TaSnRK2.4 is a vital regulator in control of thousand-kernel weight and response to abiotic stress in wheat
MIAO Li-li ,LI Yu-ying,ZHANG Hong-juan,ZHANG Hong-ji,LIU Xiu-lin,WANG Jing-yi,CHANG Xiao-ping,MAO Xin-guo,JING Rui-lian
1 Heilongjiang Academy of Agricultural Sciences Postdoctoral Programme,Harbin 150086,P.R.China
2 Institute of Crop Resources,Heilongjiang Academy of Agricultural Sciences,Harbin 150086,P.R.China
3 Institute of Crop Sciences,Chinese Academy of Agricultural Sciences,Beijing 100081,P.R.China
4 Institute of Soybean,Heilongjiang Academy of Agricultural Sciences,Harbin 150086,P.R.China
Abstract Sucrose non-fermenting 1-related protein kinase 2 (SnRK2) is a plant-specific serine/threonine kinase involved in response to adverse environmental stimuli. Previous studies showed that TaSnRK2.4 was involved in response to abiotic stresses and conferred enhanced tolerance to multiple stresses in Arabidopsis. Further experiments were performed to decipher the underlying mechanisms and discover new functions. The genomic sequences of TaSnRK2.4s locating on chromosome 3A,3B and 3D were obtained. Sequencing identified one and 13 variations of TaSnRK2.4-3A and TaSnRK2.4-3B,respectively,but no variation was detected in TaSnRK2.4-3D. The markers 2.4AM1,2.4BM1 and 2.4BM2 were developed based on three variations. Association analysis showed that both TaSnRK2.4-3A and TaSnRK2.4-3B were significantly associated with thousand-kernel weight (TKW),and that SNP3A-T and SNP3B-C were favorable alleles for higher TKW. Yeast two-hybrid and split luciferase assays showed that TaSnRK2.4 physically interacted with abiotic stress responsive protein TaLTP3,suggesting that TaSnRK2.4 enhanced abiotic stress tolerance by activating TaLTP3. Our studies suggested that TaSnRK2.4 have potential in improving TKW and response to abiotic stress.
Keywords:TaSnRK2.4,association analysis,TKW,abiotic stress
1.Introduction
Common wheat (Triticum aestivumL.) is the most widely cultivated crop in the world. However,its production is frequently impacted by abiotic stresses,such as drought,salt,heat,and low temperature. Reversible protein phosphorylation orchestrated by protein kinases and phosphatases,plays vital roles in intracellular signal transduction and multi-environmental stress responses(Kuliket al.2011). The sucrose non-fermenting (SNF) 1-related protein kinase (SnRK) family is a highly conserved core component of kinase cascades in plants. Based on sequence similarity,gene structure and expression pattern,SnRKs are divided into subfamilies SnRK1,SnRK2 and SnRK3,thereinto SnRK2 is a relatively small plant-specific subfamily,encoding serine/threonine kinases. SnRK2s contain two canonical domains,an N-terminal catalytic domain and a C-terminal regulatory region. Evidence indicates that the C-terminal domain is involved in proteinprotein interactions and abscisic acid (ABA) signaling(Kobayashiet al.2004). Moreover,SnRK2s are further divided into subclasses I,II and III based on different activation patterns in response to ABA. However,inconsistencies in gene expression patterns within the same subclass in different plant species suggest functional diversification during evolution (Huaiet al.2008; Zhaoet al.2017).
Mounting evidence shows that SnRK2s are involved in regulation of gene expression at different levels,including RNA splicing (Wanget al.2013),mRNA degradation (Somaet al.2017),microRNA accumulation (Wanget al.2013),DNA methylation and histone deacetylation (Peirats-Llobetet al.2016),and protein phosphorylation. These multifarious roles can be classified into several categories according to the implicated biological processes:(1) determination of sex differentiation in ferns (McAdamet al.2016); (2)control of plant growth and development,including seed dormancy and germination,seedling and root growth,flowering,fruit ripening,and leaf senescence (Nakashimaet al.2009; Jiaet al.2013; Wanget al.2013; Zhaoet al.2016); (3) involvement in biotic and abiotic stresses signaling(Tonet al.2009; Kuliket al.2011; Leeet al.2015); (4)crosslinking of various phytohormone signaling (Linet al.2015; WangHet al.2018); (5) regulation of phosphorus(P) and sulphur (S) absorption (Moseleyet al.2009); and(6) orchestration of plant growth or inhibition in response to different environmental conditions (Wang Pet al.2018).Previous research has mainly focused on subclass III members,and consequently the core ABA signaling pathway is well understood in model plants,whereas the functions of subclass I and II members are largely unknown,especially in the staple crops.
