Assessment of Heavy Metals, Polycyclic Aromatic Hydrocarbons,and Perfluorinated Alkyl Substances in two Marine Crustaceans (Oratosquilla oratoria and Portunus trituberculatus) in the Zhoushan Fishing Ground of China East Sea
MEI Guangming, ZHANG Xiaojun, GU Jie, FANG Yi, and YANG Wenge
Assessment of Heavy Metals, Polycyclic Aromatic Hydrocarbons,and Perfluorinated Alkyl Substances in two Marine Crustaceans (and) in the Zhoushan Fishing Ground of China East Sea
MEI Guangming1), 2), ZHANG Xiaojun2), GU Jie2), FANG Yi2), and YANG Wenge1), *
1)College of Food and Pharmaceutical Sciences and Key Laboratory of Animal Protein Food Deep Processing Technology of Zhejiang Province, Ningbo University, Ningbo 315800, China 2) Zhejiang Marine Fisheries Research Institute, Zhoushan 316021, China
Heavy metals, polycyclic aromatic hydrocarbons (PAHs), and perfluorinated alkyl substances (PFASs) polluted in 155 samples of two marine crustaceans (andharvested in the Zhoushan fishing ground in the East China Sea were assessed. The results showed that Pb, Hg, and iAs in the whole edible parts ofandwere at trace levels, far below the limits set by the China’s national standard, whereas the exceeding standard rates of Cd in the whole edible parts ofandwere 98.6% and 75.6%, respectively. Moreover, the average Cd levels and the exceeding standard rates of Cd in the visceral tissues ofwere significantly higher than those in the muscle tissues (4.64 mgkg−1. 0.49mgkg−1and 96.7%30%, respectively). PAHs with high detection frequency in the two marine crustaceans included NA, AC, FL, and PHE. Total contents of 15 PAHs (abbreviated as ∑15PAHs) in whole edible parts ofandwere 10.75–65.9μgkg−1(mean=18.7μgkg−1) and 13.26–181μgkg−1(mean=24.2μgkg−1), and those in the muscle and the visceral tissues ofwere 9.58–160μgkg−1(mean=21.1μgkg−1) and 18.22–201μgkg−1(mean=32.7μgkg−1), respectively. Furthermore, PFASs that were found at high contents included PFOA, PFOS, and PFOSA, and total contents of 23 PFASs (∑23PFASs ) ranged 0.0162–5.104μgkg−1(mean=0.915μgkg−1).Collectively, the present work provides new data on the contamination in two marine crustaceans captured in the Zhoushan fishing ground in the China East Sea, which can be useful for making suggestions on proper consumption amounts of the two marine crustaceans.
Zhoushan fishing ground;;; heavy metal;polycyclic aromatic hydrocarbons; perfluorinated alkyl substances
1 Introduction
Trace elements can generally be classified into non- essential trace elements,.., arsenic (As), mercury (Hg), and lead (Pb), cadmium (Cd), and essential trace elements,.., iron (Fe), zinc (Zn), manganese (Mn), and chromium (Cr) (Fraga, 2005). Whereas essential trace elements form a part of enzymes involving in metabolic or biochemical processes in organisms, non-essential trace elements have no known benefits and are rather considered as contaminants as they are toxic, persistent, and non-biodegradable, and can inevitably be accumulated and biomagnified in a food chain (Nghia., 2009; Spanopoulos-Zarco., 2014). Polycyclic aromatic hydrocarbons (PAHs) are a group of organic compounds containing 2 to 7 fused aromatic rings arranged in various configurations; the compounds are originated from anthropogenic and natural sources (Kim., 2013; Purcaro., 2013; Drwal., 2019). Because of their mutagenicity, carcinogenicity, teratogenicity, and genotoxicity, PAHs cause significant environmental concerns (Xia., 2012; Kim., 2013; Purcaro., 2013; Drwal., 2019). Currently, more than 200 types of PAHs have been identified, and 16 of which are characterized as priority pollutants by the US Environmental Protection Agency (Sverdrup., 2002; Mohseni-Bandpei., 2019; Omwoma., 2019). Perfluorinated alkyl substances (PFASs), which are highly fluorinated aliphatic compounds with high chemical stability and surface activity, are considered as a new class of persistent organic pollutants; they have been shown to interact with blood proteins and to cause pathological conditions and cancer (Grandjean., 2015; Rappazzo., 2017; Ghisi., 2019).Ocean has accumulated all types of pollutants, including the heavy metal and persistent organic pollutants (Rainbow, 1995; Mckinney., 2015; Yap., 2016; Brown., 2018; Rigét., 2019). Following entering into the sea, pollutants enter into aquatic organisms through a series of processes, such as ingestion, adsorption, absorption, and accumulation, and are then enriched and biomagnified through a food chain; they finally enter into human exerting their adverse effects on human health. As bottom feeders, marine crustaceans have restricted mobility, filter out water-borne organic matters, and deposit biological fragments or smaller benthic organisms as foods. For this reason, they can easily bioaccumulate environmental pollutants through a food chain, and are therefore often referred to as integrators of the ecological condition (Chou., 2000; MacFarlane., 2000; Lidwina., 2018).are two representatives of marine crustaceans that have high values as they are popular among consumers because of their good taste and richness in high-quality proteins, minerals and other nutritional ingredients (Jiang., 2003; Wang., 2013). In 2019, the fishing yields ofandin China were reported to be 0.221 and 0.458 million tons, respectively (Ministry of agriculture and rural affairs of the People’s Republic of China., 2020). The Zhoushan fishing ground, which is among the world famous fishing grounds and is located in the mouth of entry into the East China Sea for the Yangtze River, Qiantang River, and Yongjiang River in the coastal water of Zhejiang Province,has been an important fishery base since ancient times. However, because the coastal of China East Sea is located in one of the most developed regions of China, where industry and agricultural activities are being rapidly developed, the marine in the areas is increasingly threatened by pollutions such as heavy metal and organic compounds (Li., 2004; Lan., 2019; Wang., 2019; Zhang., 2020).
Although the contamination of heavy metals (Hao., 2019), PAHs (Wang., 2019), PCBs (Zhou., 2019) and organochlorine pesticides (Zhou., 2018) in seafood from the Zhoushan fishing ground have been investigated, the contamination of these pollutants inhave not been studied to date. For this reason, the distribution characteristics of pollutants in different tissues or different genders of these organisms and the polluted status of the new pollutant PFASs are rarely known. In the present work, we assessed the concentrations of Cd, Pb, Hg, inorganic arsenic (iAs),PAHs, and PFASs in two marine crustaceansharvested from the Zhoushan fishing ground in Zhejiang Province, China.
2 Materials and Methods
2.1 Sampling Details
From April 8, 2016 to October 20, 2016, 86 samples ofand 69 samples ofwere collected from 85 different marine fishing vessels docked at the Zhoushan international aquatic trading market. All the fishery cargoes were from the core area of Zhoushan fishing ground (Coordinates: 29˚30´–31˚00´N, 120˚30´– 125˚00´E), as shown in Fig.1. Thesamples consisted of 42 females and 44 males, each with a body length between 8 and 16cm and a weight between 180 and 300g. Thesamples consisted of 34 females and 35 males, each with a body length between 6 and 13cm and a weight between 14 and 22g. The sample with a total weight of about 1.0kg was placed in a clean plastic bag, frozen at −18℃, packed in polystyrene boxes, and finally immediately shipped to the laboratory.

Fig.1 Location of the fishing area.
