Isoprenylated Flavonoids as Ca v3.1 Low Voltage-Gated Ca2+ Channel Inhibitors from Salvia digitaloides
Jian-Jun Zhao ·Song-Yu Li ·Fan Xia·Ya-Li Hu ·Yin Nian ·Gang Xu
Abstract Saldigones A–C ( 1,3,4), three new isoprenylated flavonoids with diverse flavanone, pterocarpan, and isofl avanone architectures, were characterized from the roots of Salvia digitaloides, together with a known isoprenylated flavanone ( 2).Notably, it’s the fi rst report of isoprenylated flavonoids from Salvia species.The structures of these isolates were elucidated by extensive spectroscopic analysis.All of the compounds were evaluated for their activities on Ca v 3.1 low voltage-gated Ca 2+ channel (LVGCC), of which 2 strongly and dose-dependently inhibited Ca v 3.1 peak current.
Keywords Salvia digitaloides ·Isoprenylated flavonoid·Ca v 3.1 low voltage-gated Ca 2+ channel (LVGCC)
1 Introduction
Salvia, one of the largest genera of the widespread family Lamiaceae, is distributed widely in the temperate and warm zones worldwide [1].The nameSalviawas derived from the Latin word “Salvare”, meaning “to heal or to be safe and unharmed”, which rose the folkloric belief of its “magical” remedial function for various indispositions [2, 3].Phytochemical studies on more than 130Salviaspecies have led to an array of secondary metabolites, such as diterpenoids, phenolic acids, triterpenoids, sesquiterpenoids, sesterterpenoids, steroids, as well as flavonoids [1– 7].Diterpenoids with abietane or clerodane skeletons are considered as the main bioactive and characteristic chemical constituents of this genus [4,5,7].Many diterpenoids fromSalviaplants have shown antimicrobial, antioxidant, anti-HIV, antibacterial, antineoplastic, antiplatelet aggregation activities [3– 8].Attracted by their diverse chemical structures and extensive biological activities, many scientists have engaged in the phytochemistry, pharmacy, chemical synthesis, and biosynthesis of the chemical constituents ofSalviaplants [2,3,5,6].
Our group has been committed into the phytochemical studies of genusSalviasince 2000, and discovered a series of novel terpenoids with diverse structures and bioactivities [9– 14].As a part of our ongoing research about the plants ofSalviaspecies from southwestern China, unexpectedly, three new isoprenylated flavonoids saldigones A–C ( 1,3,4) (Fig.1) with various flavanone, pterocarpan, and isofl avanone architectures, were isolated from the roots ofS.digitaloides, along with a known isoprenylated flavonone, 5,7,3′-trihydroxy-4′,5′-(2⁗,2⁗-dimethylpyran)-8,2′-di(3-methyl-2-butenyl)-(2S)-fl avanone ( 2), obtained fromDendrolobium Ianceolatum[15].Terpenoids (accounted for more than 80%) and phenolic acid are the main chemical constituents of theSalviaspecies, while isoprenylated flavonoids fromSalviawere reported for the fi rst time [3– 6].For the two characterized isoprenylated flavanones ( 1 and 2), compound 1 owns both methylation and isoprenylation substitutions, and 2 has three isoprenyl groups in which one was further cyclized to form a 2,2-dimethylpyran unit.Moreover,3 and 4 were characterized as acisconfi gurated pterocarpan and an isofl avone skeletons with two isoprenyl substituents, respectively.

Fig.1 Structures of compounds 1– 4
Previous investigation indicated that some isoprenylated flavonoids led to an enhancement of activities or properties compared to the nonprenylated ones, such as cytotoxicity and bacterial neuraminidase inhibitory activities, but no low voltage-gated Ca 2+ channel (LVGCC) activators have been identifi ed [15– 18].Interestingly, isoprenylated substituents also appear to be important for anchoring certain natural products to the LVGCC [19].In this study, the biological studies revealed that compound 2 can strongly and dose-dependently inhibited the peak currents of Cav3.1 low voltage-gated Ca 2+ channel (LVGCC), an important therapeutic target for absence epilepsy, neuropathic pain, Parkinson’s disease, and insomnia [20– 22], with an IC 50 value of 3.5μM.Meanwhile,1,3, and 4 were inactive at the concentration of 30μM.In this note, the isolation and structural elucidation of these compounds were described, as well as their inhibitions on Cav3.1 LVGCC.
