An in vitro and in silico study of anti-dermatophytic activity of gossypol from fruits of Thespesia populnea (L.) Sol. ex Correa
Anuthara R, Sebastian Jose Midhun, Jyothis Mathew
Microbiology Research Lab, School of Biosciences, Mahatma Gandhi University, Kottayam, Kerala, India
ABSTRACT
Objective: To isolate, purify, and characterize gossypol from the fruits of Thespesia populnea (L) Sol. ex Correa, test its antidermatophytic activity, identify its targets on the dermatophyte, and confirm the binding of gossypol with the fungal target by molecular docking study.
Methods: Gossypol from Thespesia populnea was characterized by high performance liquid chromatography, liquid chromatographmass spectrometry, Fourier transform infrared spectroscopy, and nuclear magnetic resonance. The anti-dermatophytic activity of gossypol was tested against four different dermatophytes, viz.Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis, and Microsporum gypseum. Trichophyton mentagrophytes was selected for further studies. The inhibitory mode of action of gossypol on Trichophyton mentagrophytes was determined by analyzing the modulation of gene expression in various pathways of the dermatophyte.
Results: Gossypol inhibited all the dermatophytes. The minimum inhibitory concentrations were 12.5 μg/mL for Trichophyton mentagrophytes and Microsporum canis and 25 μg/mL for Trichophyton rubrum and Microsporum gypseum. The minimum fungicidal concentrations were 50 μg/mL for Trichophyton mentagrophytes,100 μg/mL for Microsporum canis and Trichophyton rubrum, and 200 μg/mL for Microsporum gypseum. Gossypol inhibited the mRNA expression of metalloprotease (MEP4) and isocitrate lyase (ICL). The binding of gossypol with the enzymes was confirmed by molecular docking studies. The best docking poses were found and the low binding energies were recorded with the two target enzymes.
Conclusions: Gossypol is a potential antifungal agent and can be further explored as an anti-dermatophytic drug.
KEYWORDS: Thespesia populnea; Gossypol; Anti-dermatophytic activity; Trichophyton mentagrophytes; Mechanism of action; Gene
1. Introduction
Dermatophytoses are cutaneous mycoses or skin infections caused by a group of keratinophilic fungi known as dermatophytes. It is otherwise known as ringworms, or tineas, which especially occur in immune-suppressed patients. Dermatophytes mainly infect the skin, hair, nail, and subcutaneous tissues in humans and animals[1,2].Many drugs of natural and synthetic origin have been developed for the treatment of mycotic infections. Only a very few drugs have been developed for the treatment of mycoses, even though there are serious works carried out for the development of new therapeutics.Many antifungal agents against invasive mycoses are classified by their site of action in fungal cells[3]. Many classes of antifungals,such as morpholine and allylamines, are used as topical agents due to their reduced efficacy or adverse effects when administered systemically. The absence of selective toxicity denies their application clinically. Since fungi have an almost similar structure and metabolism as mammalian cells, these drugs indiscriminately disrupt the normal processes of both fungal and host cells[3]. Since the majority of the antifungal drugs used in the treatment of such infections have several drawbacks, novel and effective antifungals from alternative sources are urgently needed. Many herbs show therapeutic potential in the treatment of various illnesses and traditional folk medicines could provide satisfactory phytoremedy for fungal infections[4-6].
Thespesia populnea (T. populnea) (L.) Sol. ex Correa, commonly known as ‘Indian tulip tree’ or ‘Portia tree’ of Malvaceae family is a small to medium-sized tree with a pantropical distribution,generally found along the coastal stretches. The bark, fruits, leaves,and flowers are useful in cutaneous infections, such as scabies,eczema, psoriasis, ringworm, and guinea worm. A bark decoction is commonly used for the treatment of skin and liver diseases. In South India, the yellow sticky sap secreted from the cut of young fruits is traditionally used for the treatment of ringworm and other skin diseases. The leaves are also used for the anti-inflammatory treatment of inflamed and swollen joints[7]. de Lima et al., reported the presence of gossypol in genera related to cotton and concluded that there is a correlation between the presence of pigment glands and gossypol[1]. In this context, T. populnea was also reported to contain pigment glands and gossypol. King and de-Silva reported the presence of + gossypol along with ± gossypol in the bark and flowers of T. populnea[8]. The fruit kernels were reported to contain a yellow pigment thespesin, which was later reported as gossypol[9].
