼体()→配合饲料。苗种胚后发育时间以日龄(day after hetching, DAH)计算,初孵仔鱼为0日龄,以此类推。3~21 DAH仔鱼投喂褶皱臂尾轮虫,日投喂2次,投喂密度为8~10 ind./mL;投喂轮虫时添加小球藻(),密度为5×105cell/mL。20 DAH开始增加投喂卤虫无节幼体,密度为1~2 ind./mL,日投喂2次;30 DAH开始投喂配合饲料,进行配合转化;40 DAH后转换投喂配合饲料。自4 DAH开始换水,换水率逐渐达100%~200%,定期清理培育池底部,保持水质清洁。
自仔鱼布池日起15 DAH前,每天从育苗池中随机取样40~60尾;15 DAH后每3 d随机取样30~40尾;30 DAH后,每5 d随机取样20~30尾。实验之前,先使用MS-222对仔鱼进行麻醉处理,然后在NIKON (MSZ800,日本)解剖镜下对仔鱼不同时期的形态特征、色素变化、器官发育、摄食情况进行观察并拍照记录,测量仔鱼的体长、眼径、油球径等。另外,使用固定液固定各日龄标本10~20尾,以备实验室补充观察使用。
1.4 温度对胚胎孵化的影响
温度梯度设置:20℃、22℃、24℃、26℃、28℃共5组,每组重复2次。实验容器为8个白色塑料桶(容积为100 L),使用300 W电热棒带控温仪控制温度(精确度为±0.5℃)作为恒温水浴。将实验烧杯(2 000 mL)加水放入,将水温调控到各自的温度组,每个温度组放置3个烧杯。发现产卵时,从采卵网箱中取受精卵,挑选发育至多细胞的卵,计数120粒分别放入每个烧杯中,微充气,定时观察并记录胚胎发育进程。每天将每个烧杯中的死卵计数后去除,并换水30%~50%左右。受精卵孵出后,统计孵化率、畸形率,确定适宜孵化水温。
1.5 数据统计分析
仔稚幼鱼生长数据、受精卵孵化率和畸形率数据采用平均值±标准差(Mean±SD)表示,使用SPSS 24.0软件进行单因素方差(one-way, ANOVA)分析方法,对受精卵孵化率、畸形率进行显著性分析,差异显著性水平设定为0.05,当<0.05时为差异显著。
2 结果
2.1 胚胎发育特征
2.1.1 卵裂前期 未受精成熟卵子原生质表层分布着复杂的网纹结构。受精后,胚盘形成,卵周隙扩大(图1A)。
2.1.2 卵裂期 受精后2 h 10 min,受精卵胚盘发生经裂。胎盘发生3次经裂,将胚盘分成8个大小均等的细胞,进入8细胞时期。每1次经裂的卵裂沟均与前1次卵裂沟垂直,分裂球等大;受精后3 h发生第5次经裂,进入32细胞时期,卵裂大小不一;受精后3 h 30 min,第1次纬裂发生,胚盘分化成排列不均的2层细胞,进入64细胞期;之后细胞不断分裂,在动物极处排成多层;至受精后5 h 15 min,胚盘动物极一侧形成表面粗糙的高帽状细胞群,进入桑葚期(图1B~图1H)。
2.1.3 囊胚期 受精后6 h 5 min,高帽状细胞群表面由粗糙变得光滑,细胞继续分裂增多,高帽状细胞群高度增加,形成高囊胚;受精后10 h 20 min,高囊胚边缘开始变薄并向扁平发展,进入低囊胚期(图1I和图1J)。
2.1.4 原肠期 受精后11 h 30 min,进入原肠早期。原肠胚边缘下包,形成原肠腔,胚盾逐渐明显;之后经历原肠中期、原肠晚期,至受精后17 h 30 min,原肠腔壁加厚,原口即将关闭,出现胚体雏形(图1K~图1N)。
2.1.5 神经胚期 受精后18 h 50 min,原口完全关闭,进入神经胚期。此时,神经胚两侧加厚隆起向内卷曲后在中间结合,形成神经脊。胚体头部呈椭圆形,胚体体节为5~6对,有棕黄色点状色素分布,出现克氏泡(图1O)。

表1 五条胚胎发育时序(22.0℃±0.5℃)