Ten SnRK2s have been identified in wheat,and all members are implicated in response to environmental stresses (Zhanget al.2016). Our previous study showed that overexpression ofTaSnRK2.4,a subclass I member,led to enhanced tolerance to drought,salt and freezing stresses inArabidopsisby improving various physiological traits,and resulted in higher yield under normal growing conditions (Maoet al.2010). The purpose of the present research was to further dissect the underlying mechanisms and discover novel functions of TaSnRK2.4 in plant growth and development,especially in development of agronomic traits. The results demonstrated that TaSnRK2.4 was associated with TKW,as well as interacted with abiotic stress responsive protein TaLTP3.
2.Materials and methods
2.1.Plant materials and measurement of agronomic traits
Common wheat Hanxuan 10,a drought-tolerant cultivar,was used for gene cloning (Xuet al.2018). A set of Chinese Spring nulli-tetrasomic lines was used for chromosomal location of genes. A panel of 32 highly variable wheat accessions selected from 262 accessions based on polymorphism analysis and population structure assay with 209 SSR markers was utilized to analyze sequence polymorphism in the target genes (Appendix A). Three populations of winter wheat were selected for different purposes. Population 1 (P1) with 262 accessions used for association analysis was mainly collected from the Northern Winter Wheat Zone and Yellow and Huai River Valleys Facultative Wheat Zone in China. Population 2(P2) consisted of 157 landrace accessions. Population 3 (P3) comprised 348 modern cultivars. The P2 and P3 were chosen from the Chinese wheat mini-core and core collections respectively (Haoet al.2008,2011),and then were used to validate functional markers and to analyze the geographic distributions of allelic variants of the target genes.
P1 was grown in 10 environments (year×site×water regime combinations) at Shunyi (116°56´E,40°23´N),Beijing,in 2010,2011 and 2012,and at Changping(116°13´E,40°13´N),Beijing,China in 2010 and 2012. Two water treatments,i.e.,well-watered (WW) and droughtstressed (DS) were applied. The WW plots were irrigated with 750 m3ha-1(75 mm) at pre-overwintering,booting,flowering,and grain filling stages,whereas DS plots were rain-fed. The amounts of rainfall in the three growing seasons were 131,180 and 158 mm,respectively,mainly occurring in May and June,that is,at flowering and grain filling stages. The experimental unit consisted of four 2-m rows with 40 plants per row and 0.3 m row space. Agronomic traits were measured by random selection of five plants per plot to calculate the mean value in each accession,including thousand kernal weight (TKW),number of spikes per plant,number of spikelets per spike,number of sterile spikelets per spike,grain number per spike,spike length,plant height,peduncle length,and length of the penultimate node.
2.2.Cloning and sequence analysis of DNA fragments from TaSnRK2.4 coding regions
Based on the known cDNA sequence ofTaSnRK2.4,fragments covering the coding region ofTaSnRK2.4sfrom different sources were amplified using the gene-specific primer pair GF/GR (Appendix B). Amplicons were inserted into thepEASY-Blunt vector,and 12 positive clones for each sample were sequenced by DNA Analyzer 3730XL. Both M13F/M13R and proper overlapping primers were used for sequence walking to obtain the full sequence of target fragments (Appendix B). The whole sequence of each clone was obtained by assembling the overlapping sequences with the SeqMan Program in the DNAStar Software package,and the consensus sequences ofTaSnRK2.4swere obtained accordingly.
2.3.Marker development
Three markers (named 2.4AM1,2.4BM1 and 2.4BM2) were developed based on variations,at 1 780 nt (T/C) in the A genome,and 3 318 nt (T/C) and 4 325 nt (15 bp/-) in the B genome. Both the dCAPS marker 2.4AM1 and CAPS marker 2.4BM1 were developed by introduction of restriction enzyme recognition sites. Target fragments were amplified by two rounds of PCR and separated by electrophoresis in 4% agarose gels after digestion by appropriate restriction endonucleases. The allele-specific PCR marker 2.4BM2 was developed based on a 15-bp InDel. Primers are listed in Appendix B.