This paper isolated, purified, and characterized gossypol from the fruit extract of T. populnea and tested its anti-dermatophytic activity against 4 different dermatophytes. The target of gossypol was explored by studying the modulation of gene expression and was confirmed by molecular docking.
2. Materials and methods
2.1. Reagents and drugs
Sabouraud’s Dextrose Agar Medium (SDA) purchased from HiMedia, India, cycloheximide (Sigma, India), chloramphenicol(HiMedia, India), sterile Petri plates (HiMedia, India), Sabouraud’s dextrose broth medium (SDB) (M033) (HiMedia, India), griseofulvin(Sigma, India), and dimethyl sulfoxide (DMSO) (HiMedia, India).
2.2. Preparation of plant material
T. populnea was collected from Marayoor, the district of Idukki,Kerala from December to April. T. populnea has been authenticated by the plant taxonomist Dr. Jomy Augustine, St. Thomas College,Palai, Kottayam, Kerala, and a voucher specimen AR 4752 was maintained at the Institute. The fruits were washed thoroughly under running tap water; shade dried, powdered in a blender, and sieved separately. This powder was used for the isolation of gossypol.
2.3. Isolation and purification of gossypol from fruits of T.populnea
Isolation and purification of gossypol were done according to the modified method of Datta et al.[10]. The shade dried fruits of T. populnea were powdered using a blender and sieved. A total of 100 g of this powder was extracted with petroleum ether until no more color was coming out. It was subsequently extracted with acetone, and the solvent was removed using a rotary evaporator.The concentrate residue obtained was dissolved in ether and poured into a column of silica gel (60-120 mesh) and eluted with 10%ether in petroleum ether. Re-crystallisation of this sample with benzene-petroleum ether yielded yellow crystals. The compound thus obtained was compared with gossypol standard (Sigma Aldrich,Bengaluru,) on high-performance thin-layer chromatography(HPTLC). Densitometric scanning was done by a Camag TLC scanner 4, and the data obtained were analyzed with the help of winCats 1.8.4.software. The chromatogram showed a single band with solvent system benzene-ethylacetate-formic acid in the ratio 5:4:1 when visualized at 366 nm[11].
2.4. Characterization of the purified compound
The isolated yellow crystals were subjected to high-performance liquid chromatography (HPLC), HPTLC, Fourier transform-infra red(FT-IR) spectroscopy, liquid chromatography-mass spectrometryquadrupole time-of-flight (LCMS-Q-ToF), and nuclear magnetic resonance spectroscopy (NMR) analysis.
2.4.1. HPLC
HPLC analysis was done on Shimadzu prominence ultra-fast liquid chromatography, HPLC system equipped with pump LC 20AD and UV-vis detector SPD M20A/ 20AV and LC solution software. The column used was EnableRPC18G with column dimensions 250 mm×4.6 mm×5 μm, and 120 Å pore size. A total of 20 μL of the sample was filtered through a syringe filter of pore size 22 μm and injected into the sample applicator. Methanol and water (9:1) at a flow rate of 1 mL/min was the mobile phase used. The purified compound was compared with standard gossypol (purchased from Sigma Aldrich,Bangalore, India).
2.4.2. HPTLC
HPTLC was carried out on a Camag HPTLC instrument,Switzerland, using Silica gel 60F 254 plates of dimensions 20 cm×20 cm (Merck, Mumbai, India). The mobile phase used was benzene-ethyl acetate-formic acid at the ratio 5:4:1 for 20 min at room temperature. The developed plates were visualized at 366 nm. Densitometric scanning was done by a Camag TLC scanner 4, and the data obtained were analyzed with the help of winCats 1.8.4.software. Gossypol standard was run along with the sample and compared.