2.1.6 器官发生期 受精后27 h 40 min,胚体包卵黄囊1/2,胚体头部和尾部明显,体节为9~12对,心脏原基形成;受精后28 h 40 min,胚体包卵黄囊2/3,形成尾芽,出现心跳活动(频率为58~79次/min),胚体两侧均匀分布零散点状黑色素,脑分化为3部分,形成晶体,体节为20~24对,胚体偶尔出现间歇性收缩(频率为1~3次/min)(图1P和图1Q)。
2.1.7 肌肉效应期 受精后30 h 25 min,胚体包卵黄囊4/5,胚体两侧分布的黑色素明显增多,形成黑色素条带,尾部较头部密集;受精后32 h,胚体完全包被卵黄囊,头部和卵黄囊色素明显增多,头部分化为5部分,尾部扭转明显,胚体间歇性收缩(肌肉效应)明显,收缩频率为12~18次/min(图1R和图1S)。
2.1.8 孵化期 受精后35 h 15 min,胚体肌肉效应幅度和频率增大(15~23次/min),卵黄囊缩小,胚体按先尾部后头部的顺序,将卵膜顶破,脱膜而出(图1T)。
2.2 仔稚幼鱼生长发育特征
2.2.1 卵黄囊仔鱼期 初孵仔鱼(0 DAH):全长为(4.03±0.27) mm,卵黄囊较大,呈椭圆形,长径为(1.53±0.13) mm,短径为(0.81±0.07) mm,油球径为(0.48±0.04) mm。头长占全长的10.9%,肛前距占全长的57.2%,眼径为头长的52.2%。初孵仔鱼刚脱膜时,身体弯曲,躯体前半部分伏在卵黄囊上,腹部朝上或横卧在水中,呈静止状态。背鳍膜和尾鳍膜之间存在明显的分界线,背鳍膜和臀鳍膜边缘分布棕黄色点状色素带,躯干、头部、油球上散布着枝状黑色素。肠道为细线状,紧贴躯干腹部(图2A)。
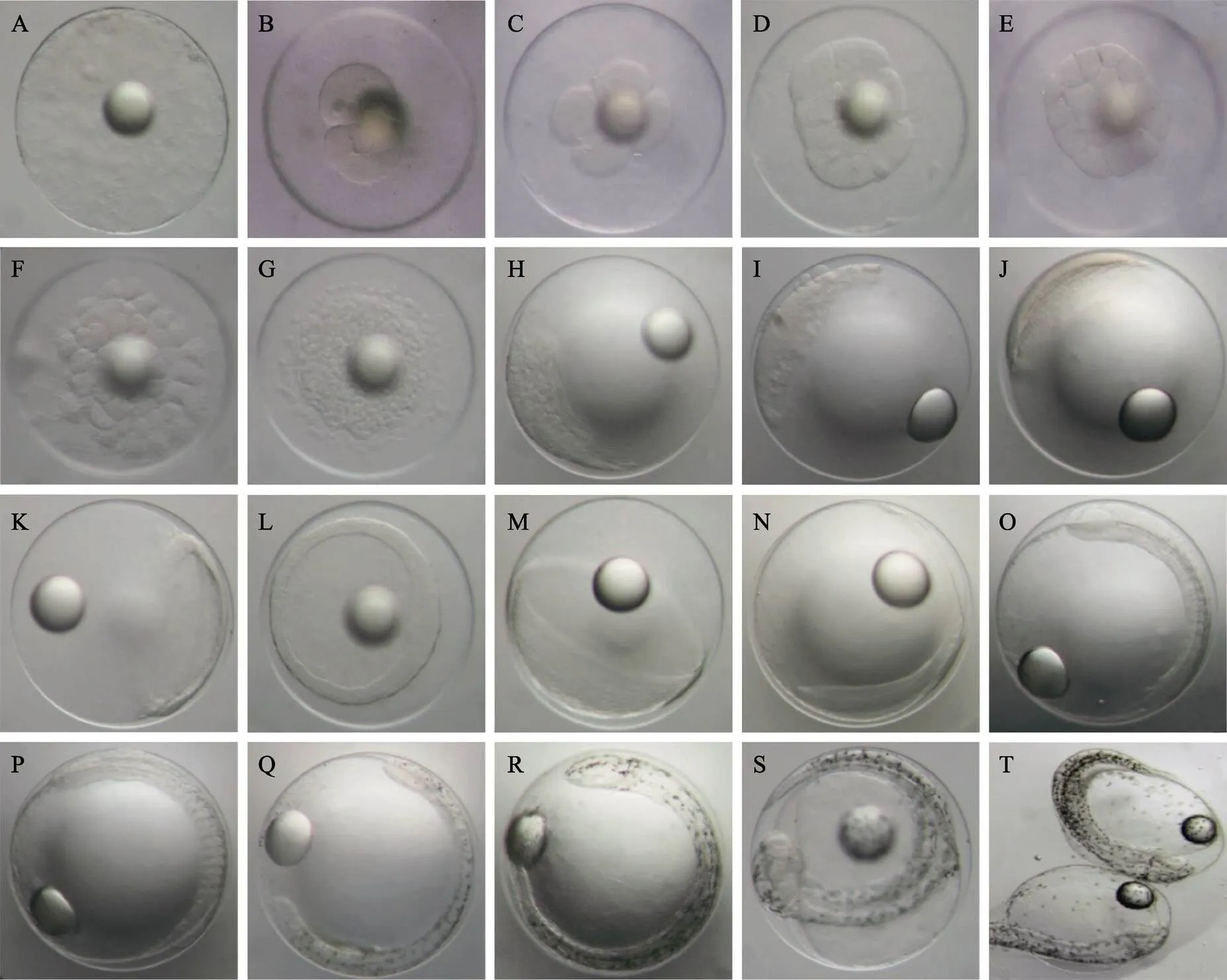
图1 五条胚胎发育形态特征
1 DAH仔鱼:全长为(4.21±0.17) mm,卵黄囊体积减少约55%,油球径为(0.44±0.06) mm。头长占全长的11.5%,肛前距占全长的57.4%,眼径为头长的54.5%。仔鱼身体完全展开,在水体中静止倒立悬浮,活动能力较弱。心脏呈L型,心跳为150~180次/min。背鳍膜和臀鳍膜边缘分布的色素细胞密度增加,呈较为明显的色素带;躯干两侧色素分布密集,尾部近乎透明。肠道尚未充气(图2B)。
2 DAH仔鱼:全长为(4.43±0.13) mm,卵黄囊体积减少约75%,油球径为(0.40±0.04) mm。头长占全长的11.6%,肛前距占全长的57.5%,眼径为头长的55.4%。仔鱼开始平游,靠尾部摆动可变换水层,且瞬时游泳速度快。在水体中分布较为均匀,分布密度水体上层较下层大。背鳍膜和臀鳍膜增高,色素带密度增加;眼囊开始分布淡黄色色素;尾鳍膜仍为透明,呈扇形。肠道加粗,中间腔扩大(图2C)。
3 DAH仔鱼:全长为(4.51±0.33) mm,卵黄囊体积减少约90%,油球径为(0.21±0.05) mm。头长占全长的15.1%,肛前距占全长的58.5%,眼径为头长的48.5%。仔鱼多静止在水体中。鳔原基形成,未充气。躯干上、下两侧枝状黑色素增多,尾部有少量点状色素,背鳍膜升高,棕黄色色素减少,胸鳍呈小叶状,活动能力不强。眼囊因黑色素分布密集而呈黑色。心脏有血液流动。仔鱼开口,下颌骨明显,膀胱腔扩大,肠道出现褶皱(图2D)。
6 DAH仔鱼:全长为(4.93±0.17) mm,卵黄囊消耗殆尽,油球仍有残余。头长占全长的23.2%,肛前距占全长的57.8%,眼径为头长的38.9%。仔鱼游泳能力提高,能躲避障碍物,主要分布在水体中、上层,有趋光性。鳔开始充气。躯干上、下两侧和眼囊黑色素分布密集,躯干中部为棕黄色,通体呈黄褐色(图2E)。
2.2.2 脊索弯曲前仔鱼期 10 DAH仔鱼:全长为(5.21±0.23) mm,油球消耗完毕。头长占全长的25.7%,肛前距占全长的59.3%,眼径为头长的32.5%。躯干黑色素分布密度加大,形成黑色色斑;两侧胸鳍分成明显的两部分,上半部为黑,下半部为棕黄。肩带骨凸显。胸鳍明显增长。鳔充气呈椭圆形。消化系统进一步发育,肠道褶皱增多(图2F)。