2 Result and Discussion
Saldigone A ( 1) was isolated as colorless gum a nd its molecular formula was determined as C27H32O6by HRESIMS (m/z451.2128 [M – H] − , calcd C27H31O 6 , 451.2126).The IR spectrum displayed bands for hydroxy (3411 cm −1 ) and carbonyl (1626 cm −1 ).The presence of a flavanone skeleton was evident from the characteristic absorption bands at λmax345 and 297 nm in the UV spectra, the ABX-type spin system (δH5.26, 1H, dd,J= 12.8, 3.0 Hz, H-2; 2.78, 1H, dd,J= 17.0, 3.0 Hz, H-3α; and 2.97, 1H, dd,J= 17.0, 12.8 Hz, H-3β) in the 1 H NMR spectrum (Table 1), conjugated with the oxygenated carbon resonance atδC78.8 (C-2), a methylene resonance atδC43.6 (C-3), and a carbonyl resonance atδC196.7 (C-4) in the 13 C NMR spectra [23– 25].The 13 C NMR and DEPT data presented a total of 27 carbon signals attributable to three signals for C-2, C-3, and C-4 mentioned above, 12 signals for two phenyls, as well as 12 other signals for one methyl (δC7.1, C-1″), one methoxyl group (δC61.5, MeO-4′), and two isoprenyls (δC22.2, C-1; 121.9, C-2; 133.6, C-3; 26.0, C-4; 18.1, C-5/28.4, C-1⁗; 122.2, C-2⁗; 136.0, C-3⁗; 26.0, C-4⁗; 18.1, C-5⁗).Except for the signals for H-2 and H-3, three hydroxy groups (δH12.25, 1H, s, OH-5; 6.14, 1H, s, OH-7; 5.64, 1H, s, OH-5′) and one methoxy group (δH3.79, 3H, s, OMe-4′) can also be easily distinguished in the 1 H NMR spectrum.
The locations of these oxygenated substituents as well as the isoprenyl and methyl groups were determined by the HSQC and HMBC experiments (Fig.2).The cross-peaks of OH-5 with C-5 (δC 160.1), C-6 (δC 104.7), and C-10 (δC103.0), and the methyl proton (δH2.02, 3H, s, H-1″) with C-5, C-6, and C-7 (δC162.2) indicated that the hydroxyl group was attached to C-5 while the methyl was substituted at C-6.The correlations of OH-7 with C-6, C-7, and