2.4.3. FT-IR
A total of 2.5 mg of purified gossypol was dissolved in 0.5 mL of methanol in a screw-caped vial. The solution was added dropwise into the attenuated total reflection attachment. The spectra were analyzed after the solution was evaporated at room temperature using a Thermo fisher scientific Nicolet IS50 FT-IR spectrophotometer in the attenuated total reflection mode. An infrared absorption frequency scale of 4 000-500 cmwas used.
2.4.4. LCMS
Ultra performance liquid chromatography (UPLC) analysis was performed on Waters Xevo G2 Q-ToF mass spectrometer coupled with a Waters Acquity H class UPLC system with ACQUITY UPLCBEH C18 column (50 mm×2.1 mm×1.7 μm; Waters,Milford, MA, USA). A binary system of HO + 0.1% formic acid(solvent A) and acetonitrile (solvent B) gradient was used for elution for 10 min. Mass spectrometer (MS) analysis was done on Xevo G2 (Waters) Q-TOF-MS. For MS analysis, the same optimized solvent system was used as the mobile phase. The scanning was done between m/z 50-1 000 KDa ranges. Electrospray operating conditions were optimized for analyzing negative ionization mode(dry temperature 350 ℃, nebulizer pressure 50 psi, capillary voltage 2.5 kV, cone voltage 35 V, and source temperature 135 ℃).
2.4.5. NMR
H NMR andC NMR spectra of the purified compound were recorded using the DMSO d6 solvent system at 400 MHz in Bruker 400 MHz Avance Ⅲ HD FT NMR spectrometer (Germany). The chemical shifts were plotted in ppm. Coupling instants (J) were plotted in Hz, and the multiplicity of the signal was indicated as single (s), doublet (d), and multiplet (m). The instrument used was Bruker 400 MHz Avance Ⅲ HD FT NMR spectrometer (Germany).
2.5. Study of anti-dermatophytic activity of gossypol purified from fruits of T. populnea
The following dermatophytes were procured from Microbial Type Culture Collection (MTCC), IMTECH (Institute of Microbial Technology, Chandigarh: Trichophyton mentagrophytes (T.mentagrophytes) MTCC 7687, Trichophyton rubrum (T. rubrum)MTCC 296, Microsporum canis (M. canis) MTCC7686, and Microsporum gypseum (M. gypseum) MTCC 2819. All cultures were grown on Sabouraud dextrose agar (SDA) medium at 28 ℃ and stored at 4 ℃, except T. rubrum, which was maintained in modified Sabouraud dextrose agar (M033) at 28 ℃. Both SDA and modified SDA (M033) media were procured from HiMedia Laboratories,Mumbai, India.
2.5.1. Preparation of fungal inoculum
The dermatophytes were grown on SDA slants by incubating at 28 ℃ for three weeks. The conidial suspension was made by flooding the surface of fungal growth with 0.9 mL sterile saline by using a Pasteur pipette. A total of 1 mL of this suspension was transferred to the test tube containing 9 mL sterile saline. A total of 40 μL of this conidial suspension was poured into the well prepared on an SDA medium plate with a cork borer. The plate was sealed and incubated at 30 ℃ for 3-7 d. After incubation, the fungal culture fully grown on the SDA plate was used as the inoculums for anti-dermatophytic assay. It was done by agar well diffusion method on SDA. SDA was prepared, and four wells were punched with a cork borer inappropriate distance. The well in the centre was inoculated with the agar block containing previously grown fungal inoculums on an SDA medium.The other three wells were filled with 40 μL of the isolated gossypol (1 mg/mL), standard antifungal agent griseofulvin as the positive control(1 mg/mL), and DMSO as a negative control. The plates were sealed to avoid contamination and incubated for 2-7 d at room temperature[12].
The conidial suspension was made by flooding 0.9% sterile saline using a Pasteur pipette and gently probing the surface of the fungal culture SDA slants. It was filtered with Whatman filter model 40 of pore size 8 μm. The cell density was adjusted to 1.5×10CFU/mL and incubated at 30 ℃ for 4-7 d. After getting visible growth in the tube, the culture was used for determination of minimum inhibitory concentration (MIC)[13].