2.4.Association analysis
Association analysis was conducted by TASSEL 5.2.13 Software following the mixed linear model (MLM),which accounts for population structure (Q) and relative kinship (K matrix). Associations were considered significantly different atP<0.05. Statistical analyses were performed using SPSS 16.0 Software.
2.5.Yeast two-hybrid (Y2H) assay
Saccharomyces cerevisiaestrain AH109 and the GAL4-based Matchmaker Two-Hybrid System (Clontech,Inc.,USA) were used in transcriptional activity assays. C-terminal truncation following the S_TKC motif coding region of TaSnRK2.4-3A was amplified and cloned into pGBKT7 to produce an in-frame fusion to the GAL4-binding domain(Appendix B). The construct was then transformed into yeast strain AH109 and cultured until absorbance at 600 nm(OD600) reached 1.0. Serial dilutions were inoculated onto SD/-Trp (SD/-1),and SD/-Trp,His,Ade (SD/-3) media to identify the transcription activity of the bait protein,and the pGBKT7 vector was used as a negative control. For the protein interaction assay,GAL4-AD and GAL4-BD derivatives were co-transformed into yeast strain AH109,and the resulting yeast cells were selected on SD/-Trp,Leu(SD/-2) deficient media,and then transferred to SD/-Trp,His,Ade,Leu (SD/-4),and SD/-4+X-α-Gal (4 mg mL-1)media for interaction assays.
2.6.Luciferase complementation imaging (LCI) assay
LCI assays were performed in tobacco (Nicotiana benthamiana) leaves as described (Chenet al.2008). The ORF ofTaLTP3and C-terminal truncation ofTaSnRK2.4-3Awere separately PCR-amplified and inserted into the pCAMBIA-cLUC and pCAMBIA-nLUC vectors to express fusion proteins,respectively (Appendix B),and expressed in tobacco leaves byAgrobacterium tumefaciens-mediated infiltration. The luciferase signal was identified with NightSHADE LB 985 (Berthold,Inc.,Germany) at 48 hours post infiltration (hpi).
3.Results
3.1.Genetic characterization of TaSnRK2.4
Genomic sequences coveringTaSnRK2.4ORFs from the A,B and D genomes were isolated from Hanxuan 10; the corresponding fragment sizes were 4 257,4 457,and 4 199 bp,consisting of nine exons and eight introns. Sequence similarities ranged from 92.5 to 96.5%. The three copies ofTaSnRK2.4were located on chromosome 3A,3B and 3D using primer pair NT-F/GR (Appendix B) and a set of Chinese Spring nulli-tetrasomic lines (Appendix C). The results were consistent with online Blast results (https://urgi.versailles.inra.fr/blast/blast.php). Therefore,the three copies ofTaSnRK2.4were named asTaSnRK2.4-3A,TaSnRK2.4-3BandTaSnRK2.4-3Daccordingly.
3.2.Sequence polymorphism assays and marker development for TaSnRK2.4
The coding regions ofTaSnRK2.4swere re-sequenced in the panel of 32 accessions. A single variation (SNPT/C) identified at position 1 780 nt inTaSnRK2.4-3Aled to an amino acid change at position 173AA (TGC→Cys,to CGC→Arg). As shown in Fig.1-A,11 SNPs and two InDels were detected inTaSnRK2.4-3B. Based on genetic linkage,the 13 variations were grouped into two categories; one group included 12 variations,locating at 1 977,2 064,2 389,2 539,2 647,2 834,2 892,2 897,2 980,3 318,3 477 nt in the sixth intron,and 4 084 nt in the ninth exon (Ex9); another contained only one variation at 4 325 nt in Ex9. In detail four variant combinations were formed (Appendix D). The SNP (T/C) at 4 084 nt in the ninth exon (Ex9) led to a synonymous mutation,and an InDel (GAAGAGGAGGATGAG/---------------) at 4 325 nt resulted in a 5-amino acid deletion. No variation was identified inTaSnRK2.4-3D.
A dCAPS marker,2.4AM1,was developed forTaSnRK2.4-3Abased on SNP-1 780 nt (T/C). When the nucleotide was C,there was a cleavage site forHpaII; when it was T,it was uncleavable (Appendix E). Two representative variations were selected to develop markers forTaSnRK2.4-3B. CAPS marker 2.4BM1 was similarly developed based on SNP-3 318 nt (T/C),which was geneticly linked to the other 11 variants (Fig.1-B). An allele-specific PCR marker 2.4BM2 was developed based on the InDel (15 bp/-) at 4 325 nt(Fig.1-B).