2.2 Sample Preparation
The collected samples were first rinsed with deionized water to remove surface adherents. The inedible parts (shells and gills forand shells of) were discarded, whereas the edible parts (about 100g per sample) were homogenized using a stainless steel blender (T18 Digital, IKA, Germany). The homogenized samples were stored in clean amber glass containers at −18℃ until subsequent use. In another batch, the edible parts of 30 unprocessedsamples were processed; the samples were divided into two parts (the visceral and the muscle), each was homogenized separately. The edible parts ofcould not be easily divided; thus, they were not further treated by the above step. As a result, a total 215 samples consisting of 86 samples of whole edible parts of, 30 samples of muscle tissues of, 30 samples of visceral tissues of, and 69 samples of whole edible parts ofwere obtained for subsequent quantitative analysis.
2.3 Quantitative Analysis
2.3.1 Quantitative analysis of Cd, Pb, Hg, and iAs
For the analysis of Cd and Pb, the samples were prepared using a microwave digestion method according to the China’s national standard with some modifications(National standard of People’s Republic of China, GB 5009.268-2016). The homogenized sample (0.5g) was added to a 50-mL PTFE digestion vessel containing 6mL of 65% concentrated nitric acid and 2mL of 30% hydrogen peroxide. After soaking overnight at room temperature, the sample was digested in a microwave oven (Ethos-1, Milestone, Italy) under the following conditions: 1) the temperature was increased at a heating power of 900 watts from room temperature to 140℃within 10min; after that, it was increased at the sane heating power to 220℃ within 8min at which it was held for 20min; 2) the temperature was decreased to room temperature within 20min. After the digestion was complete, the digestion vessels were placed in an electric heating plate (EH20B, LabTech, China), on which they were heated at 150℃ for 10min. Subsequently, the samples were diluted with deionized water to a final volume of 25mL and were then subjected to an analysis by ICP-MS (7900, Agilent, USA).
The analysis of Hg was carried out by direct assays described in our previous work (Chang., 2016). Briefly, 100mg of sample was accurately weighed into a sample boat and was then subjected to analysis by a direct mercury analyzer (DMA-80, Milestone, Italy). The content of Hg was quantified by an absorbance at 253.7nm.
The analysis of iAs was conducted by an HPLC- ICP-MS method described in our previous work (Yan., 2019). Briefly, 1.0-g aliquot of homogenized sample was extracted with 20mL of 0.15molL−1HNO3in a 50mL polypropylene centrifuge tube. After vortexing at 3000rmin−1for 60s (Vortex2, IKA, Germany), the sample was shaken at 150rmin−1on a vapor-bathing vibrator (CHA-S, China) at 50℃ overnight. After cooling down to room temperature, the sample was centrifuged at 6000rmin−1for 8min (5810, Eppendorf, Germany). The supernatant (5mL) was mixed with 3mL of-hexane in a 15mL polypropylene centrifuge tube. The mixture was vortexed at 3000rmin−1for 60s and was then centrifuged at 6000rmin−1for 8min. The lower layer was collected, filtered through a 0.45-μm hydrophilic syringe filter (PES, ANPE, China), and then subjected to analysis. The analysis was carried out by an HPLC system (1260 Infinity II, Agilent, USA) equipped with an ICP-MS (7900, Agilent, USA). The chromatographic separation was carried out on an anion exchange column (particle size: 10μm, dimensions: 4.1×250mm, Hamilton PRP-X100, Switzerland).
2.3.2 Quantitative analysis of PAHs
Fifteen PAHs, including acenaphthene (AC), naphthalene (NA), acenaphthylene (A), fluorene (FL), phenanthrene (PHE), anthracene (AN), fluoranthene (FA), benz[a]anthracene (BaA), benzo[b]fluoranthene (BbFA), pyrene (PY), benzo[k]fluoranthene (BkFA), benzo[a] pyrene (BaP), indeno[1,2,3-cd]pyrene (IP), dibenz[a,h] anthracene (DBahA), chrysene (CHR), and benzo[g,h,i] perylene (BghiP), were analyzed by HPLC following a previously described method (Sun., 2012) with some modifications. Five grams of the sample were weighed into a 50-mL polypropylene centrifuge tube, into which 25mL of methanol and 10mL of 50% NaOH were added foralkaline hydrolysis. The hydrolysis was carried out for 60min at 80℃in a water bath (HH-S2-A, China). After cooling down to room temperature, the mixture was centrifuged at 6000rmin−1for 5min (5810, Eppendorf, Germany), and the supernatant S1 was transferred to a 150 mL separatory funnel. The residue was further extracted with 20mL of cyclohexane and centrifuged at 6000rmin−1for 5min, from which the supernatant S2 was obtained. The supernatant S2 was mixed with S1 in the separatory funnel. After vigorous shaking, the mixture was allowed to stand and separate. The upper organic layer S3 was collected, while the lower layer was transferred to another 150-mL separatory funnel to further extract with 10mL of cyclohexane. The upper organic layer S4 was obtained and combined with S3. The mixture was respectively washed with 10mL of 50% methanol, 10mL of water, and 1mL of 60% sulfuric acid, and as thereafter washed repeatedly with water until its pH became neutral. The mixture was subsequently dehydrated with sodium sulphate and then concentrated to a volume of 1–2mL at 40℃ using a decompression evaporator (R210, Buchi, Switzerland). Finally, the extract was purified by a Florisil-SPE column (weight of column stuffing=250mg, column volume=3mL, CNW, Germany) activated first with 3mL of dichloromethane and 5mL of cyclohexane. After adsorption, the sample was eluted with 9mL of cyclohexane-dichloromethane (v:v, 3:1), and the eluted sample was dried at 45℃ using a decompression evaporator. The dried sample was dissolved in 2mL of acetonitrile using ultrasound-assisted mixing method and was filtered through a 0.45-μm PTFE syringe filter (ANPEL, China). The sample was then analyzed using an HPLC system (2695, Waters, USA) equipped with an LC-PAH column (5.0μm, 250mm×4.6mm, Supelcosil, USA) and a fluorescence detector (2475, Waters, USA).
2.3.3 Quantitative analysis of PFASs
Twenty-three PFASs, including perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohe- xanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUdA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTrDA), perfluorotetradeca- noic acid (PFTeDA), perfluorohexadecanoic acid (PFHx DA), perfluorooctadecanoic acid (PFODA), perfluorobutanesulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS), perfluoroheptanesulfonic acid (PFHpS), perfluorooctanesulfonic acid (PFOS), perfluorodecanesulfonic acid (PFDS), perfluorooctanesulfonamide (PFOSA), N-methyl perfluorooctane sulfonamide (N-MeFOSA), N- ethyl perfluorooctane sulfonamide (N-EtFOSA), N-me- thyl perfluorooctane sulfonamidoethanol (N-MeFOSE), and N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE)), were analyzed. The analysis was conducted according to a described previously method (Guo., 2019) with some modifications. In brief, 1.0g of sample was mixed with 2ng each of the isotopically-labeled internal standards (13C4-PFOA,13C4-PFOS, and13C8- PFOSA) in a 15-mL polypropylene centrifuge tube. After vortexing, the mixture was extracted with 5mL of acetonitrileat room temperaturefor 15min in an ultrasonic bath (20KHz, FS-2000T, China) and then centrifuged at 6000rmin−1for 10min (5810, Eppendorf, Germany). The supernatant was transferred to a fresh 15-mL polypropylene centrifuge tube, and the residue was further extracted with 5mL of acetonitrile and then centrifuged at the same speed and time. The two supernatants were combined, concentrated to 1mL at 40℃ undernitrogen stream (N- VEAP-45, Organomation, USA), and then dispersively cleaned up by vigorously mixing with 25mg of C18(CNWBOND HC-C18, 40–63μm, ANPEL, China) and 25mg of graphitized carbon (CNWBOND Carbon-GCB 120/400, ANPEL, China). Finally, 0.5mL of the purified supernatant was dissolved in 0.5mL of methanol-water (1:1, v/v), and the solution was filtered through a 0.22-μm Polypropylene filter (ANPEL, China) before subjecting to HPLC-MS/MS analysis (Acquity UPLC H-CLASS-xevo TQS, Waters, USA).