2.5.2. Determination of MIC of isolated gossypol
MIC was determined in sterile flat-bottom microplates, according to CLSI 2008 using Sabouraud’s dextrose broth (HiMedia, MO33),griseofulvin as the positive control, DMSO as negative controls, and fungal inoculums (1.5×10CFU/mL) in a total volume of 100 μL. The test was done within the concentration range of 3.13 to 1 600 μg/mL of medium (doubling dilutions) for gossypol as well as griseofulvin.The plates were incubated for 72 h at 28 ℃. MIC is the lowest drug concentration at which there is no visible fungal growth after incubation.After incubation, 50 μL of samples from well with no visible growth were subcultured on SDA plates to determine the minimum fungicidal concentration (MFC). The experiment was done in duplicate and repeated thrice.
2.5.3. Determination of MFC of gossypol purified from fruits of T. populnea
MFC was determined by sub-culturing contents from wells with no visible growth onto SDA plates in triplicates. The contents of the wells were gently mixed, and 50 μL of the samples from each well were spread over the surface of SDA plates by tilting the plate. The plates were incubated for 7 d at 30 ℃. There will not be any growth in the subcultures if the compounds are fungicidal. The MFC was determined as the lowest concentration of the compound at which the subcultures are negative.
2.6. Scanning electron microscopic (SEM) analysis of T.mentagrophytes treated with gossypol
SEM analysis was done to know the effect of gossypol on change in the morphology of T. mentagrophytes[14]. Hyphae of T.mentagrophytes was excised from the edge of the antifungal zone from the agar well diffusion plate and fixed in 2.5% glutaraldehyde at 40 ℃ for 2 h, washed in phosphate buffered saline (PBS) for 4 times, dehydrated in ethanol, air-dried, and viewed under JEOL 6390 SEM 252 JSM at 20 kV.
2.7. Study of modulation effect of gossypol on gene expression in T. mentagrophytes
The fungal strain of T. mentagrophytes with and without the test compound (gossypol) was used for the study. A set of four genes of T. mentagrophytes were selected to evaluate the modulation of gene expression. The genes were selected based on their potential as antifungal targets such as ergosterol biosynthetic pathway,glyoxylate pathway, and response to drugs. The modulation of gene expression of dermatophytes against gossypol was conducted[15].RNA of both “control” and “test” was isolated, synthesized the cDNA, and amplified using PCR. The PCR products were separated by agarose gel electrophoresis. The stained gel was visualized using a gel documentation system (E gel imager, Invitrogen. The relative intensities of bands of interest were measured on a GelDoc 2000 scanner (Bio-Rad, CA, USA) with scan analysis software. Finally,the modulation of gene expression was studied by comparing the “control” and “test”. The cycling conditions used for cDNA synthesis and inactivation were 42 ℃ for 30 min and 95 ℃ for 2 min per cycle respectively. β-tubulin was used as the housekeeping gene.The primer sequences used are given in Supplementary Table 1.
2.8. In silico analysis of effect of gossypol on selected genes of T. mentagrophytes
The effect of gossypol on gene expression that codes for particular enzymes of various pathways of dermatophyte has been confirmed by binding energies exhibited in molecular docking studies; i.e.,isocitrate lyase (ICL), and metalloprotease (MEP4).
2.8.1. Selection of protein receptor
The glyoxylate cycle is one of the main metabolic pathways in fungi which are essential for its virulence. It is a modified tricarboxylic acid cycle that bypasses the CO-generating steps to conserve carbons as substrates for gluconeogenesis. This metabolic pathway enables the fungus to survive in nutrient-limited host niches and is a prerequisite for the virulence of the fungus. Isocitrate lyase is the critical enzyme of the glyoxalate pathway. Hence it was used as one target of the present study. MEP4 (metalloprotease) was proved to play a major role in the pathogenesis of T. mentagrophytes.Both ICL and MEP4 genes were found to be down-regulated in the gene expression study using gossypol. Hence these two genes were selected as receptors for docking.