3.3.Association between the TaSnRK2.4-3A marker and agronomic traits
With SNP-1 780 nt (T/C) inTaSnRK2.4-3A,P1 was divided into two genotypes. The proportions of genotypes T and C alleles (namely SNP3A-T and SNP3A-C) were 8.5 and 91.5%,respectively. Association analysis showed that marker 2.4AM1 was significantly associated with TKW in nine of 10 environments (Table 1). SNP3A-T was associated with a significantly higher TKW relative to SNP3A-C (Fig.2).Thus,SNP3A-T was considered to be a favorable allele for higher TKW.
3.4.Association between TaSnRK2.4-3B markers and agronomic traits
Association analysis was conducted between the two markers (2.4BM1 and 2.4BM2) and agronomic traits. The frequencies of genotypes possessing T and C identified by 2.4BM1,i.e,SNP3B-T and SNP3B-C at 3 318 nt,were 27.5 and 72.5% in P1. Significant associations were identified between marker 2.4BM1 and TKW in three environments(Table 2). SNP3B-C has significantly higher TKW than SNP3B-T (Fig.3). Therefore,SNP3B-C might be a favorable allele in terms of TKW.
Marker 2.4BM2,based the 15-bp InDel at 4 325 nt,was also used for scanning P1,but due to the low frequency(4.5%) of genotypes possessing the 15-bp deletion in P1,it was not used in further association assays.
3.5.Geographic distributions of allelic variation in TaSnRK2.4-3A/3B across the 10 wheat production zones