2.4 Statistical Analysis
Statistical analyses were performed using SPSS 19.0 (IBM Corporation). The difference with a probability of less than 0.05 (<0.05) was considered statistically significant, and that with<0.01 was considered highly statistically significant. Since all the samples were analyzed in fresh muscle forms, the pollution detected results were calculated using wet weight, except for special instructions, and the datas are expressed as means of three replicate samples. Concentrations below the LODs were assigned as not detected (n.d.).
3 Results and Discussion
3.1 Quality Control of Analysis
To reduce background interferences, the containers used in the analysis of trace elements were first soaked overnight in 20% HNO3before rinsing with ultrapure water, and those used for analysis of PAHs and PFASs were soaked in methanol. Teflon containers were used in the analysis of PFASs to avoid interferences during chromatographic separations.
The limit of detection (LOD) for each analyte was defined as the concentration that yielded the signal-to-noise ratios of equal or greater than 3 (S/N≥3). To verify the accuracy and precision of the method, standard references or spiked samples were used; and the analysis was carried out according to the methods as described in Section 2.3. The LODs for Cd, Pb, Hg, and iAs were 0.005, 0.05, 0.002, and 0.035 (for As3+) and 0.055 (for As5+) mgkg−1, respectively. The test result of Cd, Pb, and Hg in standard reference (prawn; GBW10050, China), and that of iAs in standard reference(tuna fish tissue; BCR-627, European National Standards Agency) showed that their levels were within the reference’s ranges.The LODs for 15 PAHs were as follows: 0.5μgkg−1for NA, AC, and FL; 1.0μgkg−1for PHE, AN, FA, PY, BaA, CHR, BkFA, BaP, and IP; and 2.0μgkg−1for BbFA, DBahA, and BghiP. The mean recoveries of PAHs at the spiked amounts of 2.0, 10.0 and 50.0μgkg−1were 75.2%–98.7% (RSDs<12.5%).The LODs for 23 PFASs were as follows: 0.003μgkg−1for PFOSA; 0.006μgkg−1for PFBS, PFHpS, PFHxS, and PFOS; and 0.020μgkg−1for all other analytes. The recoveries of PFASs at the spiked levels of 0.02 and 0.2μgkg−1ranged from 85.0% to 110.4% (RSDs<15.0%).
3.2 Distribution Characteristics of Pb, Hg, iAs, and Cd
The levels of Pb, Hg, iAs,and Cd in whole edible parts ofandare shown in Fig.2. The levels in 69samples were as follows: Pb =<0.005–0.26mgkg−1, Hg = 0.0043–0.041mgkg−1, and iAs=<0.04–0.13mgkg−1, and the corresponding average values were 0.038, 0.020, and 0.044mgkg−1, respectively. The levels in 86 samples ofwere: Pb=<0.005–0.40mgkg−1, Hg=0.00223–0.067mgkg−1, and iAs=<0.04–0.20mgkg−1, and the corresponding average values were 0.062, 0.028, and 0.080mgkg−1, respectively. The levels of Pb in the two marine crustacean species were significantly different (<0.05), whereas the levels of Hg and iAs were highly significantly different (<0.01)Additionally, the accumulations of Pb, iAs, and Hg bywere higher than those by.Fortunately, all the levels of Pb, Hg, and iAs were below the maximum value (0.5mgkg−1for crustacean species) allowed by the China’snational standard (National standard of People’s Republic of China GB 2762- 2017). The levels of Cd in 69 samples ofvaried from 0.16 to 4.20mgkg−1with an average value of 2.26mgkg−1. Of the 69 samples, the Cd levels in 68 samples (98.6%) surpassed the maximum value (0.5mgkg−1for crustacean species) set by the China’s national standard GB 2762-2017. Similarly, the Cd levels in 86 samples ofranged from 0.055 to 4.20mgkg−1with an average value of 1.44mgkg−1, and the levels in 65 out of 86 samples (75.6%) exceeded the standard limit. These data show that bothandhad strong Cd accumulation abilities, but the ability of the former was significantly stronger (<0.01) than that of the latter.
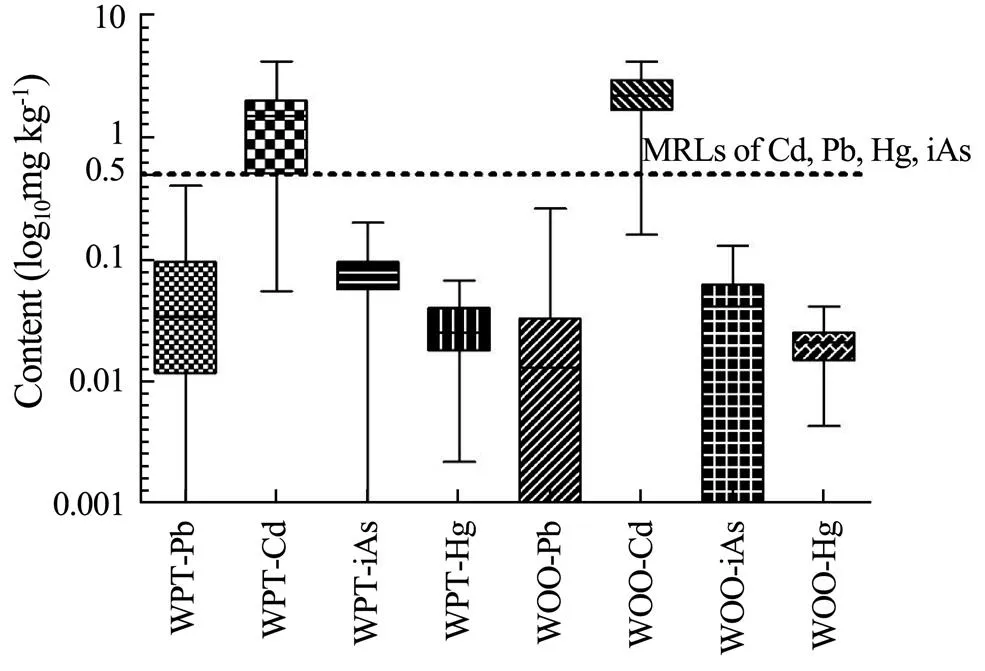
Fig.2 Contents of heavy metals in whole edible parts of the two marine crustaceans. WPT, whole edible parts of P. trituberculatus; WOO, whole edible parts ofO. oratoria.
In further study on accumulation of trace elements in different tissues, the levels of Pb, Hg, iAs,and Cd in the two edible parts (muscle tissues and visceral tissues) of 30 samples ofwere measured. As shown in Fig.3, the levels of Pb in muscle tissues were 0.005–0.25mgkg−1, those of Hg were 0.013–0.076mgkg−1, and those of iAs were <0.04–0.18mgkg−1, and the corresponding average values were 0.062, 0.033, and 0.083mgkg−1, respectively. In addition, the levels of Pb in visceral tissues were 0.011–0.34, those of Hg were 0.042–0.04, and those of iAs were <0.04–0.17mgkg−1, and the corresponding average values were 0.12, 0.019, and 0.074 mg.kg-1, respectively. The levels of iAs content in the two types of tissues were not significantly different (> 0.05), but those of Pb and Hg were highly significant different (<0.01). Similar to those of the whole edible parts, the levels of Pb, Hg, and iAs in each type of tissue ofwere below the maximum values allowed by the China’s national standard.Compared with those of Pb, Hg, and iAs, the levels of Cd were highly significantly different (<0.01). The Cd levels in muscle tissues ranged from 0.078 to 2.60mgkg−1, and those in visceral tissues ranged from 0.15 to 9.6mgkg−1; and the corresponding average values were 0.49 and 4.64mgkg−1, respectively. Additionally, the Cd levels in 30% of the muscle samples and those in 96.7% of the visceral samples exceeded the standard limit.Comparing the Cd levels in the two types of tissues revealed that the Cd level in the visceral tissues was about 9.5 times higher than that in the muscle tissues. Thus, it can be inferred that Cd can largely be accumulated in the visceral tissuesof, thus causing the Cd content in this marine crustacean to be high exceeding the standard limit. This also indicates that the accumulation of Cd mainly occurs in the visceral tissues of
Fig.3 Contents of heavy metals in different tissues of P.trituberculatus. MPT, muscle tissues of P. trituberculatus; VPT, isceral tissues of P. trituberculatus.