C-8 (δC105.3), and a set of isoprenyl protons (δH3.35, 2H, t, H-1) with C-7, C-8, and C-9 (δC 157.3) indicated that the isoprenyl group located at C-8 while the hydroxyl group was attached to C-7 of A-ring.In addition, B ring was established as a 3′-hydroxy-4′-methoxy-5′-isoprenyl substitutions by the HMBC correlations of OH-3′ with C-2′ (δC111.2), C-3′ (δC149.3), and C-4′ (δC145.3), of the methoxyl with C-4′, and of the isoprenyl protons (δH3.35, 2H, t, H-1⁗) with C-2′, C-3′, and C-4′.The 1 H- 1 H COSY correlation between H-2 and H-3, and HMBC correlations of H-2 with C-4, C-9, C-2′, and C-6′ (δC119.1), and of H-3 with C-4, C-10 (δC103.0) and C-1′ (δC135.8) confi rmed the existence of C ring furthermore.
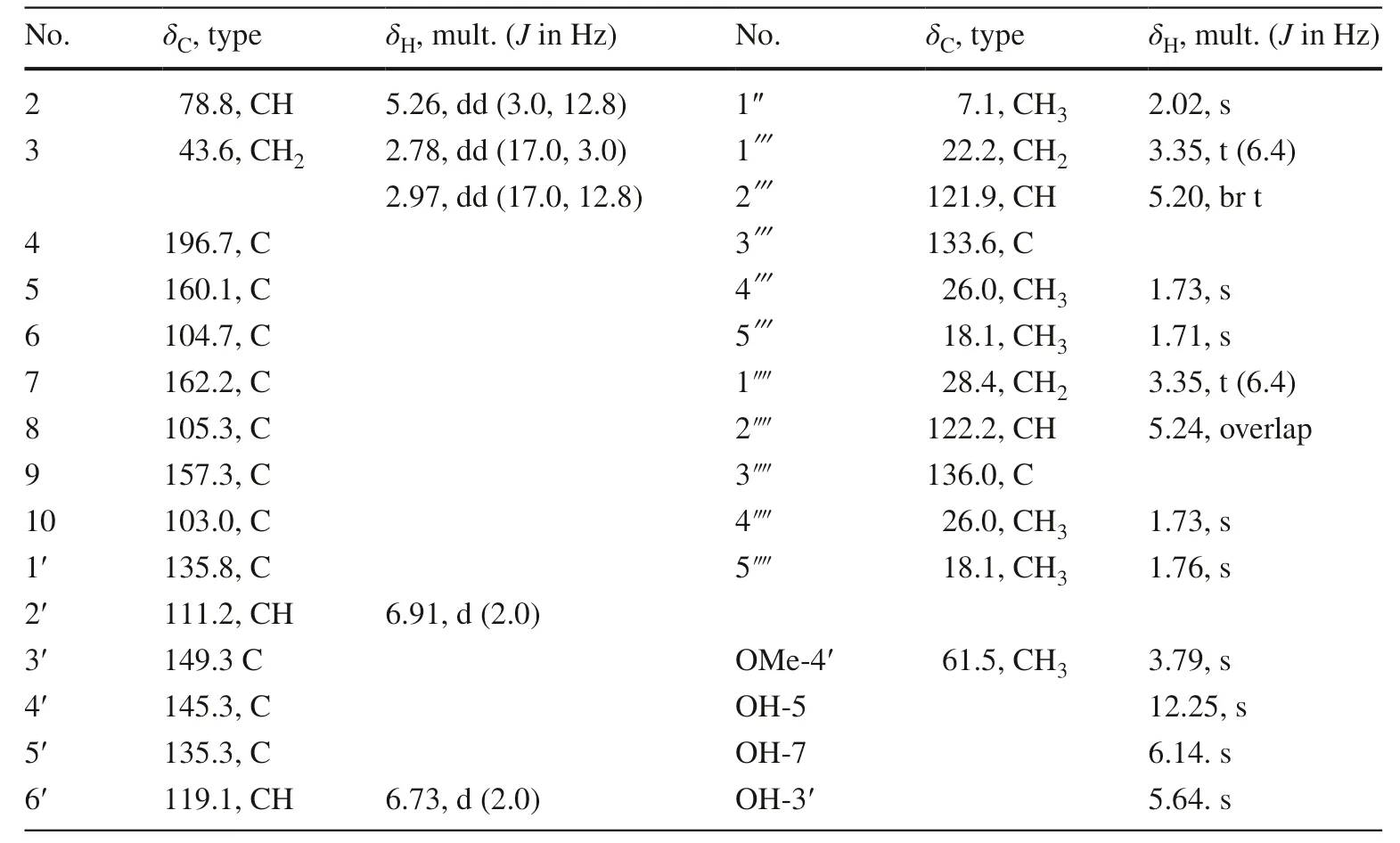
Table 1 13 C (150 MHz) and 1 H (600 MHz) NMR data of 1 (in CDCl 3 )
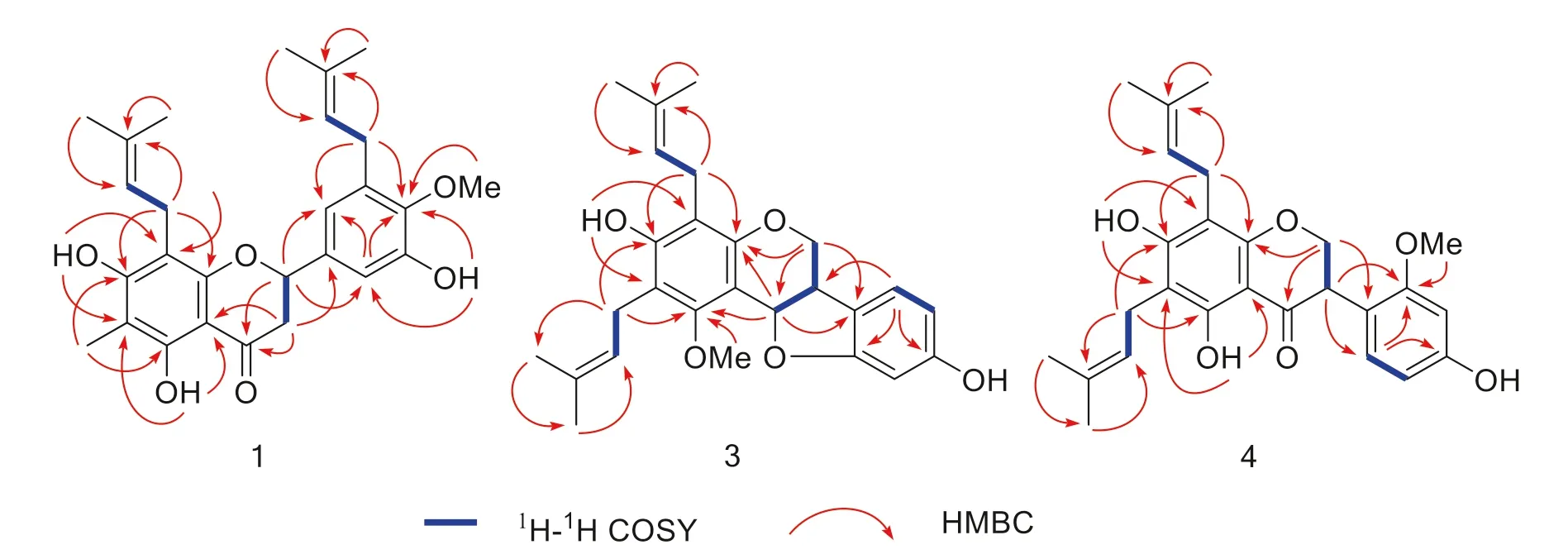
Fig.2 Key HMBC and 1 H- 1 H COSY correlations of 1,3,4
Biogenetically, flavanones are formed by cyclization of chalcones mediated by the enzyme chalcone isomerase (CHI) in a highly stereospecifi c manner through a Michael nucleophilic reaction involving the 2′-OH group and theα,β-unsaturated ketone of the chalcone, leading almost exclusively to the (2S)-isomer [26,27].As for compound 1, the circular dichroism (CD) spectra gave the sequential positive and negative Cotton eff ects near 330 and 280 nm for then-π* andπ-π* electronic transitions (Fig.3), respectively, which were consistent with the absolute confi guration 2Saccording to the reported data [25,28– 31].Therefore, the structure of 1 was determined as (2S)-5,7,3′-trihydroxy-6-methy-8,5′-diisoprenyl-4′-methoxy flavanone named saldigone A.
The structure of isoprenylated flavonone 2 was elucidated by analysis of 1D, 2D NMR, and CD spectral data confi rmed by literature data comparison [15].
