Preparation of hollow silica microsphere and its effect on the thermal conductivity of polymer composites
Shu Jingjing, Chen Jian, Chen Yawei, Zhao Chu, Wang Mozhen*, Ge Xuewu*
1.CAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei 230026, China;2.CAS Key Laboratory of Materials for Energy Conversion, Department of Materials Science and Engineering,University of Science and Technology of China, Hefei 230026, China
Abstract: The thermal conductivity of polymer composites filled with hollow microspheres is closely related to the content and structure of hollow microspheres. In this paper, micron-sized monodisperse polystyrene (PS) microspheres are synthesized as the sacrificial template to prepare a series of hollow SiO2 (H-SiO2) microspheres with different inner and outer radius ratios (r/R).The r/R value is controlled by the relative content of PS microspheres and tetraethyl orthosilicate (TEOS). The chemical composition and morphology of H-SiO2 microspheres are characterized by infrared spectroscopy, scanning electron microscopy and transmission electron microscopy. Further, H-SiO2 microspheres are blended with polydimethylsiloxane (PDMS) at a certain content to obtain H-SiO2/PDMS composite rubbers. The effect of the content and the r/R value of H-SiO2 microspheres on the thermal conductivity of the composite rubber are investigated.Combined with the theoretical model calculation on the thermal conductivity of the silicone rubber,it can be concluded that the addition of H-SiO2 microspheres with a complete hollow structure and an r/R value higher than 0.963 can reduce the thermal conductivity of H-SiO2/PDMS composite rubbers.The more the H-SiO2 microspheres, the smaller the thermal conductivity of the composite rubber.At the same time, when the mass fraction of H-SiO2 microspheres is no more than 5%, the mechanical properties of the H-SiO2/PDMS composite rubber are also enhanced with the increase of the weight content of H-SiO2 microspheres. This work provides theoretical and experimental guidance for the design and preparation of high-performance hollow microspheres filled with polymer thermal insulation materials.
Keywords: hollow silica microspheres; silicone rubber; thermal conductivity; thermal insulation material
1 Introduction
With the rise of environmental protection concepts and the development of science and technology, thermal insulation materials have been widely used in various fields[1, 2], such as housing construction[3, 4], aircraft and aerospace equipment[5], and mobile phones[6]. Thermal insulation materials must have a low thermal conductivity.For example, air has a thermal conductivity as low as 0.0257 W·m-1·K-1, resulting in an excellent thermal insulation performance. However, the thermal conductivity of organic and inorganic solid materials is much higher than that of air. Therefore, a special structural design is necessary to obtain a solid matrix with a low thermal conductivity. The introduction of closed cavities inside the material, such as the formation of a porous foam structure with a large amount of air stored, can greatly reduce the thermal conductivity of the material[7-10]. This method has been widely used to prepare a variety of polymer-based thermal insulation materials[11]. However, the porous structure will make the mechanical properties of the matrix weakened, resulting in a poor toughness and low strength, thus limiting its practical application fields[12-16]. The addition of hollow structure fillers, such as hollow polymer microspheres[17], hollow glass microspheres[18], and hollow silica microspheres[19-22],into the substrate is another alternative in order to obtain a composite material with low thermal conductivity and good mechanical properties. However, many theoretical and practical research have shown that the addition of hollow microspheres doesn’t always reduce the thermal conductivity of the matrix and increase its thermal insulation performance[23, 24]. In fact, the thermal conductivity of this kind of filled composite material is closely related to both the content and the structure of the hollow structure fillers[25, 26]. Therefore, the precise structural design on the hollow microspheres is the premise of their use as the filler in the preparation of thermal insulation materials.
Hollow silica (H-SiO2) microspheres are non-toxic and eco-friendly[27],and possess good thermostability and mechanical properties,which are expected to be an ideal filler for the preparation of thermal insulation materials[28, 29]. Although there are myriads of studies on the preparation of H-SiO2microspheres[30, 31], the correlation between the structure of the hollow microspheres (the microsphere size, the shell thickness, the surface structure and properties, etc.) and the thermal conductivity of the final composite has been rarely discussed. On the other hand, the mechanical properties of the particle-filled composite materials (such as the flexural strength and modulus, radial tensile strength, and fracture toughness) are also dependent on the particle size and content, as well as the area occupied by the particles[32]. Generally,nano-scaled fillers will have a much larger contact area with the matrix than micron-scaled filler at the same mass ratio, which can improve the mechanical properties of composite materials more efficiently[33-35]. However, the synthesis process of nano-scaled H-SiO2microspheres is more complicated, and it is also difficult to disperse nano-scaled H-SiO2microspheres uniformly in the polymer matrix. At the same time, as a filler of thermal insulation materials, the spherical shell is required to be as thin as possible to allow more air, which in turn makes the mechanical strength of the hollow microspheres worse. Therefore, a precise control of the size and the shell thickness of H-SiO2microspheres is required for the preparation of thermal insulation materials with excellent thermal insulation performance and better mechanical strength, which is a challenge in the synthesis of H-SiO2microspheres.