2.2.3 脊索弯曲仔鱼期 15 DAH仔鱼:全长为(6.24±0.66) mm。头长占全长的27.6%,肛前距占全长的66.3%,眼径为头长的35.1%。躯干左右两侧明显加宽。除头部下半部分和尾部,全身分布黑色和棕黄色色斑,通体为深褐色。仔鱼摄食轮虫良好,摄食率(feeding rate, FT, %)(摄食率=摄食仔鱼数/仔鱼总数×100%)达90%以上,肠道前端和胃部后方形成第1个生理弯曲。仔鱼脊索末端开始向上弯曲,下方尾扇形成,鳍条为12根,上有少量棕黄色点状色素,背鳍膜明显后移(图2G)。
20 DAH仔鱼:全长为(7.55±1.12) mm。头长占全长的28.7%,肛前距占全长的60.5%,眼径为头长的41.6%。除尾部和眼囊,全身密集分布黑色色素,通体呈淡黑色,眼囊变成深蓝色。脊索末端弯曲完成。背鳍鳍条16根,臀鳍鳍条8根,覆盖着棕黄色色素。尾鳍扇形面积增大,鳍条17根,仍为透明。仔鱼开始摄食卤虫无节幼体,肠道形成2个生理弯曲(图2H)。
2.2.4 脊索弯曲后稚鱼期 25 DAH稚鱼:全长为(10.25±1.35) mm。头长占全长的28.8%,肛前距占全长的59.5%,眼径为头长的33.1%。脊索末端演化成尾鳍条,尾鳍条17根,背鳍条23根,臀鳍条13根。通体除尾鳍外呈黄绿色。鱼体能顺流游动,有集群现象,分布在水体中、上层。个体生长差异加剧。苗种摄食卤虫无节幼体良好(图2I)。
30 DAH稚鱼:全长为(16.23±1.61) mm。头长占全长的24.7%,肛前距占全长的55.6%,眼径为头长的34.9%。尾鳍分叉明显,鳍条19根,尾鳍始端有黑色色素斑点。背鳍鳍条30根,臀鳍条21根,腹鳍条5根,胸鳍条12根。通体呈黑金色,眼囊为金黄色,体表开始形成色素带。可逆流游动。全部摄食卤虫无节幼体,开始进行配合饲料诱导。出现攻击与残食行为(图2J)。
35 DAH稚鱼:全长为(22.45±1.42) mm。头长占全长的26.6%,肛前距占全长的53.6%,眼径为头长的33.8%。鳍条数量与成鱼一样。鱼苗呈梭形,通体呈金黄色。生长速度加快,个体差异加大,残食现象严重,个体大的鱼苗撕咬吞食个体小的鱼苗,造成鱼苗死亡率增高(图2K)。
40 DAH稚鱼:全长为(28.07±2.32) mm。头长占全长的27.8%,肛前距占全长的54.3%,眼径为头长的32.3%。鱼体体色发生巨大变化,开始生成鳞片,通体呈黑金色,光照下躯干部为银白色,尾鳍上、下端出现褐色色斑。体表形成3~4条纵向的色素带。鱼苗在水中集群快速游动,遇有外部刺激快速散开逃逸(图2L)。
2.2.5 幼鱼期 50 DAH幼鱼:全长为(45.75±4.03) mm。头长占全长的28.9%,肛前距占全长的53.64%,眼径为头长的30.1%。色素带颜色加深,背部为黑褐色,腹部为棕黄色,背鳍、尾鳍边缘、臀鳍边缘为棕黄色,眼囊为棕黄色。纵向色素带增加至5~6条(图2M)。
65 DAH幼鱼:全长为(81.49±5.11) mm。头长占全长的25.9%,肛前距占全长的49.2%,眼径为头长的37.7%。体色再次发生变化,背部为青色,腹部为银白色,背鳍、尾鳍、臀鳍为棕黄色,体表鳞片形态同成体。眼囊为黄色,纵向色素带褪去,苗体态与成体相似(图2N)。
2.3 仔稚幼鱼摄食与生长特性
仔鱼开口前,生长主要靠内源性营养;3 DAH时,仔鱼开口后转入混合营养期(图3);在4 DAH时,卵黄囊消耗96%;在6 DAH时,卵黄囊吸收完毕,仔鱼开始发育并完全依赖外源营养,生长速度加快。油球吸收速度较卵黄囊慢,至10 DAH吸收完毕。3 DAH时,仔鱼初次摄食轮虫;6 DAH仔鱼摄食率为60%~70%;20 DAH仔鱼摄食卤虫无节幼体;30 DAH仔鱼进行配合饲料转化;40 DAH仔鱼全部摄食配合饲料。