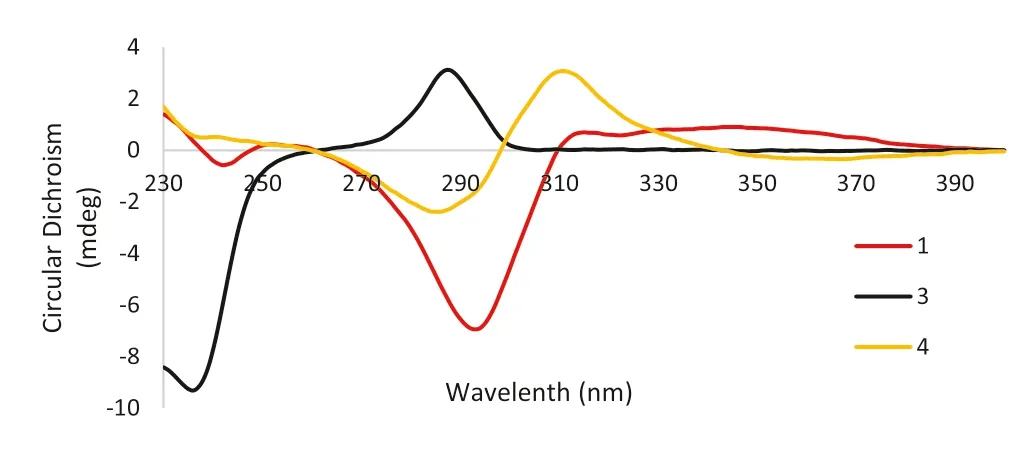
Fig.3 CD spectra of compounds 1 (red),3 (black) and 4 (yellow)
Saldigone B ( 3) was isolated as a white powder and was assigned the molecular formula C26H30O5, based on HRESIMS (m/z421.2021 [M – H] − , calcd.C26H29O5, 421.2020).The IR spectrum displayed bands for hydroxy (3427 cm −1 ).The UV spectrum showed absorption bands at λ max 286 nm.In the 1 H NMR spectrum (Table 2), the characteristic set of signals atδH3.35 (1H, br t,J= 6.6 Hz, H-6a),δH4.19 (1H, dd,J= 11.0, 4.9 Hz, H-6α),δH3.57(1H, br t,J= 11.0 Hz, H-6β), andδH5.65 (1H, d,J= 6.6 Hz, H-11a), together with the corresponding carbon signals atδC39.2,δC66.7,δC76.7 for the typical C3unit (C-6a, C-6, and C-11a) in the 13 C NMR displayed that 3 possessed a pterocarpan skeleton [18,32,33].Its 13 C and DEPT NMR spectra displayed 26 carbon resonances attributable to the C3unit mentioned above, 12 signals for two phenyls, as well as 11 other signals for one methoxyl (δC63.2, MeO-1), and two isoprenyls groups (δC 23.4, C-1′; 122.7, C-2′; 134.8, C-3′; 18.2, C-4′; 18.1, C-5′/22.5, C-1″; 122.5, C-2″; 133.6, C-3″; 26.0, C-4″; 26.0, C-5″).The pterocarpan framework can be further evidenced by the 1 H- 1 H COSY cross peaks of H-6/H-6a/H-11a, couple with the HMBC correlations from H-6 to C-4a (δC152.9), C-11a, and C-6b (δC119.8) as well as from H-11a to C-1 (δC157.1), C-4a, C-11b (δC106.7), and C-6b (Fig.2).
According to the molecular formula (C26H30O 5 ), there should be two OH groups in the structure except for the signals for the two isoprenyls, one methoxy, and 9 signals (including H-6, 2H; 6a, 1H; H-7, 1H, d,J= 7.7 Hz; H-8, 1H, d,J= 6.33; H-10, 1H, s; H-11a, 1H, and two oxygen atoms) for the pterocarpan skeleton.One of the two OH groups were located on C-3 (δC155.6) by the HMBC correlations of OH-3 (δH5.66, s) with C-2 (δC114.2), C-3, and C-4 (δC112.1).Then, the remaining OH group was attached on C-9 evidenced by the side-by-side comparison of the NMR spectral data with those of eryvarin J [32], as well as the HMBC correlations of H-7/8/10 with distinguished oxygenated quaternary carbon signal for C-9 atδC 157.1.
Structurally, there are two chiral centers (C-6a, 11a) in the pterocarpans skeleton whose absolute confi gurations are exclusivelycis(6aR/11aRor 6aS/11aS) over thetransin nature [23,28].As reviewed previously, the positive 1 Lb-band (260–310 nm) and negative 1 La-band (220–240 nm) indicated the (6aR, 11aR)-cisconfi guration of pterocaroans [22,28].As for compound 3, the diagnostic positive Cotton eff ect at 287 nm (Δε+ 8.35) and negative Cotton eff ect at 236 nm (Δε− 25.07) observed in the CD spectrum (Fig.3) conformed thecisconfi guration of 3 as well as both theRabsolute confi gurations of C-6a and C-11a [19,22,23].Then,3 was characterized as (6aR,11aR)-3,9-dihydroxy-2,4-diisoprenyl-1-methoxy pterocarpan named saldigone B.