Silicone rubber materials such as polydimethylsiloxane (PDMS) have good electrical insulation and chemical stability[36-39].They are often used as thermal insulation materials on aircraft and rocket engine components[5, 40]due to their inherent poor thermal conductivity (the thermal conductivity is 0.1519 W·m-1·K-1)[18]. However, the thermal conductivity of silica is as high as 1.3-1.5 W·m-1·K-1[24].Thus, how to identify an optimal size, shell thickness,and content of H-SiO2microspheres is the key problem for the successful preparation of high-performance thermal insulation silicone rubber materials.
In this paper, micron-sized H-SiO2microspheres with different inner and outer radius ratios(r/R) were prepared using the polystyrene (PS)microspheres as the sacrificial template.Then, the H-SiO2microspheres were blended with PDMS in different proportions to obtain H-SiO2/PDMS composite rubbers. The thermal conductivities of H-SiO2/PDMS composite rubbers were measured, and their dependence on ther/Rvalue and the content of H-SiO2microspheres was investigated. Combined with the theoretical model calculation on the thermal conductivity of H-SiO2/PDMS composite rubbers, the addition of H-SiO2microspheres which have a complete hollow structure and anr/Rvalue higher than 0.963 can reduce the thermal conductivity of H-SiO2/PDMS composite rubbers.At the same time, the mechanical properties of H-SiO2/PDMS composite rubbers are also enhanced.Therefore, this work provides theoretical and experimental guidance for the preparation of high-performance H-SiO2/PDMS thermal insulation materials.
2 Materials and methods
2.1 Materials
Methacrylatoe thyltrimethyl ammonium chloride (MTC) aqueous solution (mass fraction 80%) was purchased from Bidepharm. Styrene (St), azobisisobutyronitrile (AIBN), polyvinyl pyrrolidone (PVP K30), tetraethyl orthosilicate (TEOS), ammonia, stannous octoate (Sn(Oct)2), and absolute ethanol were purchased from Sinopharm Chemical Reagent Co., Ltd. St and AIBN were refined by vacuum distillation and recrystallization respectively before use. Hydroxy-terminated polydimethylsiloxane (PDMS, viscosity: 2000 mm2·s-1) was purchased from Hubei Xinsihai Chemical Co., Ltd. Deionized water was used in all experiments.
2.2 Synthesis of hollow silica (H-SiO2) microspheres
H-SiO2microspheres were prepared by a sacrificial template method, as shown in Figure 1. First,monodispersed polystyrene (PS)template microspheres were synthesized according to the previous work of Wu et al[41]. PVP (1.5 g) was ultrasonically dissolved in a mixture of 45 g of ethanol and 10 mL of water, followed by the addition of 10 g of St and 400 mg of AIBN under magnetic stirring.After being deoxygenated by bubbling nitrogen for 20 min, the system was heated to 70 ℃ and reacted for 1.5 h. A mixture of 45 g of ethanol, 0.5 g of MTC, and 10 g of St was added to the system, and the reaction continued for 14 h at 70 ℃ to obtain PS microspheres.Next, the system was cooled naturally to 50 ℃, followed by the addition of 2 mL of ammonia and a certain amount of TEOS, then continued to react at 50 ℃ for 8 h to let the formation of a silica shell around PS microspheres (PS@SiO2).Finally, the PS@SiO2microspheres were separated by centrifugation (4000 r/min, 3 min), washed with ethanol, and dried in an oven at 60 ℃ to a constant weight, then calcinated at 600 ℃ in a muffle furnace for 5 h to obtain H-SiO2microspheres.

Figure 1. Preparation principle of H-SiO2 microspheres.

Figure 2. Preparation process of H-SiO2/PDMS composite rubber.