Fig.4 shows the levels of Pb, Cd, iAs, and Hg inandwith different genders (male and female). According to the result, the accumulations in both genders were not significantly different (> 0.05).

Fig.4 Contents of heavy metals in different gender of the two marine crustaceans. Fe-WPT, hole edible parts of female P. trituberculatus; Ma-WPT, whole edible parts of male P. trituberculatus; Fe-Woo, whole edible parts of femaleO. oratoria; Ma-WOO, whole edible parts of male O. oratoria.
Hao. (2019) have analyzed Pb, Cd, and Hg in the abdominal muscle of 22 crab samples captured from the coastal area of Zhoushan, from which they found that the average Pb, Cd, and Hg contents were 0.203±0.045, 0.248±0.130, and 0.019±0.006mgkg−1, respectively, in the spring and were 0.228±0.061, 0.196±0.168, and 0.028±0.006mgkg−1, respectively, in the autumn.These Pb and Cd contents were significantly higher and lower, respectively, than those presented in the present work, possibly due to the differences of the sampling methods, the species, the fishing location and the fishing time. Aquatic organisms can easily enrich Cd in the environment; for example, the Cd accumulation in shellfish was up to 105to 106times, and after enriching, Cd can be transferred and accumulated along the food chain (Johansen., 2000). Based on our results, the two marine crustacean speciesandhad a strong Cd accumulation ability, but the ability of the former was higher than that of the latter. In addition, the Cd accumulation took place in the visceral tissues. The Cd concentrations in the whole edible parts ofcaptured from the Yellow Sea in northern China have been found to range from 0.052 to 8.89mgkg−1(mean = 2.26mgkg−1). In addition, the concentrations in 85% of these samples exceeded the standard limit, and the concentrations in different genders were not significantly different (Zhao., 2020). Investigation on the accumulation of heavy metals in marine food network in the coastal area of Jiangsu province, China, found that the Cd content in the body ofwas high with a value of 5.00±0.92mgkg−1; the biomagnification effect was also high, but the discharge rate was low (Li., 2019). Several other studies have also demonstrated that marine crustaceans have a strong ability to enrich heavy metals, and the accumulation is more pronounced in the digestive gland or the hepatopancreas tissues (Karar., 2019; Kumar., 2019; Shalini., 2020).These observations are consistent with our results, which showed that Cd was found to largely accumulate in the edible tissue ofand. Cd in the organisms can be combined with proteins, organic acids,. into organic Cd, and can also be ionized presence. Toxicology studies have shown that Cd toxicity is related to its presence species, and organic Cd is less toxic than ionized Cd (Genchi., 2020). Zhao. (2017) had shown that Cd in liver pancreas ofand muscle tissue ofwere mainly in the forms of Cd-metallothionein, Cd-cysteine and other organic Cd. Although the total Cd polluted level in marine crustaceans is high, the presence of most Cd in organic species with little toxicity may reduce the health risks on the population after consumption. Therefore, it is necessary to do further in-depth study on the distribution characteristics and toxicology of different Cd morphological compounds in order to scientifically evaluate the risk of Cd pollution in marine crustaceans.
3.3 Distribution Characteristics of PAHs
Thedetection frequency of 15 PAHs inandis shown in Fig.5. Based on the graph, NA, AC, FL, PHE, and FA were found in 69 samples of, and their positivity rates were 27.54%, 4.35%, 4.35%, 24.64%, and 10.14%, respectively. Additionally, 11 PAHs, including NA, AC, FL, PHE, AN, FA, PY, BaA, BbFA, BkFA, and BaP were detected in 86 samples of whole edible parts of; and among these PAHs, the most frequently detected were NA, AC, FL, and PHE with positivity rates of 61.63%, 46.51%, 62.79%, and 56.98%, respectively. Furthermore, the analysis of the muscle tissues of 30samples showed that NA, AC, FL, PHE, and FA were found in the muscle tissues with detection frequency of 53.33%, 30%, 50%, 50%, and 3.33%, respectively. The analysis of visceral tissues showed that NA, AC, FL, PHE, AN, FA, and BaA were detected,and the positivity rates of NA, FL, PHE, and AC (the top 4 most frequently detected) were 73.33%, 73.33%, 66.67%, and 40%, respectively.

Fig.5 Detection frequency of PAHs in tissues of the two marine crustaceans.
The contents of 15 PAHs detected inandare tabulated in Table 1. In all 69 samples of the whole edible parts of, the average values of 5 PAHs that were detected could be ranked as follows: NA>PHE>FL>AC>FA; and the total concentration of PAHS ranged from 10.75 to 65.9μgkg−1(mean= 18.7μgkg−1). Of 86 whole edible parts ofsamples, 11 PAHs were detected; and the average values of the top 4 PAHs could be ranked in the following order: NA > PHE > AC > FL, the total concentration of which ranged from 13.26 to 181μgkg−1(mean=24.2μgkg−1). In addition, the detection of PAHs in the specific tissues of 30 samples ofshowed that FL, AC, PHE, and NA were the main PAHS pollutants in the muscle and the visceral tissues (their average values increased in the same order), and∑15PAHs ranged from 9.58 to 160μgkg−1(mean=21.1μgkg−1) and from 18.22 to 201μgkg−1(mean=32.7μgkg−1), respectively. The above results show that PAHs accumulated inandharvested from the Zhoushan fishing ground mainly included NA, AC, FL, PHE. In addition, the detection frequency and concentration of PAHs in.were significantly higher than those in(<0.01), and those in the visceral tissues ofwere significantly higher than those in the muscle(<0.01). As fat-soluble substances, PAHs can more easily accumulate in fat-rich tissues or organisms, including the visceral tissues of,which has a higher fat content than those of. BaP, which is the most carcinogenic substance among all PAHs, was detected only in one sample ofwith a concentration of 1.86μgkg−1. This concentration is below the maximum allowable BaP concentration (5.0μgkg−1in smoked and roasted aquatic products) set by GB 2762-2017, as well as below the limit allowed by the EU’S commission regulation No 835/2011 (content of BaP<2.0μgkg−1, or sum contents of BaP, BaA, BbFA, and CHR<12μgkg−1for smoked crustaceans). This indicates that the two marine crustaceans captured from the Zhoushan fishing ground are safe to be consumed. The levels of PAHs reported in the present study are comparable to those in(83.1 to 174.7μgkg−1) farmed in the Zhoushan archipelago and the Xiangshan harbor in East China (Wang., 2019), as well as to those in marine fish and cephalopods in the coast of the South China Sea (total contents of PAHs ranged from 94.88 to 557.87μgkg−1dry weight, mean = 289.86μgkg−1dry weight (Ke., 2017), if calculated by wet weight using the moisture content of about 80%, which is the content usually found in fresh aquatic products.