TL=0.024 32–0.390 7+5.855 7 (²=0.997 2)
式中,TL为仔鱼全长,为日龄。


图2 五条胚后发育形态特征
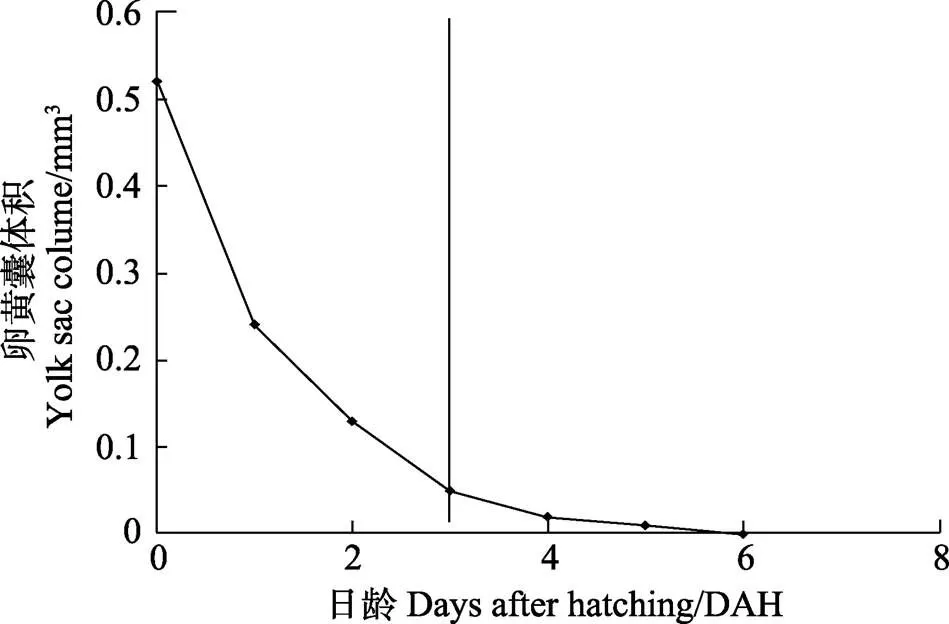
图3 五条仔鱼卵黄囊吸收过程
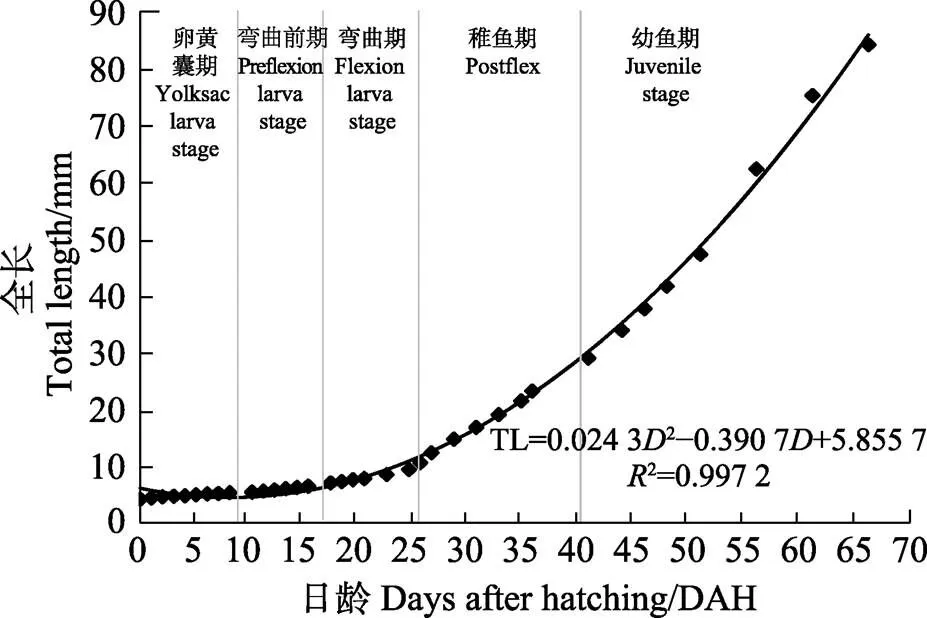
图4 五条早期生长曲线(0~65日龄)
2.4 温度对胚胎孵化的影响
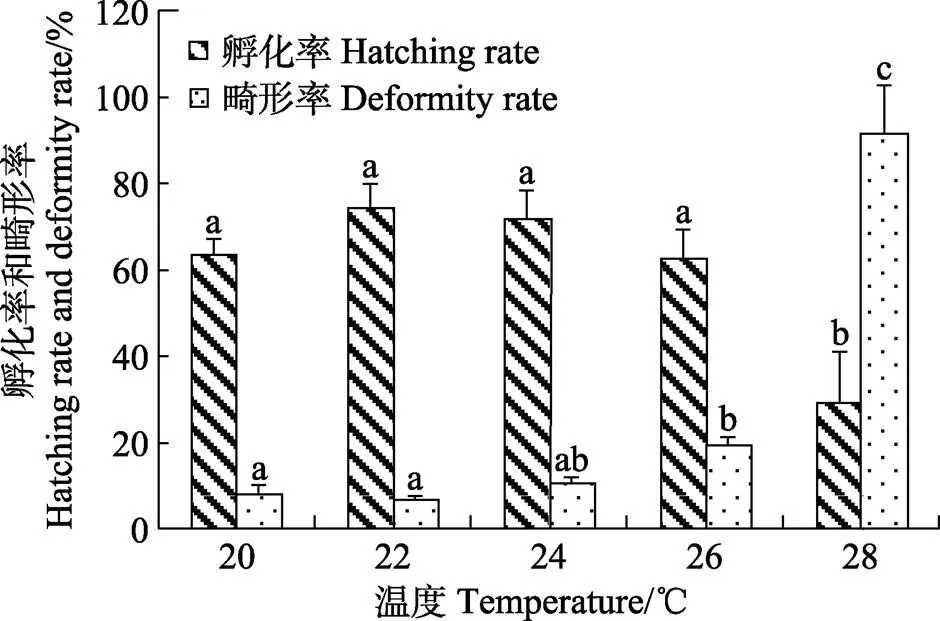
图5 温度对五条胚胎孵化和初孵仔鱼畸形的影响

3 讨论
3.1 五条胚胎发育特征
3.2 仔稚幼鱼摄食与生长

表2 温度对五条胚胎发育的影响
3.3 温度对胚胎孵化的影响


ERIKO O, TAKUYA N, YOSHITOMO N,. Genetic linkage maps of two yellowtails (and). Aquaculture, 2004, 244(1/2/3/4): 41– 48
EMMANUEL M M, KARINA G Á, JUAN P L,. Morphological development and allometric growth of yellowtail kingfishV. Larvae under culture conditions. Aquaculture Research, 2016, 47(4):1277–1287
FAN Y J, SHI Z P. Research progress and application prospects of fish hatching enzymes.Marine Limnology Bulletin, 2002(1): 48–56 [樊廷俊, 史振平. 鱼类孵化酶的研究进展及其应用前景. 海洋湖沼通报, 2002(1): 48–56]
FUKUHARA O, NAKAGAWA T, FUKUNAGA T. Larval and juvenile development of yellowtail reared in the laboratory. Nippon Suisan Gakkaishi, 1986, 52(12): 2091–2098
HIGUCHI K, YOSHIDA K, GEN K,. Effect of timing of restricted feeding on sexual maturation in female yellowtail,. Aquaculture, 2017, 479: 609–615
HUANG X K, SHAN L Z, YAN M C,. Embryonic development of yellow aquarium and its relationship with temperature and salinity. Marine science, 2017, 41(7): 44–50 [黄贤克, 单乐州, 闫茂仓, 等. 黄姑鱼胚胎发育及其与温度和盐度的关系. 海洋科学, 2017, 41(7): 44–50]
KEIICHI M, KAZUTOSHI K, TAKASHI K,. Advanced spawning in yellowtail,, by manipulations of the photoperiod and water temperature. Fisheries Science, 1998, 64(5): 727–731