2.3 Preparation of H-SiO2/PDMS composite rubber
The preparation process of the H-SiO2/PDMS composite rubber is shown in Figure 2. Firstly,a certain amount of the as-prepared H-SiO2microspheres and PDMS were mixed in a homogenizer (THINKY ARE-310) by stirring at 1500 r/min for 5 min, and deaeration at 2200 r/min for 1 min. Next,Sn(Oct)2(0.5% of the mass of PDMS) was added.After the same stirring and degassing as above, the mixture was cooled at -18 ℃ in a refrigerator for 20 min. Then, TEOS (0.75% of the mass of PDMS) was added into the system. After being stirred and degassed again, the mixture was poured into a square plastic mold, degassed in vacuum for 10 min,and then cured in an oven at 60 ℃ for 6 h.

Figure 3. SEM image of PS microspheres (a), TEM images of PS@SiO2 microspheres (b) and H-SiO2microspheres (c)prepared by the calcination of the sample in (b). (The sample in (b) was prepared at a condition of the relative feed volume of TEOS to St (VTEOS∶VSt) of 0.7∶2.2.).

Figure 4. IR spectra of PS, PS@SiO2 and H-SiO2 microspheres.
2.4 Characterizations for products
The morphology of the microspheres was characterized by a transmission electron microscope (TEM, JEOL2011 (H-7650), 100 kV) and a scanning electron microscope (SEM, JEOL (JSM-6700), 10 kV). The average particle size (Dn), the weight-average particle size (Dw) and the polydispersity index (PDI) of the microspheres were measured using the Adobe Photoshop CS3 software, and calculated according to the following equations with the diameters of at least 100 particles measured in the SEM images.
(1)
(2)
(3)
Whereniis the number of the microspheres with a diameter ofDi.
The infrared spectra of PS, PS@SiO2and H-SiO2were performed on attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR, ALPHA II)in a range of 400-4000 cm-1with a resolution of 2 cm-1and 96 overlay scans.
The thermal conductivity of the composite rubber was measured by the thermal conductivity meter (Hot Disk 2500s) at 25 ℃.Three cylindrical specimens with a diameter of 50 mm ± 1 mm and a thickness of 30 mm ± 1 mm were cut out for each sample. The thermal conductivity of the sample is the average of the measured thermal conductivities of the three specimens. The tensile properties of the composite silicone rubber were characterized on the electronic universal testing machine (UTM2502) with a crosshead rate of 10 (mm·min-1) according to GB/T528-2009.The tested specimens were dumbbell-shaped with the width of the narrow middle part of 4 mm ± 0.2 mm and the thickness of 2 mm ± 0.2 mm. Five specimens were tested for each composite rubber sample. The tensile strength and elongation at break of the specimens were recorded and averaged as the tensile strength and the elongation at break of the sample.
3 Results and discussion
3.1 Preparation and morphology control of H-SiO2 microspheres
In this work,monodispersed PS microspheres were prepared by the dispersion polymerization of St and a small amount of comonomer MTC in an aqueous alcohol solution using PVP as the dispersant. The morphology of the PS microspheres is shown in Figure 3(a). The average particle size and PDI of PS microspheres are 1.4 μm and 1.001 respectively.
The infrared spectrum of PS microspheres is shown in Figure 4, on which the characteristic absorption peaks of the benzene ring skeleton (1450 cm-1, 1500 cm-1, 1600 cm-1) can be clearly seen. The bands at 700 cm-1and 750 cm-1are identified as the deformation vibration absorption peaks of the C-H bonds on the monosubstituted benzene ring.
Obviously, the water-soluble PVP and hydrophilic quaternary ammonium ions are distributed on the surface of the PS microspheres, which is conducive to the adsorption of TEOS and its hydrolysate on the surface of the PS template microspheres[42-44]. So a silica layer can be coated on the PS microsphere to obtain the PS@SiO2composite microsphere, as shown in Figure 1. Figure 3(b) displays a typical TEM image of PS@SiO2composite microspheres, which are obtained at a condition of relative feed volume of TEOS to St (VTEOS∶VSt) of 0.7∶2.2. It can be observed that the shape of the microspheres hardly changed after the coating of the silica layer except that the diameter of PS@SiO2microsphere increases slightly to 1.75 μm. The infrared spectrum of PS@SiO2composite microspheres is shown in Figure 4. In addition to the characteristic absorption peaks of PS, a strong and broad absorption band around 1091 cm-1assigned to the anti-stretching vibration of Si-O-Si occurs, indicating the presence of SiO2in the prepared PS@SiO2microspheres. After being calcinated at high temperature, the solid PS@SiO2composite microspheres are transformed into H-SiO2microspheres, as shown in Figure 3(c). The striking contrast between the edge and the middle of H-SiO2microspheres indicate a typical hollow structure. At the same time, the characteristic absorption peaks of silica can be seen in the infrared spectrum of the H-SiO2microspheres in Figure 4(the peak at 800 cm-1can be identified as the symmetric stretching vibration of Si-O bonds), and the characteristic absorption peaks of PS nearly disappeared, indicating that H-SiO2microspheres are successfully prepared.