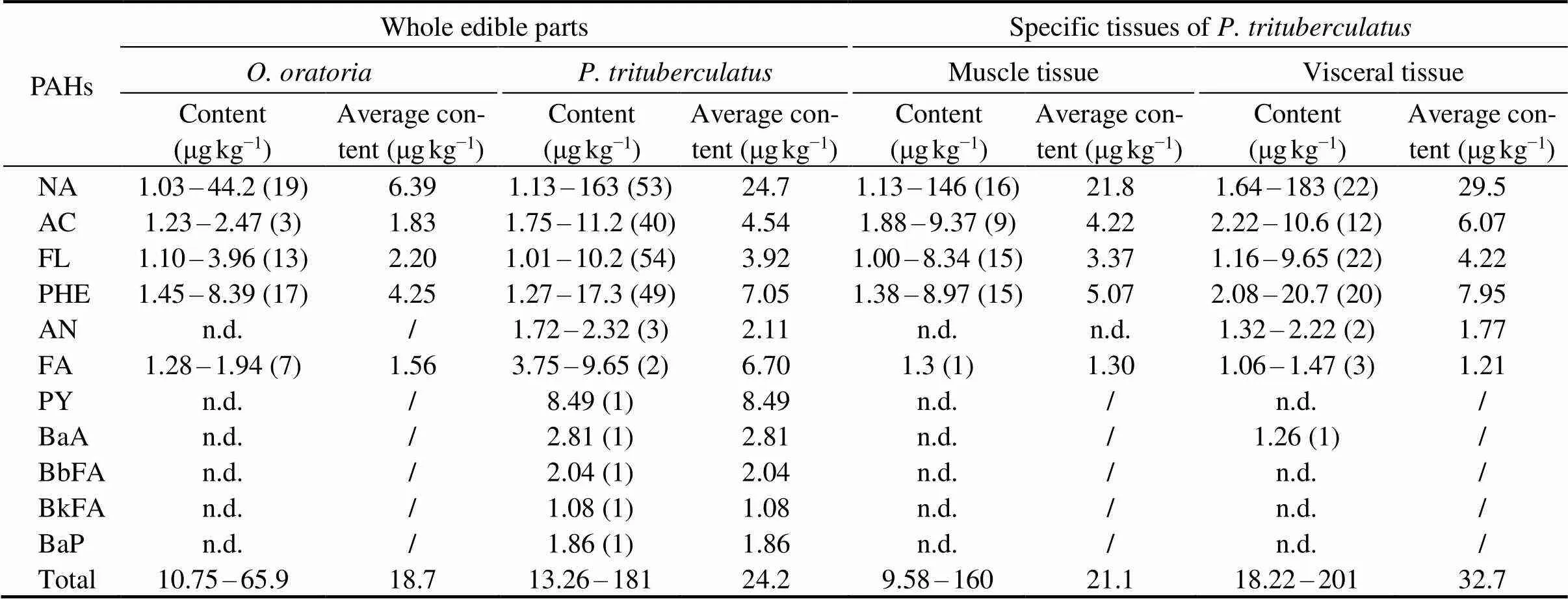
Table 1 Contents of PAHs in P. trituberculatus and O. oratoria
Notes: Numbers in the parentheses indicate the numbers of samples in which PAHs were detected. n.d. = not detected or the detected values were below the LODs. ‘/’ stands for not calculated.
3.4 Distribution Characteristics of PFASs
The contents of PFASs were detected in the whole edible parts of 60 samples ofand(30 samples for each species), of which 58 samples were positive. As shown in Fig.6, 16 PFAS, including PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUdA, PFDoA, PFTrDA, PFTeDA, PFHxDA, PFODA, PFHxS, PFOS, and PFOSA, were identified.The detection frequency could be ranked as follows: PFOA (66.7%) >PFOS (58.3%)>PFOSA (50%)>PFUdA (36.7%)> PFPeA (26.7%)>PFNA (25.0%)>PFDA (23.3%). Whereas the detection frequency of PFBA, PFHxA, PFHpA, PFDoA, PFTrDA, PFTeDA, PFHxDA, PFODA, and PFHxS were all below 12%, PFBS, PFHpS, PFDS, N-MeFOSA, N-EtFOSA, N-MeFOSE, and N-EtFOSE were not detected in any samples.As shown in Fig.7,comparing the average content of the 7 most important PFASs detected showed that they could ranked in the following order: PFOA (ranged from 0.141to3.87μgkg−1, mean=0.771μgkg−1)>PFNA (0.125–0.48μgkg−1, mean=0.238μgkg−1)>PFDA (0.101–0.38μgkg−1, mean=0.213μgkg−1)>PFUdA (0.0992–0.258μgkg−1, mean=0.177μgkg−1)>PFPeA (0.0924–0.239μgkg−1, mean=0.16μgkg−1)>PFOS (0.0162–0.49μgkg−1, mean=0.149μgkg−1)>PFOSA (0.0062–0.39μgkg−1, mean=0.127μgkg−1). ∑23PFASs ranged from 0.0162–5.104μgkg−1with a mean value of 0.915μgkg−1. As the dominant pollutants, PFOA, PFOS, and PFOSA had the total contents that could be accounted for 82.8%–89.5% of total PAFSs detected in both types of marine crustaceans.
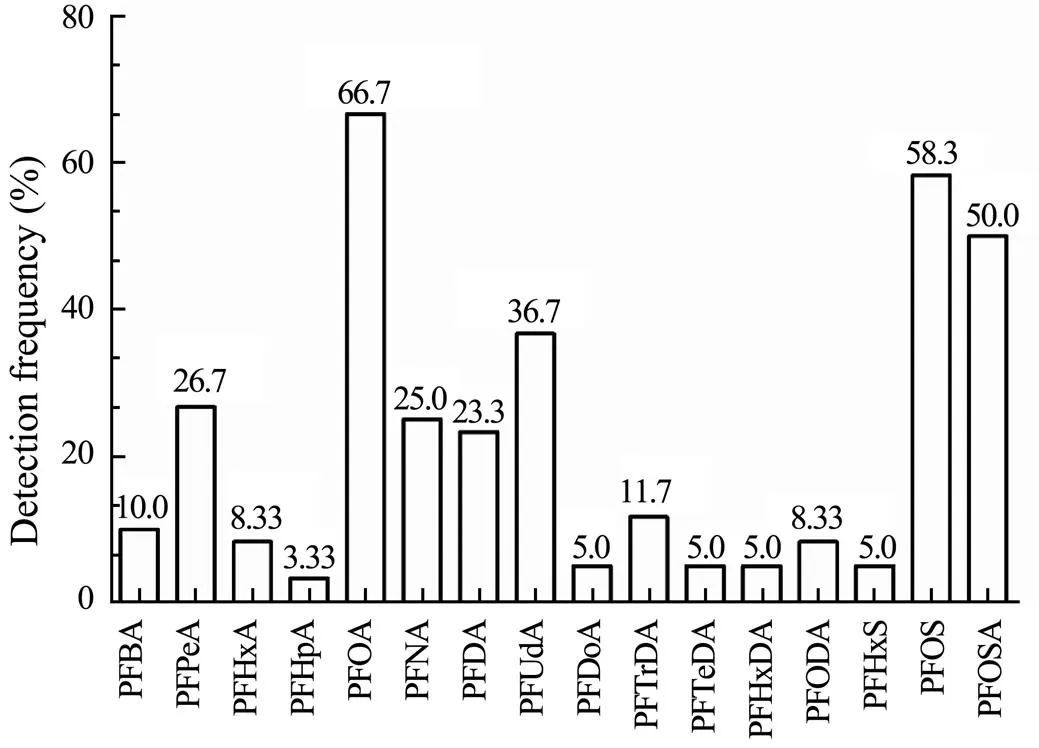
Fig.6 Detection frequency of PFASs in tissues of P. trituberculatus and O. oratoria.