2.8.2. Preparation of receptor and ligand
The crystal structures of the targets have been obtained from the Protein Data Bank with PDB id 5E9H and 4k90 (http://www.rcsb.org). Some modifications were made for molecular docking such as energy minimization for the protein structures. The molecular structure of the ligand molecule used in the present study was retrieved from the NCBI PubChem database (http://pubchem.ncbi.nlm.nih.gov/search/search.cgi), and Chemspider (http://www.chemspider.com). The geometries of these compounds were optimized using Autodock 4.
2.8.3. Molecular docking simulation
The molecular docking studies were performed using open-source software (AutoDock 4). The binding interaction between the receptor and ligand was monitored using the Discovery Studio Visualize 2017. The type of interactions and the number of hydrogen bonds formed were also retrieved[16].
2.9. Statistical analysis
For the anti-dermatophytic assay, the statistical significance of the difference between control and test was analyzed using Fisher’s exact test using Graph Pad software 5.03 (GraphPad QuickCalcs 2X2). To determine the MIC, the experiment was done in duplicate and repeated independently three times. A P-value < 0.05 showed that the difference was significant.
3. Results
3.1. Isolation of gossypol
The isolated compound was yellow on recrystallization from petroleum ether (yield 1.9%).
3.2. Characterization of the purified compound
3.2.1. HPLC
HPLC analysis of the isolated crystal showed a single peak at retention time 5.649 (for methanol and water 9:1 as mobile phase)(Supplementary Figure 1A). The sample was compared with standard gossypol and observed the same result (Rt: 5.651) (Supplementary Figure 1B).
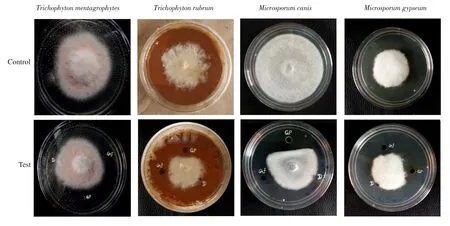
Figure 1. Inhibitory effect of gossypol on the growth of different dermatophytes. GP: Gossypol, Gf: Griseofulvin, D: DMSO.
3.2.2. HPTLC
The chromatogram showed a single band, which indicated the purity of the isolated compound and was also compared with the standard gossypol (Rvalue: 0.9) (Supplementary Figure 2).

Figure 2. Scanning electron microscopic analysis of Trichophyton mentagrophytes with and without gossypol. A: control cells; B & C: shortening of interseptal distance, non-uniform widths, and flattening and globular swelling (Black arrow); D: A thick mesh of fibers and globular material was seen on the surface of hyphae (Black arrow); E: Each segment of hyphae became round and constricted. Grainy material was produced along the length of the hyphae. Clusters of hyphae and small vesicles were seen on the surface (Black arrow); F: The structure of the surface was altered and swollen. It lost its original tubular shape. A cluster of hyphae was seen with compression (Black arrow).
3.2.3. FT-IR
From Supplementary Figure 3, the predominant peaks observed were 3 514 cm, 3 420 cm, 2 963 cm, 2 872 cm, 1 615 cm,1 589 cm, 1 439 cm, 1 386 cm, 1 333 cm, 1 050 cmand 752 cm. The band at 3 514 cmand 3 420 cmare assigned for -OH associated in phenol. The bands at 2 963 cmcorrespond to unsaturated and aromatic (phenyl) -CH vibrations. The band at 2 872 cmsuggests the presence of aldehydic -CH, and the bands at 1 615 cmand 1 589 cmcorrespond to aromatic C=C bond. The bands at 1 439 cmand 1 386 cmare of methyl bending vibrations, while 1 333 cmcan be assigned to the in-plane -CH bending of the phenyl group. The peak at 1 050 cmcorresponds to the in-plane -CH of 1,2-substituted phenyl, while the band at 752 cmcorresponds to -CH out of plane bending vibrations. The FTIR spectrum validates the predictive model with the actual gossypol content.

Figure 3. Gene expression. A: Agarose gel image of ICL and ICL expression; B: Agarose gel image of gene MEP4 and MEP4 expression; C: Agarose gel image of gene ERG1 and ERG1 expression; D: Agarose gel image of gene CS and CS expression. c: Untreated control, s: Sample gossypol. *P<0.05, vs. control.