Figure 5. SEM (a1-d1) and TEM (a2-d2) images of H-SiO2microspheres prepared at different VTEOS∶VSt. (a1-a3): 0.6∶2.2; (b1-b3): 0.7∶2.2; (c1-c3): 0.8∶2.2; (d1-d3):0.9∶2.2.a3-d3 are the enlarged TEM images of the corresponding a2-d2.
The shell thicknesses of the H-SiO2microspheres are controlled by adjusting the relative content of TEOS and PS microspheres.Figure 5 shows the morphologies of H-SiO2microspheres prepared at differentVTEOS∶VSt. The corresponding structure parameters of the H-SiO2microspheres are listed in Table 1. It can be seen that the shell thickness of H-SiO2increases with the relative amount of TEOS. When the relative amount of TEOS is low, i.e.,VTEOS∶VSt=0.6∶2.2 (Figure 5(a1-a3)), a large number of broken H-SiO2microspheres can be observed. It means that when the relative amount of TEOS is small,it is not enough to form a complete silica shell on the surface of all PS template microspheres.Therefore, broken silica shells are left after the inner PS cores are removed by calcination.WhenVTEOS∶VSt≥ 0.7∶2.2, almost all the surface of PS microspheres can be covered by silica, thus H-SiO2microspheres with complete morphology can be obtained.However, the diameter of the cavity (1.4 μm) of the obtained H-SiO2microspheres is unchanged with the TEOS content, which is consistent with the diameter of the PS template microsphere.

Table 1. The structure parameters of H-SiO2 microspheres prepared at different VTEOS∶VSt.
[Note]*inner radius of H-SiO2microspheres;**outer radius of H-SiO2microspheres
3.2 The influence of the content and the structure of H-SiO2 microsphere on the thermal conductivity of H-SiO2/PDMS composite
The as-prepared H-SiO2microspheres and PDMS were uniformly mixed in a certain proportion, then a small amount of TEOS was added as the crosslinking agent to form H-SiO2/PDMS composite rubber under the catalysis of Sn(Oct)2. When the mass fraction of H-SiO2microspheres is no more than 5%, they have good dispersibility in PDMS without any agglomerations, as shown in the SEM images of the cross-section of the composite silicone rubbers in Figure 6. Moreover,the hollow microspheres have a certain strength since no deformation or fragmentation can be observed during the mixing and curing process. These indicate that the as-prepared H-SiO2microspheres have enough structure stability and good compatibility with PDMS, which make them be used as a promising filler to prepare composite silicone rubbers with good performance.
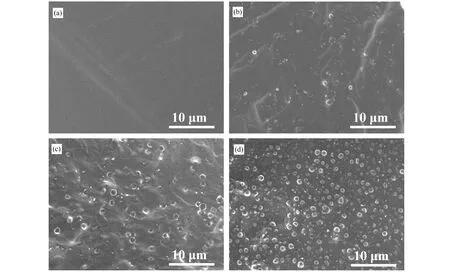
Figure 6. The SEM images of the fracture surfaces of H-SiO2/PDMS with different mass fraction of H-SiO2: (a) 0%, (b) 1%, (c) 3%, and(d) 5%.(Samples were brittle broken after being immersed in liquid nitrogen for 20 min).