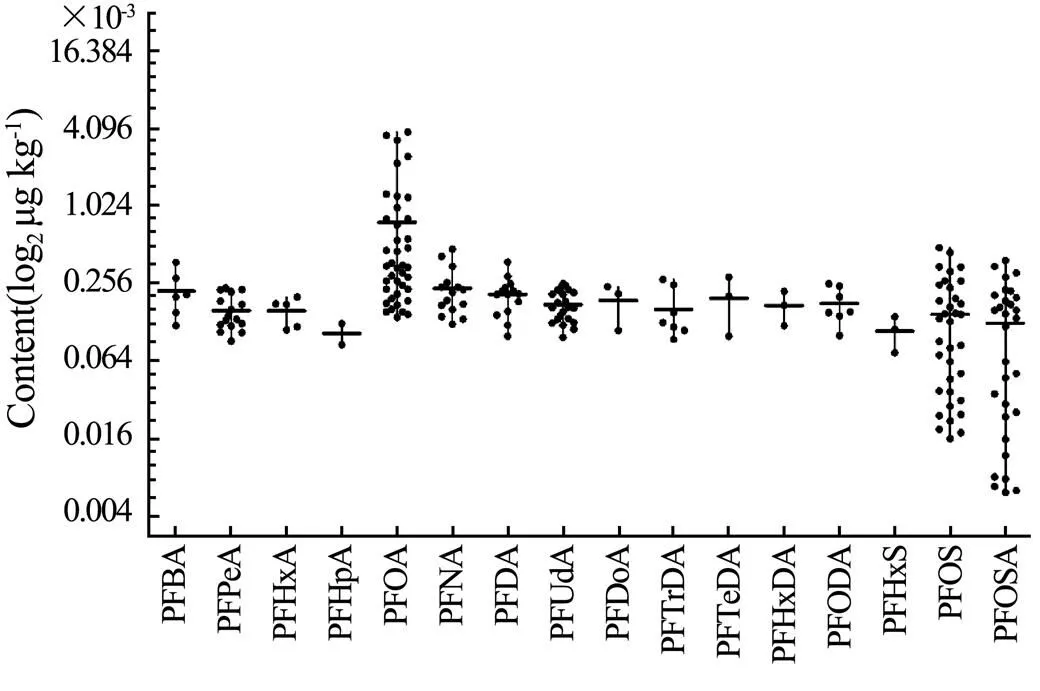
Fig.7 Detection results of PFASs in samples of P. trituberculatus and O. oratoria.
According to the results reported by the European Food Safety Authority (EFSA), PFASs in food were more frequently found in seafood, meat, and meat products, and the PFASs that are mainly detected are PFOA and PFOS (European Food Safety Authority, 2012). In marine shellfish captured from the China’ Bohai Sea, the highly frequently detected PFASs ranked by their detection frequency was as follows: PFOA (98.3%) > PFNA (67.4%) > PFOSA (57.8%) > PFOS (57.4%) and PFOA posed the highest threat to the safety of shellfish products, as it contributed to 87.2% of total PFASs (the concentration of total PFASs ranged from 0.0156 to 64.0μgkg−1) (Guo. 2019).Our results are similar to the results reported in literature shown above; however, there are some differences as follows: (1) PAFSs detection frequency in present work was not as high as those reported in the literature; the four highest detection frequencies were 66.7% (PFOA), 58.3% (PFOS), 50% (PFOSA), and 25% (PFNA); and (2) the detected concentrations of total PFASs (0.0162–5.104μgkg−1) were slightly lower than those reported in the literature, which may be attributed to different sampling locations (the Bohai Sea and the East China Sea) or different marine species; these differences can result in different accumulations of PFASs and their sensitivity to the detection. The fifth edition of the Chinese total diet study (TDS) conducted in 20 provinces from 2011 to 2013 has demonstrated that the detection frequency of PFOA, PFNA, PFDA, PFUdA, PFDoA, PFTrDA, PFHxS, and PFOS in aquatic food are 25%, 80%, 100%, 100%, 60%, 60%, 25%, and 95%, respectively, and their average concentrations were 0.042, 0.168, 0.115, 0.269, 0.054, 0.116, 0.038, and 0.419μgkg−1(Wang., 2019), respectively. Compared to that demonstrated in the present work, the detection frequency reported in the TDS was much higher, which is likely due to that the aquatic food samples in the TDS were obtained from markets, and these samples were processed aquatic products that can be polluted by PFASsduring the food processing.
Because of the persistency, widespread distribution, bioaccumulative properties, and health concerns of PFASs, especially for PFOA and PFOS, the Stockholm Convention listed PFOS, as well as its salts and perfluorooctane sulfonyl fluoride, as compounds with restricted use (United Nations Environment Programme, 2009, 2015, 2017). Based on human epidemiological studies, the tolerable weekly intakes (TWIs) of PFOS and PFOA established by the EFSA Panel on contaminants in the food chain were 13 and 6ngkg−1of bwweek−1, respectively (Knutsen., 2018). The calculation using the mean concentrations of PFOS (0.149μgkg−1) and of PFOA (0.771μgkg−1) in the two marine crustaceans captured from the Zhoushan fishing ground showed that for a person with an average body weight of 60kg and the average seafood consumption amount of 42.3gd−1(Li., 2013). The estimated weekly intakes (EWIs, ngkg−1of bwweek−1) of PFOS and PFOA for residents dwelling in the areas around the Zhoushan region should be 0.74 and 3.80ngkg−1of bwweek−1, respectively, which are much lower than the TWIs established by the EFSA. This indicates that the exposure to PFOS or PFOA through consumption ofandmay not pose threat to human health. However, considering that a person can acquire PFASs through consumption of other foods, the dietary intake of PFASs may increase, therefore causing the potential health risk to the residents with excessive seafood consumption.
4 Conclusions
In conclusion, we characterized the distribution of heavy metals and organic pollutants in two marine crustaceans,and. We found that the contents of Pb, Hg, and iAs in the two marine crustaceans harvested from the Zhoushan fishing ground in China East Sea were at trace levels that are far below the limits set by the China’s national standard GB2762-2017. By contrast, the exceeding standard rates of Cd in the whole edible parts ofandwere 98.6% and 75.6%, respectively. The accumulation of Cd inwas significantly higher than that in, and the accumulation mainly occurred in the visceral tissues of
Moreover, PAHs that were mainly found in the samples included NA, AC, FL, and PHE, and total concentrations of 16 PAHs detected in all whole edible parts ranged from 10.75 to 181μgkg−1. Both the detection frequency and the concentration of PAHs inwere significantly higher than those in. The concentration of total PAHs in the visceral tissues ofwas significantly higher than in the muscle. BaP, the most toxic compound among all PAHs, was detected only in one sample ofat a low content of 1.86 μgkg-1, which is a safe level.
Furthermore, we observed that the concentrations of total PFASs ranged from 0.0162 to 5.104μgkg−1with a mean value of 0.915μgkg−1. Main PFASs detected in the two marine crustaceans were PFOA, PFOS, and PFOSA; the contents of which in each sample were accounted for 82.8%–89.5% of total PAFSs. Estimated weekly intakes of PFOS and PFOA through consumption of the two marine crustaceans for residents residing around the Zhoushan region were 0.74 and 3.80ngkg−1of bwweek−1, respectively, which are much lower than the TWIs established by the EFSA. The data indicate that exposures to PFOS and PFOA through consumption ofandmay not pose threat to the health of consumers.
Overall, we may conclude that the health risks caused by Pb, Hg, iAs, PAHs, and PFASs contaminated inandcaptured in the Zhoushan fishing ground in China East Sea are low; however, attention should be paid to risks caused by Cd.
Acknowledgements
This work was supported by the National Key R&D Programof China (No. 2020YFD0900900), the Public Interest Science andTechnology Plan of Ningbo (No. 2019C10007), and the Basic Public Welfare Project of Zhejiang Provincial Department of Science and Technology (No. LGN20C200015).