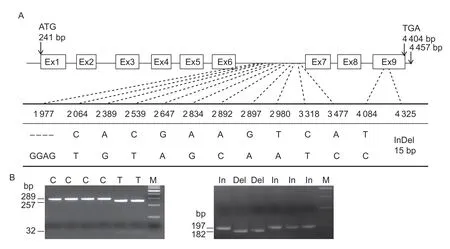
Fig.1 Nucleotide polymorphisms and functional marker development for TaSnRK2.4-3B. A,11 SNPs and two InDels were detected in TaSnRK2.4-3B. B,A CAPS marker 2.4BM1 was developed based on SNP 3 318 nt (T/C) in the left image. Digestion of the amplified 289-bp fragment with BmtI produced fragments of 257 and 32 bp for accessions with SNP-T,but not for accessions with SNP-C. An allele-specific PCR marker 2.4BM2 was developed based on the InDel-4 325 nt (a 15-bp insertion (In) or deletion(Del)) in the right image. A 197-bp fragment was amplified in accessions with the 15-bp insertion; a 182-bp product was detected in accessions lacking the insertion. M,100-bp DNA ladder.

Table 1 Association between TaSnRK2.4-3A marker 2.4AM1 and thousand kernel weight (TKW)
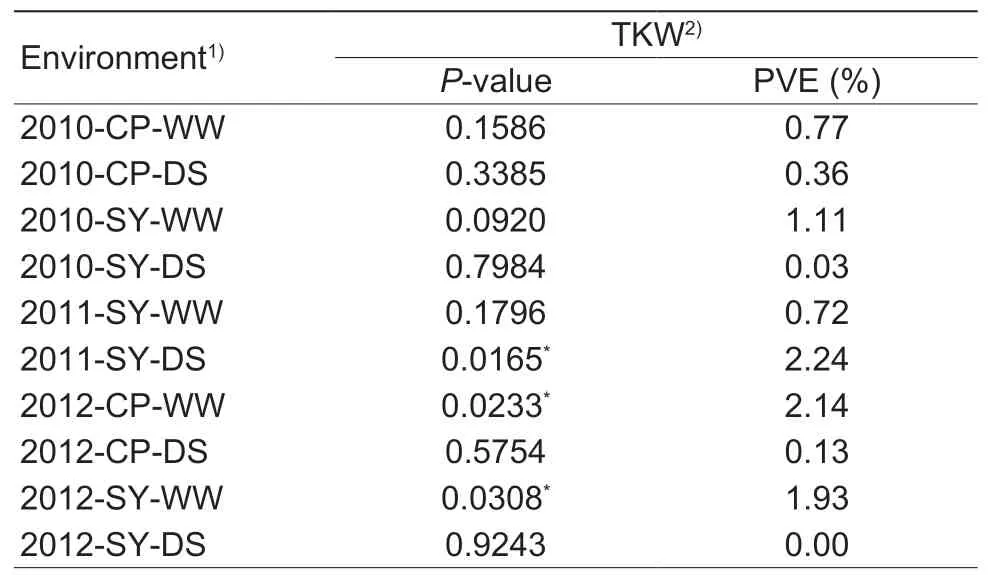
Table 2 Association between TaSnRK2.4-3B marker 2.4BM1 and thousand kernel weight (TKW)
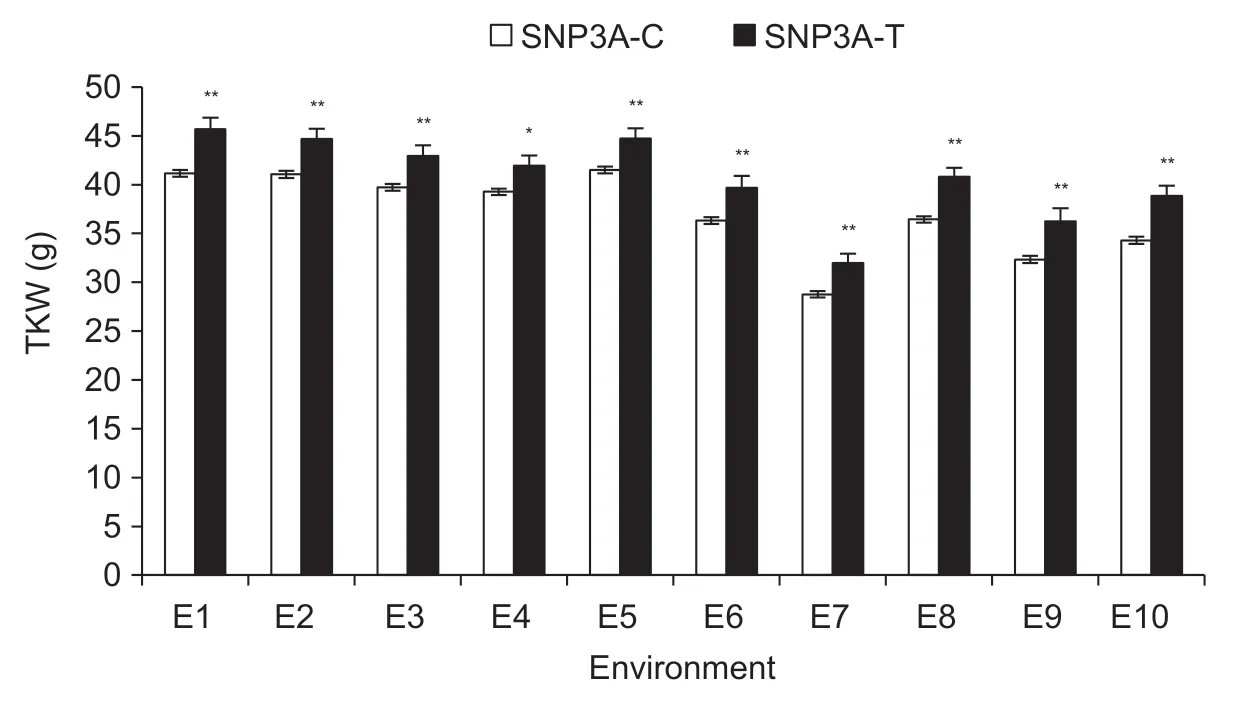
Fig.2 Comparisons of thousand-kernel weight (TKW) between SNP3A-T and SNP3A-C in 10 environments. Environments were Changping (CP) and Shunyi (SY) under well-watered (WW) and drought-stressed (DS) treatments in 2010,2011 and 2012. E1,2010-CP-WW; E2,2010-CP-DS; E3,2010-SY-WW; E4,2010-SY-DS; E5,2011-SY-WW; E6,2011-SY-DS; E7,2012-CP-WW; E8,2012-CP-DS; E9,2012-SY-WW; E10,2012-SY-DS. *,P<0.05; **,P<0.01. Error bars denote SE (n=3).