Many studies on the theoretical calculation on the influence of the content and structural parameters of hollow microsphere on the thermal conductivity of composite materials have been reported[11, 23]. The heat conduction process of the hollow microspheres filled polymer composite material includes: ①the heat conduction process of the solid matrix ofthe composite material and the gas inside the microsphere; ②the heat radiation process of the solid; ③the gas convection process in the microsphere. It is generally believed that the heat radiation process ②can be ignored when the temperature is not too high. In addition, the process ③does not need to be considered,since the gas cannot undergo convection in small microspheres. Therefore, only the heat conduction processes of each solid material and gas are used to calculate the overall thermal conductivity of the material. According to the principle of minimum thermal resistance and the assumption that the specific equivalent thermal conductivity is equal, Liang et al.[46, 47]deduced that the equivalent thermal conductivity (keff) of polymer composite materials filled with the hollow microspheres could be calculated by the following Equation (4):
(4)
Wherekp,kg, andkaare the thermal conductivities of the polymer matrix, the shell of the filled microsphere and the gas in the microsphere, respectively.φfis the volume fraction of the filled microsphere.ρs,ρg, andρaare the equivalent density of the microsphere, the shell density, and the density of the gas in the microsphere, respectively.ρsis defined as
(5)
whereVgandVsare the volumes of the spherical shell and the entire microsphere, andrandRare the inner and outer radiuses of the hollow microsphere. The gas densityρais negligible compared withρsandρg, that is,ρs-ρa≅ρs,ρg-ρa≅ρg. After simplification, Equation (4) can be expressed as
(6)
Generally, the hollow microspheres are added to the matrix based on the mass fraction (w).Thus,φfcan be calculated fromwaccording to the following Equation(7):

(7)
whereMis the total mass of the composite material,wis the mass fraction of the filled microspheres, andρpis the density of the polymer matrix. Thekeffof the composite material can be calculated from Equations (6) and (7). For the H-SiO2/PDMS composite rubber, the required parameters are listed as follows:kp=0.1519 W·m-1·K-1(measured by experiment),kg= 1.4 W·m-1·K-1[30],ka= 0.0257 W·m-1·K-1,ρp= 0.97 g·cm-3,ρg= 2.32 g·cm-3. By substituting them into Equations (6) and (7), the dependence ofkeffof the composite rubbers with different H-SiO2mass fraction(w=1%, 3%, and 5%) onr/Rof the hollow microspheres can be theoretically calculated, as shown by the solid lines in Figure 7.It can be seen that whenr/R=0.963, no matter how much H-SiO2microspheres added, the thermal conductivity of the composite rubber is as same as that of the pure PDMS matrix, in other words,keff=kp. These results can also be deduced from Equation (6), whenkeff=kp:

Figure 7. The relationship between the theoretical equivalent thermal conductivities (solid lines) and the measured thermal conductivities(symbols) of the H-SiO2/PDMS composite rubber with different H-SiO2 mass fraction and the r/R of H-SiO2.
(8)
Whenr/R<0.963,the addition of H-SiO2microspheres can only increase the thermal conductivity of the composite rubber. The more the H-SiO2microspheres added, the higher the thermal conductivity. Only whenr/R> 0.963, the addition of H-SiO2microspheres can reduce the thermal conductivity of the composite rubber. The more the H-SiO2microspheres added,the smaller the thermal conductivity of the composite rubber.
The actual thermal conductivity of H-SiO2/PDMS composite rubber was measured by the thermal constant analyzer,as shown by the data points of different colors in Figure 7.It can be seen that when the H-SiO2microspheres withr/Rof 0.948 (< 0.963) are used,the actual thermal conductivity of the composite rubber is indeed higher than that of the silicone rubber matrix, and increase with the content of H-SiO2microspheres. The thermal conductivity of H-SiO2/PDMS composite rubber prepared from H-SiO2microspheres withr/Rof 0.958 (less but close to 0.963) is close to that of pure PDMS matrix. The thermal conductivity of H-SiO2/PDMS composite rubber prepared by H-SiO2microspheres withr/Rof 0.966 (> 0.963) is less than that of the silicone rubber matrix.And the more the H-SiO2microspheres, the smaller the thermal conductivity of the composite rubber. When the mass fraction of H-SiO2microspheres reaches 5%, the thermal conductivity of the sample is only 0.118W·m-1·K-1, which is 22.3% lower than that of the PDMS matrix. However, the thermal conductivity of H-SiO2/PDMS composite rubber prepared by adding H-SiO2microspheres with anr/Rvalue of 0.974 is surprisingly increased. This can be attributed to that a large amount of broken silica shells exist in this case, as shown in Figure 5(a1-a3), which means the actual gas volume introduced by the addition of H-SiO2microspheres is much less than the theoretical prediction. The high thermal conductivity of silica itself (1.4 W·m-1·K-1) leads to the thermal conductivity of the final composite rubber is much higher than the theoretical prediction.