Saldigone C ( 4) was isolated as pale-yellow gum and assigned a molecular formula of C26H30O6, based on HRESIMS (m/z437.1968 [M – H] − , calcd.C26H29O6, 437.1970).The IR spectrum displayed bands for hydroxy (3429 cm −1 ) and carbonyl (1618 cm −1 ).It was deduced to be an isofl avanone based on the characteristic absorption bands in the UV spectrum (λmax254 and 296 nm) together with three typical aliphatic proton signals (δH4.47, 1H, br t,J= 11.1 Hz, H-2α; 4.41, 1H, dd,J= 11.1, 5.5 Hz, H-2β;4.27, 1H, dd,J= 11.1, 5.5 Hz, H-3) in the 1 H NMR spectrum (Table 3).This deduction can be further confi rmed by the corresponding oxygenated methylene resonance (δC70.7, C-2), a methine resonance (δC46.9, C-3), and a carbonyl resonance at (δC198.2, C-4) exclusively for an isofl avanone skeleton observed in the 13 C NMR spectra [34,35].Furthermore, the 13 C NMR and DEPT data displayed that the presence of totally 26 carbon signals owing to the signals for C-2, C-3, and C-4 described above, 12 signals for two phenyls, as well as 11 other signals for one methoxyl (δC55.8, MeO-2′) and two isoprenyls (δC21.5, C-1″; 122.1, C-2″; 134.9, C-3″; 18.1, C-4″; 26.1, C-5″/21.9, C-1; 122.4, C-2; 134.2, C-3; 18.1, C-4; 26.1, C-5).
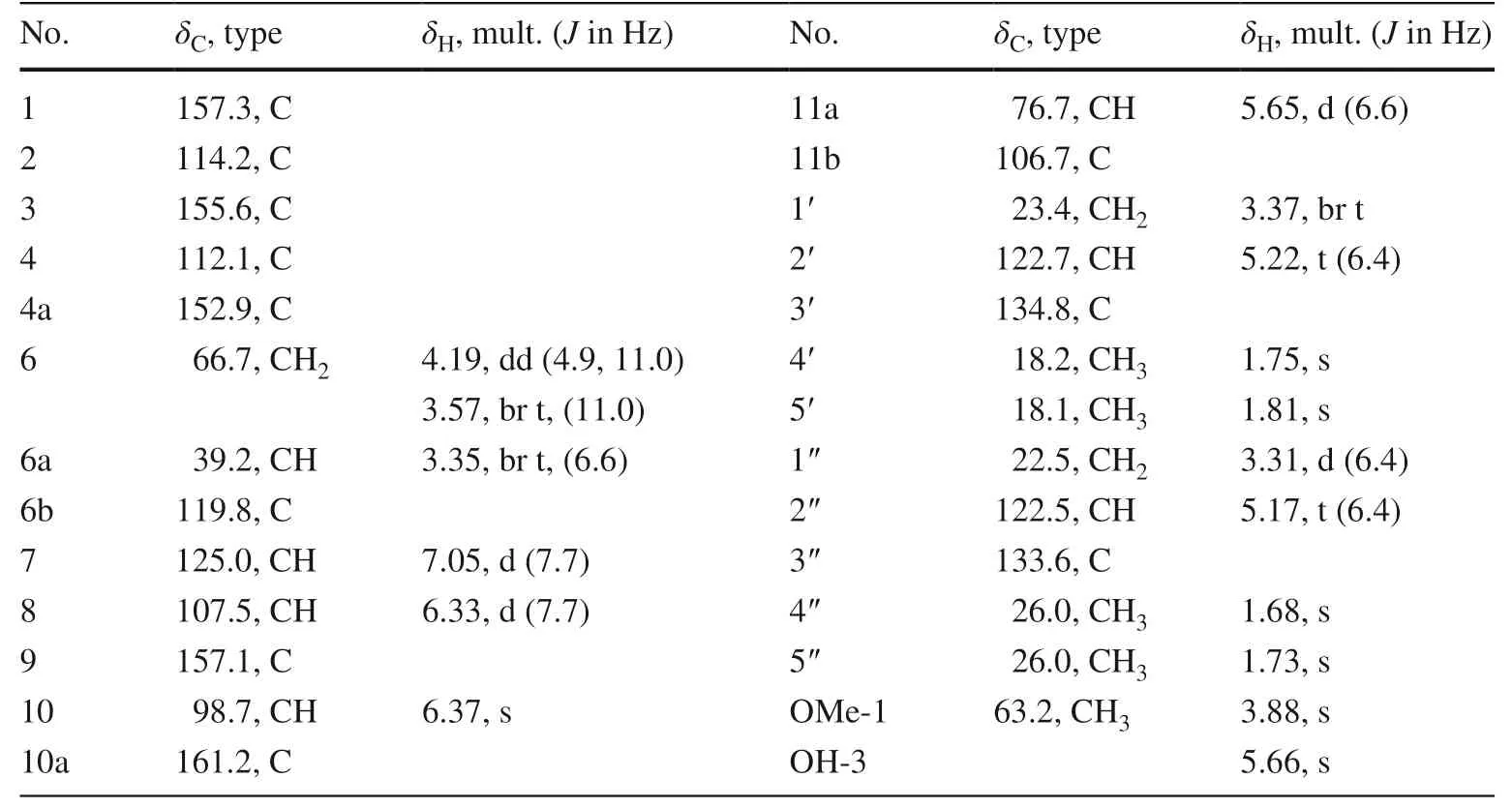
Table 2 13 C (150 MHz) and 1 H (600 MHz) NMR data of 3 (in CDCl 3 )
Side-by-side comparison of the 1D NMR data of 4 with those of eryzerin B indicated their structures were closely similar to each other except for the existence for an additional hydroxyl group (δH12.53) in 4 as evidenced by the MS and 1D NMR [35].And the location of this OH group on C-5 was deduced by its HMBC correlations with C-5 (δC159.9), C-6 (δC107.3), and C-10 (δC103.4).In addition, the HMBC correlations of the isoprenyl protons atδH3.33 (1H, d,J= 7.1 Hz, H-1″) andδH3.27 (1H, d,J= 7.1 Hz, 1) with C-5/6/7(δC 162.2) and C-7/8 (δC 106.3)/9 (δC 158.4), respectively, as well as of methoxy protons (δH 3.74, 3H, s, MeO-2′) to C-2′ (δC158.8) indicated that the isoprenyls and one methoxyl groups were attached to C-6, C-8, and C-2′, respectively.