Brown, T. M., Macdonald, R. W., Muir, D. C. G., and Letcher, R. J., 2018. The distribution and trends of persistent organic pollutants and mercury in marine mammals from Canada’s eastern Arctic., 618: 500- 517, DOI: org/10.1016/j.scitotenv.2017.11.052.
Chang, J. Q., Zhang, X. J., and Mei, G. M., 2016. Determination of total mercury in aquatic products by cold atomic absorption spectrometry., 35 (4): 310-314, DOI: 10.3969/j.issn.1008-830X.2016.04.007.
Chou, C. L., Paon, L. A., Moffatt, J. D., and Zwicker, B., 2000. Copper contamination and cadmium, silver, and zinc concentrations in the digestive glands of American lobster () from the inner Bay of Fundy, Atlantic Canada., 65: 470-477,DOI: 10.1007/s001280000148.
Drwal, E., Rak, A., and Gregoraszczuk, E. L., 2019. Review: Polycyclic aromatic hydrocarbons (PAHs)–Action on placental function and health risks in future life of newborns., 411: 133-142,DOI: 10.1016/j.tox.2018.10.003.
European Food Safety Authority, 2012. Perfluoroalkylated substances in food: Occurrence and dietary exposure., 10 (6): 2743, DOI: 10.2903/j.efsa.2012.2743.
Fraga, C. G., 2005. Relevance, essentiality and toxicity of trace elements in human health., 26 (4-5): 235-244, DOI: 10.1016/j.mam.2005.07.013.
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A., and Cata- lano, A., 2020. The effects of cadmium toxicity., 17 (11): 3782, DOI: 10.3390/ijerph17113782.
Ghisi, R., Vamerali, T., and Manzetti, S., 2019. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review., 169: 326-341, DOI: 10. 1016/j.envres.2018.10.023.
Grandjean, P., and Clapp, R., 2015. Perfluorinated alkyl substances: Emerging insights into health risks., 25: 147-163, DOI: 10.1177/1048291115590506.
Guo, M. M., Zheng, G. C., Peng, J. X., Meng, D., Wu, H. Y., Tan, Z. J.,., 2019. Distribution of perfluorinated alkyl substances in marine shellfish along the Chinese Bohai Sea coast., 54: 271-280, DOI: 10.1080/03601234.2018.1559570.
Hao, Z., Chen, L. H., Wang, C. L., Zou, X. Q., Zheng, F. Q., Feng, W. H.,., 2019. Heavy metal distribution and bioaccumulation ability in marine organisms from coastal regions of Hainan and Zhoushan, China., 226: 340-350, DOI: 10.1016/j.chemosphere.2019.03.132.
Jiang, X. M., Qian, Y. X., and Wang, C. L., 2003. Analysis and evaluation of the nutritional composition in muscle of three species of., 25 (2): 175-177, DOI: 10.3321/j.issn:0512-7955.2003.02.023.
Johansen, P., Pars, T., and Bjerregaard, P., 2000. Lead, cadmium, mercury and selenium intake by Greenlanders from local marine food., 245: 187-194. DOI: 10.1016/s0048-9697(99)00443-x.
Karar, S., Hazra, S., and Das, S., 2019. Assessment of the heavy metal accumulation in the Blue Swimmer Crab (), northern Bay of Bengal: Role of salinity., 143: 101-108, DOI: 10.1016/j.marpolbul. 2019.04.033.
Ke, C. L., Gu, Y. G., Liu, Q., Li, L. D., Huang, H. H., Cai, N.,., 2017. Polycyclic aromatic hydrocarbons (PAHs) in wild marine organisms from South China Sea: Occurrence, sources, and human health implications., 117: 507-511, DOI: 10.1016/j.marpolbul. 2017.02.018.
Kim, K. H., Jahan, S. A., Kabir, E., and Brown, R. J., 2013. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects., 60: 71-80, DOI: 10.1016/j.envint.2013.07.019.
Knutsen, H. K., Alexander, J., Barregard, L., Bignami, M., Bruschweiler, B., Ceccatelli, S.,., 2018. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food., 16 (12): e05194, DOI: 10.2903/j.efsa.2018.5194.
Kumar, B. S., Padhi, R. K., and Satpathy, K. K., 2019. Trace metal distribution in crab organs and human health risk assessment on consumption of crabs collected from coastal water of South East coast of India., 141: 273-282, DOI: 10.1016/j.marpolbul.2019.02.022.
Lan, J., Jia, J., Liu, A., Yu, Z., and Zhao, Z., 2019. Pollution levels of banned and non-banned pesticides in surface sediments from the East China Sea., 139: 332-338, DOI: 10.1016/j.marpolbul.2019.01.006.
Li, D. J., and Daler, D., 2004. Ocean pollution from land-based sources: East China Sea, China., 33: 107-113, DOI: 10.1639/0044-7447(2004)033[0107:OPFLSE]2.0.CO;2.
Lidwina, B., Monferrán, M. V., Mouneyrac, C., and Amé, M. V., 2018. Native crustacean species as a bioindicator of freshwater ecosystem pollution: A multivariate and integrative study of multi-biomarker response in active river monitoring., 206: 265-277, DOI: 10.1016/j.chemosphere.2018. 05.002.
Li, P., Feng, X. B., Liang, P., Chan, H. M., Yan, H. Y., and Chen, L. G., 2013. Mercury in the seafood and human exposure in coastal area of Guangdong Province, South China., 32: 541-547, DOI: 10. 1002/etc.2113.
Li, Y. K., Zhang, R., Zhang, S., and Zhang, H., 2019. Assessment of heavy metal bioaccumulation in food web of the coastal waters of Jiangsu Province, China, based on stable isotope values (δ13C and δ15N)., 30 (7): 2415-2425, DOI: 10.13287/j.1001-9332. 201907.029.
MacFarlane, G. R., Booth, D. J., and Brown, K. R., 2000. The Semaphore crab,: Bio-indication potential for heavy metals in estuarine systems., 50: 153-166, DOI: 10.1016/s0166-445x(00)00083-7.
Mckinney, M. A., Pedro, S., Dietz, R., Sonne, C., Fisk, A. T., Roy, D.,., 2015. A review of ecological impacts of global climate change on persistent organic pollutant and mercury pathways and exposures in arctic marine ecosystems., 61: 617-628, DOI: 10. 1093/czoolo/61.4.617.
Ministry of Agriculture and Rural Affairs of the People’s Republic of China, National Fisheries Technology Extension Center and China Society of Fisheries, 2020. 2020. China Agriculture Press, Beijing.
Mohseni-Bandpei, A., Majlesi, M., Rafiee, M., Nojavan, S., Nowrouz, P., and Zolfagharpour, H., 2019. Polycyclic aromatic hydrocarbons (PAHs) formation during the fast pyrolysis of hazardous health-care waste., 227: 277- 288, DOI: 10.1016/j.chemosphere.2019.04.028.
National standard of People’s Republic of China, 2017.. China Standard Press, Beijing.
National Standard of People’s Republic of China, 2016.. China Standard Press, Beijing.
Nghia, N. D., Lunestad, B. T., Trung, T. S., Son, N. T., and Maage, A., 2009. Heavy metals in the farming environment and in some selected aquaculture species in the Van Phong Bay and Nha Trang Bay of the Khanh Hoa Province in Vietnam., 82: 75-79, DOI: 10.1007/s00128-008-9561-z.
Omwoma, S., Mbithi, B. M., Pandelova, M., Ssebugere, P., Lalah, J. O., Wang, Y. W.,., 2019. Comparative exposomics of persistent organic pollutants (PCBs, OCPs, MCCPs and SCCPs) and polycyclic aromatic hydrocarbons (PAHs) in Lake Victoria (Africa) and Three Gorges Reservoir (China)., 695: 133789,DOI: 10. 1016/j.scitotenv.2019.133789.