Wheat production in China was divided into 10 major agroecological production zones based on cultivars,growing season and response to temperature and photoperiod(Zhanget al.2002). The geographic distributions of theTaSnRK2.4-3A/3Ballelic variation were determined in both landraces (P2) and modern cultivars (P3) covering all zones. From Chinese landraces to modern cultivars,the frequencies of the two favorable alleles SNP3A-T and SNP3B-C increased in many zones,except for zones III(13.04 to 3.92%),V (25.00 to 12.50%),VI (33.33 to 27.27%),and IX (38.46 to 0.00%) forTaSnRK2.4-3A; and V (100.00 to 87.50%),VI (100.00%,no change) and X (90.00 to 88.89%)forTaSnRK2.4-3B(Table 3). The distribution patterns indicated that favorable alleles SNP3A-T and SNP3B-C were selected in most wheat zones,but the degree of selection varied between zones.
3.6.Identification of proteins interacting with TaSnRK2.4
The protein sequences of TaSnRK2.4-3A and TaSnRK2.4-3B were same. There was only one amino acid difference between TaSnRK2.4-3A/3B and 3D at the 334th AA. The identity of TaSnRK2.4-3A/3B and TaSnRK2.4-3D was 99.91%. In TaSnRK2.4-3A/3B,the 334th AA was Ala,and in TaSnRK2.4-3D it was Val (Appendix F). Both Ala and Val are non-polar amino acids. Protein secondary structure prediction showed the variation led to no alteration (https://swissmodel.expasy.org/). Further variation effect assay was performed by Protein Variation Effect Analyzer (http://provean.jcvi.org/index.php). The results revealed the AA variation had a neutral effect on TaSnRK2.4’s function.TaSnRK2.4-3A,-3B and -3D had the same S_TKC motif located at 4 to 260 AA as predicted (http://smart.emblheidelberg.de/),including an ATP binding site,a polypeptide substrate binding site,an active site,and an activation loop.To discover potential substrates and decipher the underlying mechanism,the C-terminal of TaSnRK2.4-3A/3B (261-363 AA) was subcloned into the pGBKT7 vector to identify its transcriptional activation ability. The results showed that it has no such ability (Fig.4). Thus,it was further used as a bait to screen a wheat cDNA library in yeast.
Seventy-two clones and 61 sequences with high quality were obtained. After removing redundancy 42 contigs were subjected to annotation by BLAST,and 37 contigs with annotation were obtained (Appendix G). A gene with relative high redundancy was selected for further assays.It encoded a lipid transfer protein,with high identity to TaLTP3 (AY226580.1).
3.7.TaSnRK2.4 interacts with TaLTP3
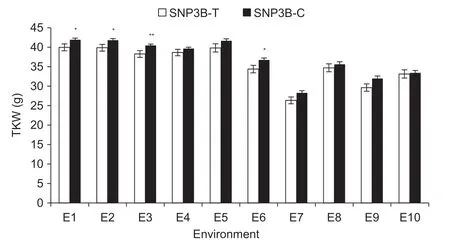
Fig.3 Comparisons of thousand-kernel weight (TKW) between SNP3B-C and SNP3B-T in 10 environments. Environments were Changping (CP) and Shunyi (SY) under well-watered (WW) and drought-stressed (DS) treatments in 2010,2011 and 2012. E1,2010-CP-WW; E2,2010-CP-DS; E3,2010-SY-WW; E4,2010-SY-DS; E5,2011-SY-WW; E6,2011-SY-DS; E7,2012-CP-WW; E8,2012-CP-DS; E9,2012-SY-WW; E10,2012-SY-DS. *,P<0.05; **,P<0.01. Error bars denote SE (n=3).