3.2.4. LCMS
The mass spectrum of the isolated compound was studied in the negative ionization mode. In this, the peak at m/z 517 (observed mass: 517.186 5; Calculated for the deprotonated parent ion of gossypol: 517.186 3) represents the parent molecule. There was also a peak at 518 (Exact mass: 518.189 6; Approximation 30% intensity to that of m/z 517) that represents the M+2 isotopic peak due to oxygen atoms (Supplementary Figure 4).

Figure 4. Docking interaction of gossypol with isocitrate lyase (ICL). A: Three-dimensional structure of ICL; B: Molecular structure of the compound; C:Docking interaction, D: Docked pose; E: Hydrogen bonding interaction.
3.2.5. NMR
A singlet for the CHO proton was observed at 11.17 ppm in DMSO d6 solvent. This result implied that in this solvent, gossypol exists in aldehyde form. The singlet at 14.62 (small peak) corresponds to intramolecular hydrogen-bonded (H-O) protons. The single sharp peak at 1.529 corresponds to methyl protons of C-(CH)groups. The overall peaks from proton NMR support the presence of gossypol (Supplementary Figures 5 & 6). Various corresponding C of gossypol is listed in Supplementary Table 2, which supports the presence and purity of solute extracted. The IUPAC name of the compound is “7-(8-formyl-1,6,7-trihydroxy-3-methyl-5-propan-2-ylnaphthalen-2-yl)-2,3,8-trihydroxy-6-methyl-4-propan-2-ylnaphthalene-1-carbaldehyde” and the molecular formula is CHO.

Figure 5. Docking interaction of gossypol with metalloprotease. A: Three-dimensional structure of metalloprotease (4k90); B: Docking interaction of metalloprotease with gossypol; C: Docked pose; D: Hydrogen bonding interaction.
3.3. Anti-dermatophytic activity of gossypol isolated from the fruits of T. populnea
The purified gossypol (1 mg/mL) showed inhibitory action on the growth of all dermatophytes tested. The fungus showed growth away from the well inoculated with gossypol. Results are shown in Figure 1.
3.4. MIC and MFC determination by broth microdilution method
Different concentrations of gossypol isolated from T. populnea inhibited the growth of dermatophytes and the results are given in Supplementary Table 3. The MIC values of gossypol against T.mentagrophytes, T. rubrum, M. canis, and M. gypseum were 12.5, 25,12.5, and 25 μg/mL, respectively and the MIC of griseofulvin for all dermatophytes was 6.25 μg/mL. The MFC values of gossypol against T. mentagrophytes, T. rubrum, M. canis, and M. gypseum were 50, 100, 100, and 200 μg/mL, respectively and the MFC of values of griseofulvin were 25, 50, 25, and 100 μg/mL, respectively.
3.5. SEM
SEM analysis was performed to detect the effect of gossypol on the morphological change in T. mentagrophytes. The hyphae of the control sample were tubular with uniform thick cell walls (Figure 2A). Comparable changes were observed in the cells treated with gossypol after 6 h, 12 h, 24 h, 48 h, and 72 h. The changes included shortening of interseptal distance, non-uniform widths, and flattening and globular swelling (Figure 2B&C). A thick mesh of fibers and globular material was seen on the surface of hyphae (Figure 2D).Each segment of hyphae became round and constricted. Grainy material was produced along the length of the hyphae. Clusters of hyphae and small vesicles were seen on the surface (Figure 2E). The structure of the surface was altered and swollen. It lost its original tubular shape. A cluster of hyphae was seen with compression(Figure 2F).
3.6. Modulation of gene expression of T. mentagrophytes to gossypol
Figure 3 shows the effect of gossypol on the modulation of gene expression in T. mentagrophytes. Gossypol significantly repressed the expression of genes ICL, which plays a vital role in the glyoxylate pathway, and MEP4, the gene encoding metalloprotease when compared to the control (P<0.05). However, there were no modulations in the expression of genes encoding ergosterol (ERG1)and citrate synthase (CS).