LIU X Z, XU Y J, LIU X F,. Early growth and development characteristics of barfin flounder (). Oceanologia et Limnologia Sinica, 2009, 40(6): 699–706 [柳学周, 徐永江, 刘新富, 等. 条斑星鲽()的早期生长发育特征. 海洋与湖沼, 2009, 40(6): 699–706]
LIU X Z, XU Y J, MA A J,. The effect of temperature, salinity, and light on embryonic development of semi-smooth tongue sole and incubation condition regulation technology. Marine Fisheries Research, 2004, 25(6): 1–6 [柳学周, 徐永江, 马爱军, 等. 温度、盐度、光照对半滑舌鳎胚胎发育的影响及孵化条件调控技术研究. 海洋水产研究, 2004, 25(6): 1–6]
LOU Y D. Hatching enzymes of fish. Zoological Journal, 1965(3): 97–101, 123 [楼允东. 鱼类的孵化酶. 动物学杂志, 1965(3): 97–101, 123]
MEN Q W, SU J X, MIU X Z. Taxonomy of fishes. Beijing: China Agriculture Press, 1995, 672 [孟庆闻, 苏锦祥, 缪学祖. 鱼类分类学. 北京: 中国农业出版社, 1995, 672]
NGUYEN N H,WHATMORE P, MILLER A,. Quantitative genetic properties of four measures of deformity in yellowtail kingfishValenciennes, 1833. Journal of Fish Diseases, 2016, 39: 217–228
OSAMU F, TOHRU N, TATSUHIRO F. Larval and juvenile development of yellowtail reared in the laboratory. Bulletin of the Japanese Society of Scientific Fisheries, 1986, 52(12): 2091–2098
RØNNESTAD I, KOVEN W, TANDLER A,. Utilisation of yolk fuels in developing eggs and larvae of European sea bass (). Aquaculture, 1998, 162(1/2): 157–170
SANG G Y, SANG W H, JI S C,. Morphological development of embryo, larvae and juvenile in yellowtail kingfish,. Development and Reproduction, 2016, 20(2): 131–140
SHIOGAKI M, DOUTSU Y. The spawning of sea culpin,. Bulletin of Fishery of Nagasaki University, 1974, 38: 71–76
SHUKEI M, MASAEI K, KAZUHISA T. Embryonic and morphological development of larvae and juveniles of the amberjack,. Japanese Journal of Ichthyology, 1990, 37(2): 164–169