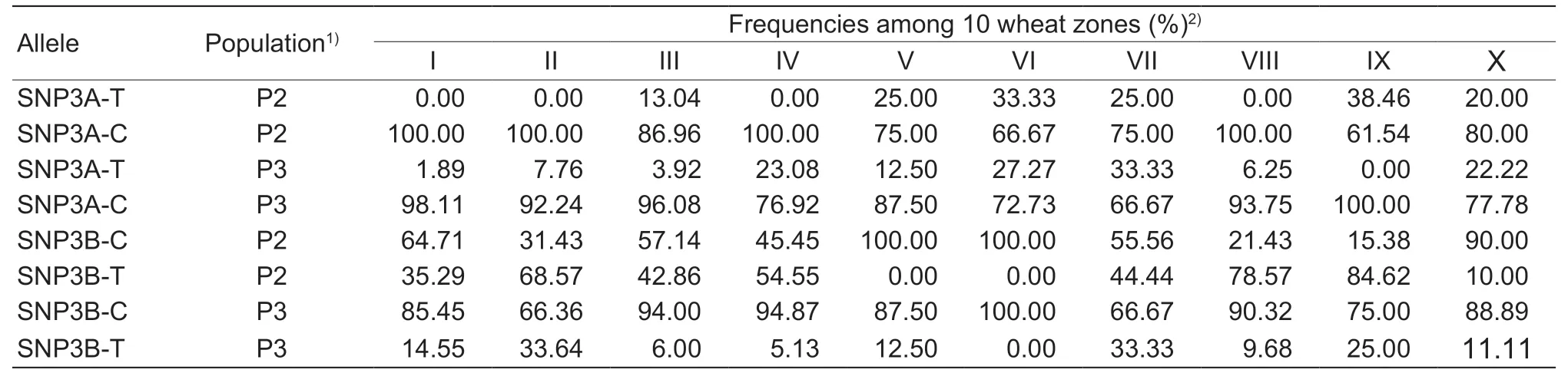
Table 3 Geographic distributions of allelic variations in TaSnRK2.4-3A/3B among the 10 major wheat zones in China

Fig.4 Transcriptional activation activity of TaSnRK2.4-3A-C in a modified yeast two-hybrid assay. The C-terminal truncation after the S_TKC motif was introduced into the pGBKT7 vector. GAL4-BD,the GAL4 DNA binding domain. The empty pGBKT7 vector was the negative control.
Yeast cells were co-transformed with pGADT7/pGBKT7,pGADT7/pGBKT7-TaSnRK2.4-C,pGADT7-TaLTP3/pGBKT7,and pGADT7-TaLTP3/pGBKT7-TaSnRK2.4-C for Y2H assays. Transformants of pGADT7-TaLTP3/pGBKT7-TaSnRK2.4-C grew well on both SD/-2 and SD/-4 media,whereas the three controls only grew on SD/-2 medium.The results were further confirmed on SD/-4 plates supplied with X-α-gal; α-galactosidase activity was observed only in pGADT7-TaLTP3/pGBKT7-TaSnRK2.4-C transformants(Fig.5-A).
In LCI assays,four combinations,i.e.,nLUC/cLUC,nLUC-TaSnRK2.4-C/cLUC,nLUC/cLUC-TaLTP3,and nLUC-TaSnRK2.4-C/cLUC-TaLTP3,were transformed into tobacco leaves by infiltration,and LUC images were captured at 48 hpi. As shown in Fig.5-B,strong LUC activity was exclusively expressed in the regions infiltrated with the nLUC-TaSnRK2.4-C/cLUC-TaLTP3 combination,yet no LUC activity was observed in the three regions infiltrated with negative controls. These results strongly supported that TaSnRK2.4 physically interacts with TaLTP3.
4.Discussion
4.1.TaSnRK2.4 has a role in control of TKW
Maximum yield is the most important breeding goal in wheat improvement. Among yield components,TKW has high heritability and phenotypic stability. Association analyses indicated that bothTaSnRK2.4-3AandTaSnRK2.4-3Bwere significantly associated with TKW. Favorable alleles SNP3A-T and SNP3B-C for TKW enhancement were positively selected across the 10 Chinese wheat production zones. The degree of SNP3A-T was selected poorly,possibly due to its under-utilization in most wheat zones,or linkage to unknown and adverse traits. Moreover,transgenicArabidopsisplants overexpressingTaSnRK2.4generated longer siliques and higher yield under normal growing conditions (Maoet al.2010). Similar studies showed thatTaSnRK2.3-1AandTaSnRK2.3-1Bwere associated with TKW (Miaoet al.2017),andTaSnRK2.10-4Awas associated with TKW,number of spikes per plant and number of spikelets per spike (Wang Qet al.2014).Overexpression ofSAPK9in rice increased grain yield under drought stress,due to a higher proportion of mature pollen,high spikelet fertility and high panicle weight (Deyet al.2016). We propose that SnRK2s have important roles in development of agronomic traits related to grain yield.