To analogy the data for similarly substituted isofl avanone systems, the positive Cotton eff ect near 310 nm and the negative Cotton eff ect near 280 nm (Fig.3) indicated that the absolute confi guration at C-3 wasRfor 4 [25,28,35].Thus,4 was characterized as (3R)-5,7,4′-thihydroxy-2′-methoxy-6,8-diisoprenyl isofl avanone named saldigone C.
As mentioned above, isoprenyl substituents appear to be important for anchoring some natural products to the low voltage-gated Ca 2+ channel (LVGCC) [19].The inhibitors of Cav3.1 LVGCC are promising agents for drug development against a number of neurological disorders [20,21].In this study, compound 2 was also tested to exhibit strong inhibitory activity on Cav3.1.In HEK 293 cells expressing human Cav3.1, 10μM 2 notably antagonized currents that elicited by depolarizing voltage steps (− 80 to + 60 mV) (Fig.4 A).It took approximate 120 s to arrive the steady-state inhibition after the perfusion of 2 and this eff ect was hard to reverse upon washout (Fig.4 B), suggesting that the binding sites of 2 may be buried deeply inside the channel.Dose–response studies showed that 2 inhibited Cav3.1 peak currents with an IC50value of 3.5μM (Fig.4 C and Table S1).While, the Hill coeffi cient of 2 is 0.9, indicating a negative cooperativity.Mibefradil, the positive control, indicates a stronger activity than 2, with an IC50value of 1.12μM (Fig.4 D and Table S1).Moreover, compounds 1,3 and 4 showed negligible eff ects on Cav3.1 at the higher concentration of 30μM (Fig.S1).
In conclusion, saldigones A–C ( 1, 3, 4), three new isoprenylated flavonoid constituents featured with diverse flavanone, pterocarpan, and isofl avanone architectures, were characterized fromS.digitanoides,along with a known isoprenylated flavonone ( 2).To our knowledge, it’s the fi rst report of isoprenylated flavonoid type metabolites from theSalviaplants despite many flavonoids have been isolated from this genus [3– 8].In detail, compounds 1 and 2 are isoprenylated flavonoids with methylation and isoprenylation substitutions, while 3 and 4 are two isoprenylated flavonoids with diff erent pterocarpan and isofl avanone skeletons, respectively.In addition, compound 2 was proved to be a potent inhibitor of Cav3.1 LVGCC, indicating thatisoprenylated flavonoids are deserved further in-depth exploration in genusSalvia.Totally, these fi ndings could enrich diversities of secondary metabolites of the genusSalvia, and increased universality of biological activities and properties.