WANG H T, ZHANG P J. Effects of environmental factors on the development of marine fish fertilized eggs and early larvae. Marine Science, 1998, 22(4): 50–52 [王宏田, 张培军. 环境因子对海产鱼类受精卵及早期仔鱼发育的影响. 海洋科学, 1998, 22(4): 50–52]

XU Y J, LIU X Z, SHI B,. Domestication and early development characteristics of Pacific cod () broodstock. Fishery Science Progress, 2017, 38(1): 159–167 [徐永江, 柳学周, 史宝, 等. 太平洋鳕()亲鱼驯化培育与早期发育特征. 渔业科学进展, 2017, 38(1): 159–167]
ZHANG T T, CHEN C, SHI Z H,. Effects of temperature on embryonic development and larval vigor of Moray grouper (). Fishery Science Progress, 2016, 37(3): 28–33 [张廷廷, 陈超, 施兆鸿, 等. 温度对云纹石斑鱼()胚胎发育和仔鱼活力的影响. 渔业科学进展, 2016, 37(3): 28–33]
Early Growth and Development Characteristics ofand the Temperature Adaptation of Embryonic Development
FANG Lu1,2, XU Yongjiang1①, LIU Xuezhou1, CUI Aijun1, WANG Kaijie1, WANG Bin1, JIANG Yan1, LI Wensheng3
(1. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture and Rural Affairs, Deep Blue Fishery Joint Laboratory, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, Shandong 266071, China; 2. College of Fishers and Life Science, Shanghai Ocean University, Shanghai 201306, China; 3. Laizhou Mingbo Aquatic Co., Ltd, Yantai, Shandong 261400, China)
Yellowtail amberjack,, is a globally distributed, economically important, marine pelagic fish species belonging to the same genusasand. It is a popular table fish worldwide because of its flavorsome flesh and high nutritional value. Recently, we made a breakthrough in triggering the natural spawning ofby manipulating