3.7. Molecular docking studies
3.7.1 Docking interaction of gossypol with ICL
Mainly two approaches have been applied in the present in silico study, i.e., molecular docking and molecular dynamics, to study the binding conformations and structural specificity of the antifungal inhibitor. The antifungal compound gossypol was significantly bound to the crucial amino acids of the active site of the target enzyme ICL. Gossypol was bound to THR 113, LYS 135, and ASN 134 with binding energy -8.02 kcal/mol. The low binding energy denotes the inhibitory interaction of the compound with the enzyme. Gossypol formed three hydrogen bonds with ICL. The hydrogen bonding interaction and docked pose of the enzyme with the compound are given in Figure 4.
3.7.2. Docking interaction of gossypol with MEP4
Gossypol formed inhibitory binding interaction with matrix metalloprotease. It significantly formed hydrogen bonding interaction with critical amino acids in the active sites of matrix metalloprotease enzyme. Gossypol was bound to arg407, asn394,and tyr409 of the matrix metalloprotease with binding energy -7.79 kcal/mol. The hydrogen bonding interaction and the docked poses of the enzyme metalloprotease with the gossypol are given in Figure 5.
4. Discussion
Since the incidence of fungal infections has been increased dramatically, the discovery of new antifungals that do not cause any damage to the host cell has become a pharmacological challenge.In this case, biologically active plant compounds got tremendous attraction especially due to their biocompatibility and less toxicity.Many researchers have tried to find out the antifungal targets of pure compounds against a variety of fungal species[17]and there are a few reports on the antibacterial and antifungal activity of gossypol[18].Most of them are rudimentary and the anti-dermatophytic activity of this compound has not been fully studied.
In the present study, MIC and MFC of gossypol confirmed its efficacy against fungal pathogens and the MFC against T.mentagrophytes was 50 μg/mL. A similar lower MFC value of gossypol has been reported against fungal pathogens of cotton root[19]. SEM analysis also revealed the anti-dermatophytic activity of the compound as seen in the preliminary antimicrobial studies.Significant morphological aberrations were observed in the dermatophyte due to the application of gossypol. The hyphae of the control sample were tubular with uniform thick cell walls. Treatment with gossypol has induced many changes in the morphology of dermatophytes. The changes included shortening of interseptal distance, non-uniform widths, and flattening and globular swelling.Similar anti-fungal activities were also reported in Fusarium oxysporum f. sp. lupini treated with heterocyclic aza-derivatives of gossypol[20]. However, gossypol-mediated anti-dermatophytic activity still needs to be explored further.
Furthermore, gene studies were conducted in the dermatophyte for deciphering the molecular action of gossypol. The genes such as ICL, MEP4, ERG1, and CS were selected based on their potential as antifungal targets because these genes are important in the pathogenesis of dermatophytes, ergosterol biosynthetic pathway,glyoxylate pathway, and response to drugs[21].
Metabolic pathways are currently being considered as potential targets of antifungal compounds since metabolic pathways of microbial cells are flexible and help them survive in nutrient deficient host niches during infection. Some microorganisms especially dermatophytes use the glyoxylate cycle to synthesize glucose from lipids and other alternative carbon sources. In the present study, gossypol repressed the expression of ICL, which plays a vital role in the glyoxylate pathway, and MEP4, the gene encoding metalloprotease. However, modulations in the expression of genes encoding ERG1 and CS were not observed. In another study, T.rubrum was co-cultured with human keratinocytes, and the proteins ICL and citrate synthase have been induced. This study suggests that the glyoxylate pathway is significant for the pathogenesis of dermatophytes[22]. So, inhibition of the expression of ICL and MEP4 effectively controlled the growth and metabolism of the pathogen. Lorenz and Fink[23]have studied the importance of three enzymes of the glyoxylate cycle: ICL, malate synthase, and CS in the virulence of Candida albicans (C. albicans). C. albicans usually are phagocytosed by macrophages and neutrophils. They have studied the events that take place in fungus during the ingestion by mammalian macrophages. They isolated the live fungal cells from the phagolysosomes and induced genes of the glyoxylate cycle. They found ICL1 and malate synthase genes (MLS1) were upregulated and the C. albicans mutants lacking the ICL1 gene were less virulent in mice than the wild type. Another study of Mycobacterium tuberculosis also suggests that the ICL gene was upregulated and was required for its virulence, which demonstrates the significance of the glyoxylate cycle in the pathogenesis of various microorganisms[24]. The group of enzyme proteases has been considered de facto a critical virulence factor for dermatophytes, as they infect keratin-containing tissues. Their genomes display a clear embellishment in protease genes[25]. Those proteases are upregulated during growth in vitro and are important in their virulence. They differ from those genes that are highly expressed during infection[26]. A study proposed the pathogenic potential of five metalloprotease genes from T.mentagrophytes. It states that MEP4 and MEP5 are most likely to affect pathogenicity, which has been proved in a guinea pig model and keratin degradation test[27]. It showed that the expression of MEP4 was significantly upregulated during growth in collagen,keratin, elastin, or human skin sections in vitro. In another study, T.rubrum is co-cultured with human keratinocytes (HaCaT) and also showed elevated gene expression of MEP4[28]. In dermatophytes, the beginning stages of keratin proteolysis are induced by endoproteases,which are expanded into subfamilies when compared with other filamentous fungi, e.g. S8 family subtilysins and M36 family fungal lysins[29-31]. The endoproteases act on keratin to release free peptides, on which the exoproteases act and hydrolyze[32]. It is then followed by the uptake of tripeptides, dipeptides, and amino acids for central metabolism[33]. In this way, the expansion of the protease family within dermatophytes helps them to utilize keratin and cause infection in keratinized tissues[34]. The downregulation of metalloprotease in the dermatophyte observed in the present study indicated its weakened metabolic activity when treated with gossypol.
The group of genes ERG plays an essential role in the biosynthesis pathway of ergosterol in fungi, which is an integral part of the fungal cell membrane. It regulates the stability, fluidity, permeability of the membrane, and regulates the enzyme activity and substrate transportation associated with the membrane[34]. Considering the high cost of processing, one gene involved in the initial step of ergosterol biosynthesis, i.e., ERG1 gene (Squalene to squalene epoxidase) was selected for our study. Gossypol neither induced nor repressed the expression of the gene ERG1 in the present study.Other genes related to each pathway are needed to be considered in the future to rule out the remaining mechanisms of activity.
Finally, the binding of gossypol with selected genes was confirmed by molecular docking studies. The isolated and purified gossypol was docked with the target proteins, ICL and MEP4, and the best docking poses were identified. ICL has been identified as an attractive antifungal target for dermatophytes. The compound showed a higher binding affinity with ICL and MEP4.
The present study demonstrates the inhibitory action of gossypol against dermatophytes, especially T. mentagrophytes, and confirms its binding with the amino acids of the target enzymes. Gossypol repressed the gene expression of ICL, which plays a vital role in the glyoxylate pathway, and MEP4, which encodes metalloprotease. But,gossypol did not modulate the gene expression of ERG1 (which is involved in the ergosterol biosynthetic pathway) or CS (which is also involved in the glyoxylate pathway). This study provides an insight into the mechanism of gossypol as an anti-dermatophytes agent.
Conflict of interest statement
The authors declare no conflict of interest.
Authors’ contributions
AR designed the study, executed the idea and analysed the results.MSJ verified and analysed the data, edited and formatted the manuscript. JM supervised the study.
 Asian Pacific Journal of Tropical Biomedicine2021年12期
Asian Pacific Journal of Tropical Biomedicine2021年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Chemical constituents and biological activities of essential oils of Amomum genus(Zingiberaceae)
- Salsola imbricata Forssk. ameliorates acetic acid-induced inflammatory bowel disease by modulating dysregulated antioxidant enzyme system and cytokine signaling pathways in mice
- Demethylbelamcandaquinone B from Marantodes pumilum var. alata (Blume) Kuntze inhibits osteoclast differentiation in RAW264.7 cells
- Ethyl acetate extract of Smilax glabra Roxb roots and its major active compound astilbin promote osteoblastogenesis in vitro by upregulating bone cell differentiationassociated genes