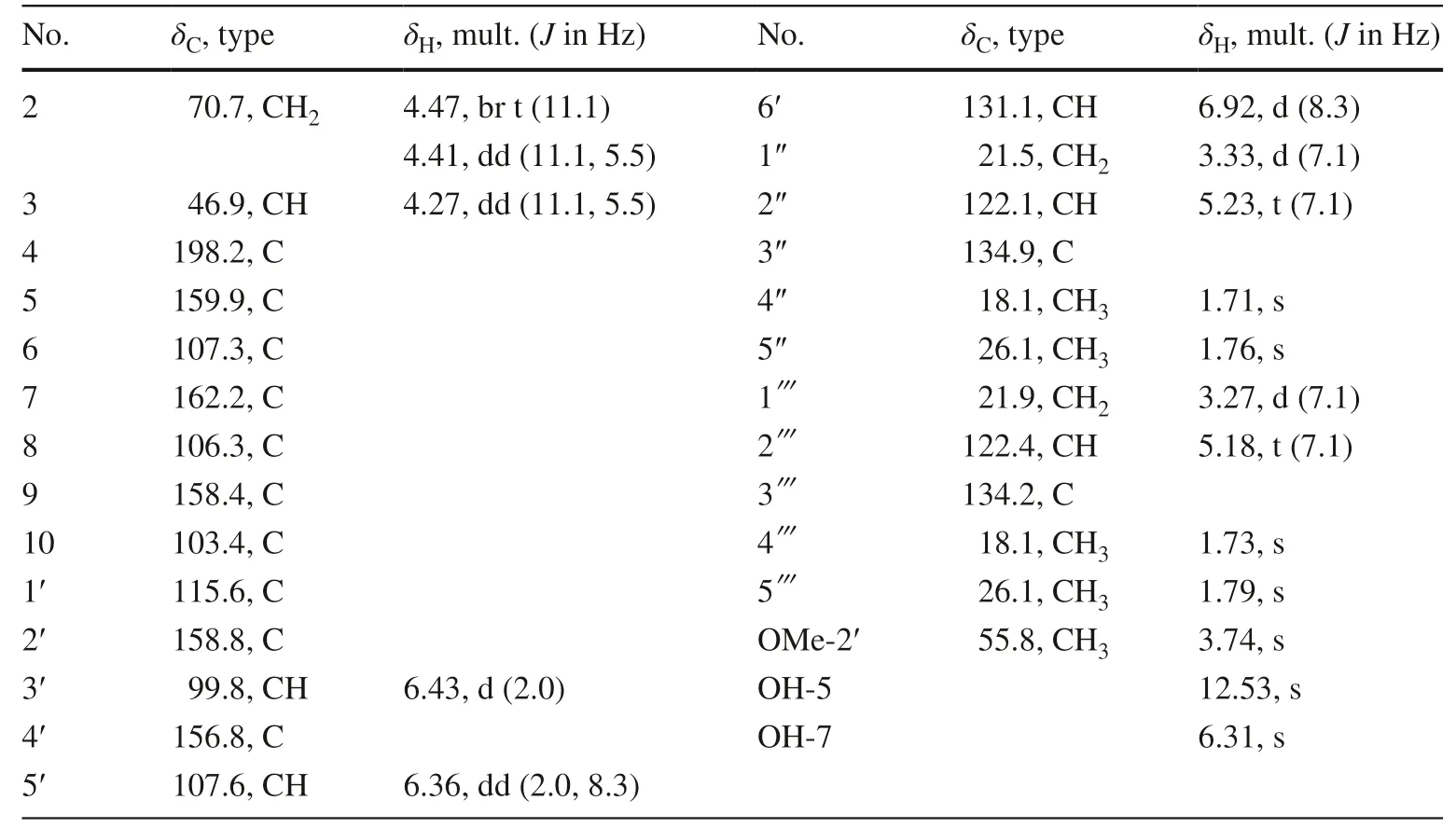
Table 3 13 C (150 MHz) and 1 H (600 MHz) NMR data of 4 (in CDCl 3 )

Fig.4 Inhibitory eff ect of Compound 2 on Ca v 3.1.A Normalized current–voltage (I–V) curves of Ca v 3.1 in the absence or presence of 10 μ M 2.Ca v 3.1 Currents were evoked from a holding potential (HP) of − 100 mV by 150 ms depolarization from − 80 to + 60 mV in 10 mV increment at 4 s intervals.Data are represented as mean ± SD (n = 3).B Time course of peak current inhibition by 10 μ M 2.Ordinate axis, peak current during exposure to 2, which normalized by the peak current after drug exposure.C Representative Ca v 3.1 peak current trace that elicited by 150 ms depolarization to − 40 mV at 4 s intervals from a HP of − 100 mV in the absence (Bath) and presence of various concentrations of 2.D Dose–response curve of inhibiton of the peak current by 2.Solid curve represents fi t to the Hill equation.Data are represented as mean ± SD (n = 3)
3 Experimental
3.1 General Experimental Procedures
Silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical Co., Ltd., Qingdao, China), MCI gel (75–150μm, Mitsubishi Chemical Corporation, Tokyo, Japan) were used for column chromatography.Fractions were monitored by TLC (GF 254, Qingdao Marine Chemical Co., Ltd.Qingdao, China), and spots were visualized by heating silica gel plates immersed in 8% H2SO4in ethanol.Semi-preparative HPLC was performed on Waters 2695 HPLC with a COSMOSIL C 18 -MS-II (4.6ID × 250 mm) packed column.1D and 2D NMR spectra were recorded on a Bruker DRX-600 spectrometer using TMS as an internal standard.Unless otherwise specif ied, chemical shifts (δ) are expressed in ppm with reference to the solvent signals.ESIMS and HRESIMS data were acquired on Agilent G6200 TOF mass spectrometer.Optical rotations were measured on a Jasco P-1020 polarimeter.UV spectra were recorded on a Shimadzu UV-2401PC spectrometer.IR spectra were recorded on a Bruker FT-IR Tensor-27 infrared spectrophotometer with KBr disks.
3.2 Plant Material
Roots ofS.digitaloideswere collected in Haba, Yunnan Province, People’s Republic of China, in August, 2011.The plant material was identifi ed by Dr.Chun-Lei Xiang, Kunming Institute of Botany, Chinese Academy of Sciences.A voucher specimen was deposited in Kunming Institute of Botany, Chinese Academy of Sciences with identifi cation number 20110788.