Purcaro, G., Moret, S., and Conte, L. S., 2013. Overview on polycyclic aromatic hydrocarbons: Occurrence, legislation and innovative determination in foods., 105: 292-305. Doi:10.1016/j.talanta.2012.10.041.
Rainbow, P. S., 1995. Biomonitoring of heavy metal availability in the marine environment., 31: 183-192, DOI: 10.1016/S0269-7491(97)84230-3.
Rappazzo, K. M., Coffman, E., and Hines, E. P., 2017. Exposure to perfluorinated alkyl substances and health outcomes in children: A systematic review of the epidemiologic literature., 14: 691, DOI: 10.3390/ijerph14070691.
Rigét, F., Bignert, A., Braune, B., Dam, M., Dietz, R., Evans, M.,., 2019. Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota., 649: 99-110, DOI: 10.1016/j.scitotenv.2018.08. 268.
Shalini, R., Jeyasekaran, G., Shakila, R. J., Arisekar, U., Sundhar, S., Jawahar, P.,., 2020. Concentrations of trace elements in the organs of commercially exploited crustaceans and cephalopods caught in the waters of Thoothukudi, South India., 154: 111045,DOI: 10.1016/j.mar polbul.2020.111045.
Spanopoulos-Zarco, P., Ruelas-Inzunza, J., Meza-Montenegro, M., Osuna-Sánchez, K., and Amezcua-Martínez, F., 2014. Health risk assessment from mercury levels in bycatch fish species from the coasts of Guerrero, Mexico (eastern Pacific)., 93: 334-338,DOI: 10.1007/s00128-014-1311-9.
Sun, X. M., Mei, G. M., Chen, X. C., Guo, Y. M., and Chen, P., 2012. Determination of 15 polycyclic aromatic hydrocarbons in aquatic products by HPLC-fluorescence., 8: 48-53, DOI: 10.3969/j.issn.2095-0780.2012. 03.007.
Sverdrup, L. E., Nielsen, T., and Krogh, P. H., 2002. Soil ecotoxicity of polycyclic aromatic hydrocarbons in relation to soil sorption, lipophilicity, and water solubility., 36: 2429-2435, DOI: 10.1021/ es010180s.
United Nations Environment Programme, 2009. The conference of the parties 4 of the stockholm convention (COP-4) in geneva placed perfluorooctane sulfonate and perfluorooctane sulfonyl fluoride (PFOS and PFOSF) in Annex B. http://www. pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx.
United Nations Environment Programme, 2015. Proposal to list pentadecafluorooctanoic acid (CAS No: 335-67-1, PFOA, perfluorooctanoic acid), its salts and PFOA-related compounds in Annexes A, B and/or C to the stockholm convention on persistent organic pollutants. http://www.pops.int/ Convention/POPsReviewCommittee/Chemicals/tabid/243/De fault.aspx.
United Nations Environment Programme, 2017. POPRC Recommendations for listing chemicals. http://chm.pops.int/ Convention/POPsReviewCommittee/Chemicals/tabid/243/De fault.aspx.
Wang, Q., Wu, X. G., Lou, B., Yang, Y. P., Liu, Z. J., and Cheng, Y. X., 2013. Comparison of nutritional composition of different muscle parts in., 35: 310-312,DOI: 10.13325/j.cnki.acta.nutr. sin.2013.03.001.
Wang, S., Liu, J., Li, J. C., Xu, G., Qiu, J. D., and Chen, B., 2020. Environmental magnetic parameter characteristics as indicators of heavy metal pollution in the surface sediments off the Zhoushan Islands in the East China Sea., 150: 110642, DOI: 10.1016/j.marpolbul.2019. 110642.
Wang, X. Y., Celander, M. C., Yin, X. L., Zhang, Z. C., Chen, Y. J., Xu, H. Z.,., 2019. PAHs and PCBs residues and consumption risk assessment in farmed yellow croaker () from the East China Sea, China., 140: 294-300, DOI: 10.1016/j.marpolbul. 2019.01.062.
Wang, Y. X., Liu, J. Y., Li, J. G., Zhao, Y. F., and Wu, Y. N., 2019. Dietary exposure of Chinese adults to perfluoroalkyl acidsanimal-origin foods: Chinese total diet study (2005– 2007 and 2011–2013)., 67: 6048-6055, DOI: 10.1021/acs.jafc.9b01108.
Xia, K., Hagood, G., Childers, C., Atkins, J., Rogers, B., Ware, L.,., 2012. Polycyclic aromatic hydrocarbons (PAHs) in Mississippi seafood from areas affected by the deepwater horizon oil spill.,46: 5310-5318, DOI: 10.1021/es2042433.
Yan, G., Mei, G. M., Chang, J. Q., Zhang, X. J., Gu, J., and Meng, C. Y., 2019. Species analysis and distribution characteristics of arsenic in sea crabs by inductively coupled plasma mass spectrometry., 40: 332-339, DOI: 10.7506/spkx1002-6630-20180424-315.
Yap, C. K., Cheng, W. H., Karami, A., and Ismail, A., 2016. Health risk assessments of heavy metal exposureconsumption of marine mussels collected from anthropogenic sites., 553: 285-296, DOI: 10.1016/j.scitotenv.2016.02.092.
Zhang, Z. M., Zhang, J., Zhang, H. H., Shi, X. Z., Zou, Y. W., and Yang, G. P., 2020. Pollution characteristics, spatial variation, and potential risks of phthalate esters in the water-sediment system of the Yangtze River Estuary and its adjacent East China Sea., 265: 114913, DOI: 10.1016/j.envpol.2020.
Zhao, Y. F., Kang, X. M., Shang, D. R., Zhai, Y. X., Ning, J. S., Ding, H. Y.,., 2020. Study of Cd content distribution and its bioaccessibility in edible tissues of crabfrom the coastal area of Shandong, China., 197 (1): 294-303, DOI: 10.1007/s12011-019-01968-0.
Zhao, Y. F., Shang, D. R., Ning, J. S., Zhai, Y. X., Ding, H. Y., and Sheng, X. F., 2017. Chemical speciation analysis of cadmium inand., 33 (5): 259-264, DOI: 10.13982/j.mfst.1673-9078.2017.5.041.
Zhou, S. S., Pan, Y. Q., Zhang, L. N., Xue, B., Zhang, A. P., and Jin, M. Q., 2018. Biomagnification and enantiomeric profiles of organochlorine pesticides in food web components from Zhoushan fishing ground, China., 131: 602-610, DOI: 10.1016/j.marpolbul.2018.04.055.
Zhou, S. S., Zhu, H. B., Huang, S. R., Zhou, J. Y., Zhang, S. W., and Wang, C. Z., 2019. Biomagnification and risk assessment of polychlorinated biphenyls in food web components from Zhoushan fishing ground, China., 142: 613-619, DOI: 10.1016/j.marpolbul.2019.04.024.
December 17, 2020;
April 14, 2021;
June 21, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2021
. E-mail: yangwenge@nbu.edu.cn
(Edited by Ji Dechun)
 Journal of Ocean University of China2021年6期
Journal of Ocean University of China2021年6期
- Journal of Ocean University of China的其它文章
- Meshless Method with Domain Decomposition for Submerged Porous Breakwaters in Waves
- Facial Features of an Air Gun Array Wavelet in the Time-Frequency Domain Based on Marine Vertical Cables
- Magma Evolution Processes in the Southern Okinawa Trough:Insights from Melt Inclusions
- Summery Intra-Tidal Variations of Suspended Sediment Transportation–Topographical Response and Dynamical Mechanism in the Aoshan Bay and Surrounding Area, Shandong Peninsula
- High-Resolution Geochemical Records in the Inner Shelf Mud Wedge of the East China Sea and Their Indication to the Holocene Monsoon Climatic Changes and Events
- Geological Guided Tomography Inversion Based on Fault Constraint and Its Application