photothermal regimes in China, and produced over 7700 juveniles with an average total length (TL) of 147 mm in an indoor tank culture system. Meanwhile, the morphological and quantitative characteristics and the growth and development ofduring the early life history stages were observed and described for the first time. The mature eggs ofare transparent, spherical, and buoyant, with a single oil globe with a diameter of approximately 1.26~1.36 mm. The embryos hatched out at approximately 37 h 40 min post hatching at a water temperature of (22.0±0.5)℃. The embryonic development process can be divided into eight stages: pre-cleavage, cleavage, blastula, gastrula, neurula, organogenesis, muscle effect, and hatching. The morphology of embryonic development has been described previously. The TL of the newly hatched larvae was (4.03±0.27) mm with an oval yolk sac, which accounted for 3/8 of the TL. The TL of larvae 3 days after hatching (DAH) was (16.23±1.61) mm, and the mouth opened and larvae entered the mixed nutrition period. The first food was a rotifer. The TL of larvae at 6 DAH was (4.93±0.17) mm; here, the yolk sac was exhausted and the swim bladder started to inflate. The TL of larvae at 10 DAH was (5.21±0.23) mm, and the larvae entered the exogenous nutrition stage. The TL of larvae at 15 DAH was (6.24±0.66) mm, and the end of the spine began to bend. The TL of larvae at 25 DAH was (10.25±1.35) mm, and the spine bending process was completed; thereafter, the larvae began to feed on. The TL of juveniles at 30 DAH was (16.23±1.61) mm, which is when commercial feed conversion