Plant SnRK1,yeast SNF1 and mammalian AMPactivated protein kinase are highly conserved,and play a key role in regulation of aspects of carbon and nitrogen metabolism closely related to yield (Hrabaket al.2003).Recent studies indicated that SnRK2 and SnRK3 were derived from duplication of SnRK1 followed by evolutionary divergence to become involved in metabolic and stress signaling (Hrabaket al.2003; Hauseret al.2011); our results support that SnRK2s still maintain their original functions in determination of grain yield.
4.2.TaSnRK2.4 confers enhanced tolerance to abiotic stress probably by interacting with TaLTP3
Previous studies indicated thatTaSnRK2.4was involved in response to abiotic stress,and its overexpression conferred significantly strengthened tolerance to drought,salt and freezing stress as measured by various physiological parameters inArabidopsis(Maoet al.2010). Here,both Y2H and LCI assays indicated that TaSnRK2.4 physically interacted with TaLTP3,an abiotic stress responsive protein,involved in response to heat,PEG,NaCl,and ABA(Wang Fet al.2014). Overexpression ofTaLTP3led to enhanced thermotolerance in yeast cells (Wang Fet al.2014). Moreover,expression ofTaLTP3inArabidopsisresulted in strengthened thermotolerance and oxidative stress resistance (Wang Fet al.2014).

Fig.5 TaSnRK2.4 physically interacts with TaLTP3. A,TaSnRK2.4 interacted with TaLTP3 in yeast cells. AD,pGADT7 vector;BD,pGBKT7 vector. B,TaSnRK2.4 interacted with TaLTP3 in tobacco leaves. The luciferase signals were detected at 48 hours post infiltration.
The homologs ofTaLTP3also have the ability to enhance tolerance to various environmental stimuli. For example,AtLTP3,a homolog ofTaLTP3inArabidopsis,was positively regulated byAtMYB96by direct binding to theAtLTP3promoter. Overexpression ofAtMYB96orAtLTP3exhibited enhanced freezing tolerance inArabidopsis(Guoet al.2013). Additionally,ectopic expression of the maize homologZmLTP3resulted in increased salt tolerance inArabidopsis(Zouet al.2013).
Various abiotic stress factors,including drought,salinity and extreme temperature,cause the accumulation of reactive oxygen species (ROS) in plants (Thaoet al.2015;Farneseet al.2016). As TaLTP3 is involved in response to multiple environmental stimuli and has the ability to enhance oxidative stress tolerance,we speculate that the enhanced tolerance to multiple stresses by TaSnRK2.4 might be partially regulated by TaLTP3 in order to reduce the injury by ROS.
5.Conclusion
To sum up,the multifunctionalTaSnRK2.4gene set has potential for increasing grain yield and multiple stress tolerances in wheat. Marker-assisted selection should be undertaken to increase the frequencies of favorable alleles in wheat improvement.
Acknowledgements
We thank Prof.Robert A.McIntosh (Plant Breeding Institute,University of Sydney,New South Wales,Australia) for revising the manuscript. We also thank our colleagues from the Institute of Crop Sciences,Chinese Academy of Agricultural Sciences,Dr.Zhang Xueyong and Dr.Hao Chenyang for providing DNA samples of two nature populations,Dr.Xia Xianchun for providing nulli-tetrasomic lines of Chinese Spring,and Dr.Sun Jiaqiang and his colleagues for help with the LCI assay. This work was supported by the National Key Research and Development Program of China (2017YFD0300202),the National Natural Science Foundation of China (31571660),the Heilongjiang Postdoctoral Financial Assistance,China (LBH-Z17200),and the Research Project of Heilongjiang Academy of Agricultural Sciences,China (2018YYYF018).
Appendicesassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2021年1期
Journal of Integrative Agriculture2021年1期
- Journal of Integrative Agriculture的其它文章
- The dynamic impact of income and income distribution on food consumption among adults in rural China
- Driving factors of direct greenhouse gas emissions from China’s pig industry from 1976 to 2016
- ATP regulates the phosphorylation and degradation of myofibrillar proteins in ground ovine muscle
- Use of two-stage dough mixing process in improving water distribution of dough and qualities of bread made from wheatpotato flour
- lmpact of climate change on maize yield in China from 1979 to 2016
- Estimating daily actual evapotranspiration of a rice-wheat rotation system in typical farmland in the Huai River Basin using a two-step model and two one-step models