Figure 8. The relationship between (a) the tensile strength, (b) elongation at break of H-SiO2/PDMS silicone composite rubber and the weight content of H-SiO2.
In summary,in order to achieve much lower thermal conductivity of the H-SiO2/PDMS composite rubber, H-SiO2microspheres should have a complete hollow structure and anr/Rvalue higher than 0.963.The more the H-SiO2microspheres, the smaller the thermal conductivity of the composite rubber, which further improves the thermal insulation performance of the composite rubber.
3.3 Mechanical properties of the H-SiO2/PDMS composite rubber
The mechanical properties of the H-SiO2/PDMS composite rubber directly affect its application prospects. The tensile strength and elongation at break of the silicone rubber filled with different contents of H-SiO2microspheres(r/Rof 0.966) were characterized, as shown in Figure 8. When the mass fraction of H-SiO2microspheres is no more than 5%,the tensile strength and the corresponding elongation at break of the composite rubber increase with the weight content of H-SiO2microspheres, indicating that the mechanical properties of the composite rubber have been significantly improved. When the mass fraction of H-SiO2is 5%, the tensile strength of the H-SiO2/PDMS composite rubber is 1.05 MPa, which is 4.6 times that of pure silicone rubber (0.23 MPa).At the same time,its elongation at break reaches ~250%, which is more than twice of that of the pure silicone rubber (120%). It has been reported in the literature that the mechanical properties of the composite will be deteriorated when hollow glass beads with a larger particle size (10-100 μm) are filled in the silicone rubber[45].It means a smaller size of the hollow silica microspheres will be helpful to improve the mechanical properties of the composite rubber.However,when the mass fraction of H-SiO2increases to more than 5%, the elongation at break of the composite rubber changes little, but the tensile strength has an obvious decrease with the increase of the content of H-SiO2.This indicates that excessive H-SiO2microspheres may hinder the formation of the cross-linked network of the silicone rubber, thereby reducing the toughness of the composite rubber. Therefore, in order to prepare composite rubber with low thermal conductivity and good mechanical properties, it is best to use the H-SiO2microspheres with a complete hollow structure and anr/Rvalue higher than 0.963. At the same time,the mass fraction of the H-SiO2microspheres is preferably no more than 5%.
4 Conclusions
In this paper, a series of monodisperse H-SiO2microspheres(~1.4 μm) with different inner and outer radius ratios(r/R) were synthesized using the PS microspheres as the sacrificial templates. The as-prepared H-SiO2microspheres have good compatibility with PDMS, and can be dispersed homogeneously in PDMS to obtain H-SiO2/PDMS composite rubbers. The thermal conductivities of the composite rubbers with different content andr/Rvalue of H-SiO2microspheres were measured. Combined with the theoretical calculation, it confirms that only the addition of H-SiO2microspheres with complete hollow structure and anr/Rvalue higher than 0.963 can make the thermal conductivity of the H-SiO2/PDMS composite rubber lower than that of the pure PDMS matrix. At the same time, when the mass fraction of the micron-sized H-SiO2microspheres is no more than 5%, the mechanical properties of the H-SiO2/PDMS composite rubber can also be enhanced as the increase of the mass fraction of H-SiO2microspheres.At present, the thermal conductivity of the commercial solid insulation silicone rubber materials ranges from 0.15 to 0.25 W·m-1·K-1. Thus, the H-SiO2/PDMS composite rubber prepared in this work not only has a much lower and adjustable thermal conductivity, but also an improved mechanical property. This work provides theoretical and experimental guidance for the preparation of high-performance polymer thermal insulation materials filled with hollow microspheres.
Acknowledgments
This work is supported by the Fundamental Research Funds for the Central Universities (WK9110000066, WK3450000005, WK3450000006), and the National Natural Science Foundation of China (51973205, 51573174, 51773189).
Conflict of interest
The authors declare no conflict of interest.
- 中国科学技术大学学报的其它文章
- 沉浸式虚拟现实技术在地球科学中的应用
- A new high-resolution δ13Ccarb record for the Early-Middle Triassic:Insights from the Tianshengqiao section, South China
- Searching for radio pulsation from SGR 1935+2154 with the Parkes ultra-wideband low receiver
- A fourth order linear parabolic equation on conical surfaces
- Transit-time scattering of radiation belt electrons by off-equatorially generated magnetosonic waves
- Application of network vector autoregression model in volatility spillover analysis