started, and the juveniles began to feed well on the commercial diet at 40 DAH when the TL reached (28.07±2.32) mm. The TL of juveniles at 65 DAH was (81.49±5.11) mm, which was when the juvenile morphology was similar to the adults. Furthermore, the suitable temperature for embryonic development was determined to be 22℃~24℃. The results provide technical support regarding artificial breeding and seedling production technology ofthat could boost the development of thefarming industry in China
; Embryonic development; Postembryonic development; Morphological characterisitcs; Growth performance; Temperature adaption
XU Yongjiang, E-mail: xuyj@ysfri.ac.cn
S936
A
2095-9869(2021)06-0194-11
10.19663/j.issn2095-9869.20210224002
http://www.yykxjz.cn/

FANG L, XU Y J, LIU X Z, CUI A J, WANG K J, WANG B, JIANG Y, LI W S. The early growth and development characteristics ofand the temperature adaptation of embryonic development. Progress in Fishery Sciences, 2021, 42(6): 194–204
徐永江,研究员,E-mail: xuyj@ysfri.ac.cn
2021-02-24,
2021-03-30
*山东省支持青岛海洋科学与技术试点国家实验室重大科技专项(2018SDKJ0303-1; 2018SDKJ0501-2)、国家重点研发计划项目(2019YFD0900901; 2018YFD0901204)、中国水产科学研究院基本科研业务费(TD47)、财政部和农业农村部:国家现代农业产业技术体系(CARS-47)共同资助 [This work was supported by the Marine Science and Technology Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018SDKJ0303-1; 2018SDKJ0501-2), National Key Research and Development Program of China (2019YFD0900901; 2018YFD0901204),Central Public-Interest Scientific Institution Basal Research Fund, CAFS (TD47), and China Agriculture Research System of MOF and MARA (CARS-47)]. 方 璐,E-mail: 342245529@qq.com
(编辑 陈 严)