3.3 Extraction and Isolation
The air-dried roots ofS.digitaloides(25.0 kg) were extracted with acetone for three times at room temperature (each 100 L, 3 days).The combined extracts were concentrated, the crude extract (730 g) was subjected to a silica gel column chromatography successively eluted with CHCl3, EtOAc and MeOH.The EtOAc fraction (180 g) was chromatographed on a silica gel column, eluted with petroleum ether/EtOAc (from 500:1 to 0:1, v/v), to yield fi ve fractions (Fr.A–E) on the basis of TLC analysis.Fraction C (130.0 g) was separated through MPLC on an MCI gel column (MeOH-H2Ofrom 60:40 to 100:0) to obtain four fractions (Fr.C1 – C4).Fraction C1 (20.0 g) was then chromatographed on a silica gel column, eluted with petroleum ether/acetone (from 200:1 to 0:1, v/v), to yield six fractions (Fr.C1-1 – C1-6).
Fraction C1-6 (4.0 g) was subjected to column chromatography over a silica gel column eluted with petroleum ether/CHCl3/EtOAc (9/0.7/0.3) to yield seven fractions (C1-6-1 – C1-6-7).Fraction C1-6–2 (900 mg) was chromatographed on Sephadex LH-20 (acetone) and further purifi ed by semi-preparative HPLC (MeCN – H3O+ , 72:28) to aff ord compounds 1 (2.9 mg,t R= 11.0 min),2 (2.7 mg,t R= 12.9 min),3 (7.5 mg,t R= 16.1 min) and 4 (2.2 mg,t R= 17.3 min).
3.3.1 Saldigone A ( 1)
Colorless gum; [α]− 28.3 (c0.08, MeOH); UV (MeOH) λmax(logε) 345 (3.63), 297 (4.27), 195 (5.01), 328 (3.57), 254 (3.55) nm; CD (MeOH;c1.19 × 10–4): Δε350 (+ 1.74), 293 (− 14.15), 252 (+ 0.47), 242 (− 1.77), 227 (+ 3.57), 215 (− 1.74), 205 (+ 11.52) nm; IR (KBr)vmax3411, 2922, 1626, 1607, 1374, 1186, 845, 554 cm−1;1H and13C NMR data, see Table 1; ESIMSm/z451 [M – H] − ; HRESIMSm/z451.2128 [M – H] − (calcd for C27H31O6, 451.2126).
3.3.2 Saldigone B ( 3)
White powder; [α]− 187.3 (c0.09, MeOH); UV (MeOH) λmax(logε) 286 (4.06), 208 (5.06), 257 (3.56), 203 (5.04) nm; CD (MeOH;c1.1 × 10 –4 ): Δε287 (+ 8.35), 236 (− 25.07), 229 (− 22.58), 215 (− 49.89), 203 (+ 19.75) nm; IR (KBr)vmax3427, 2924, 1608, 1496, 1354, 1264, 1142, 1124, 1099, 959, 839, 445 cm−1;1H and13C NMR data, see Table 1; ESIMSm/z421 [M – H] − ; HRESIMSm/z421.2021 [M – H] − (calcd for C26H29O5, 421.2020).
3.3.3 Saldigone C ( 4)
Pale yellow gum; [α]+ 10.9 (c0.08, MeOH); UV (MeOH) λmax(logε) 194 (4.79), 196 (4.81), 254 (3.56), 296 (4,08), 420 (2.35) nm; CD (MeOH;c2.2 × 10–4): Δε367 (− 0.48), 311 (+ 4.24), 285 (− 3.29), 215 (+ 6.69), 199 (− 10.45) nm; IR (KBr)vmax3429, 2924, 1618, 1468, 1178, 989, 567, 486 cm −1 ; 1 H and 13 C NMR data, see Table 1; ESIMSm/z437 [M – H] − ; HRESIMSm/z437.1968 [M – H] − (calcd for C26H29O6, 437.1970).
3.3.4 Cell Preparation and Expression
Human embryonic kidney (HEK) 293 cells were grown in DMEM (HyClone) plus 10% newborn calf serum (Gibco) and penicillin (100 U/ml)/streptomycin (0.1 mg/ml) (Biological Industries).HEK 293 cells were transiently transfected with pCDNA3.1-T type and pCDNA3.1-EGFP plasmids together using LipoD293™ (SignaGen Laboratories) and used in 48 h.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s13659- 021- 00307-y.
AcknowledgementsThe work was fi nancially supported by the Foundation of Supported by National Natural Science Foundation of China (32070392 and 32070393) and the Second Tibetan Plateau Scientifi c Expedition and Research (STEP) program (2019QZKK0502-0303).
Declarations
Conflict of interestThe authors declare no competing fi nancial interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http:// creat iveco mmons.org/ licen ses/ by/4.0/.
 Natural Products and Bioprospecting2021年6期
Natural Products and Bioprospecting2021年6期
- Natural Products and Bioprospecting的其它文章
- Scutellarin Reduces Cerebral Ischemia Reperfusion Injury Involving in Vascular Endothelium Protection and PKG Signal
- Furostanol Saponins from Asparagus cochinchinensis and Their Cytotoxicity
- Six New 3,5-Dimethylcoumarins from Chelonopsis praecox,Chelonopsis odontochila and Chelonopsis pseudobracteata
- Anticancer Properties and Mechanism of Action of Oblongifolin C, Guttiferone K and Related Polyprenylated Acylphloroglucinols
- Natural Products as Potential Lead Compounds for Drug Discovery Against SARS-CoV-2
- Advances in Research on Chemical Constituents and Their Biological Activities of the Genus Actinidia
