Why use pre-differentiated cells to address complex multi-factorial neurodegenerative diseases?
Alex Kopyov, Toni L. Uhlendorf, Randy W. Cohen
Past four decades have seen a concerted push to develop regenerative treatments for incurable neurodegenerative diseases such as Parkinson’s disease (PD). PD’s pathophysiology is primarily characterized by dopamine deficiency caused by the progressive depletion of neurons in substantia nigra (Bernheimer et al., 1973). So,it is not very surprising that most attempts at regenerative treatment of PD focus on using predifferentiated, dopaminergic neurons. However,PD’s pathogenesis spans far beyond strictly dopamine deficiency and is still not completely known (Chaudhuri et al., 2006). Thus, attempts at implanting pre-differentiated dopaminergic neurons that are locked into a single, inflexible function, predictably ran into the same problems as dopamine replacement therapy: 1) These cells are not curative; 2) They cannot address all PD deficits, and 3) They tend to cause side effects(Kordower et al., 2017). In order to address the multifactorial nature of this disease, we suggest the use of non-tumorigenic undifferentiated stem cells. Unlike adult cells, undifferentiated cells, due to their inherent plasticity, have the potential to respond to microenvironmental cues (Martínez-Cerdeño et al., 2017) from the Parkinsonian brain, target multiple systems and pathways, and eventually restore both structure and function(Figure 1).
Multifactorial nature:Traditionally it has been thought that the movement disorders of PD result from degeneration of dopamineproducing neurons in the substantia nigra pars compacta that feed into and regulate the complex connections among the cerebral cortex,cerebellum, thalamus, subthalamic nucleus,and basal ganglia. The latest experimental data suggest that the pathogenesis of PD involves more than the dopamine pathway. In fact,the pathological substrate of symptoms of Parkinsonism varies widely (Marras and Lang,2008). Different anatomical abnormalities can result in similar Parkinsonian symptoms while the same type of lesion can result in markedly differing symptoms in different patients (Marsden and Obeso, 1994).
Standard of treatment:To date no single treatment for PD has been shown to have an unequivocal disease-modifying effect, and many treatments have been shown to be ineffective.The possible explanation for this fact is that previous treatments aimed for only a single fragment of the pathogenic mechanism of PD.
Pre-differentiated cells by their single-targeted nature are only able to address one deficit.Even when addressing this deficit, there is still an issue of dosing and placement.Undifferentiated cells have an option to differentiate or not to differentiate, to multiply or to not multiply depending on influence of the microenvironment where they are implanted.Adult pre-differentiated cells do not have such freedom of diversification. Moreover, since predifferentiation is required, they are not able to address deficits that the treating physician is not currently aware of. Thus, this pre-differentiated approach will never be completely curative like any other dopamine-replacing therapies.
Proposed solution:Undifferentiated embryonic cells were one of the first regenerative treatments considered (Thomson et al., 1998).However, these cells have a propensity to develop teratomas (Adewumi et al., 2007)which precluded these unmodified embryonic cells from being used in human PD treatments.However, undifferentiated neural progenitor cells(NPCs) are more advanced developmentally than embryonic cells and have lost their tumorigenicity(Madrazo et al., 2019). At the same time, these cells are not locked into a single fate like adult stem cells. In addition, undifferentiated cells may be influenced by the new host environment,affecting further maturation into the correct replacement cell. Their plasticity, potentially,allows NPCs to provide a disease-appropriate measured response to the microenvironmental cues and to be able to address multiple deficiencies. Consequently, the nature and severity of each disease may impact how these undifferentiated cells respond as each disease state establishes a unique biochemical profile.The NPC studies that we have carried out in the last few years lead us to believe that such cellular behavior may indeed occur.
Spastic Han-Wistar (sHW) model:Our preferred choice of animal model for studying this phenomenon is not a traditional PD model such as 6-hydroxydopamine (6-OHDA)-injection model. The 6-OHDA model, like other toxinbased PD models, works by selectively destroying dopaminergic neurons in the substantia nigra,mimicking the dopamine deficiency part of PD pathophysiology. However, unlike a gradual continuous dopaminergic neuron death observed in human PD, cell destruction in toxic models is almost instantaneous. In effect, these models poorly recreate the progressive multifactorial pathophysiological process like a human PD,but rather model a frozen-in-time snapshot of dopamine deficiency. In addition, 6-OHDA model presents a challenge when evaluating NPCs.Undifferentiated cells are expected to self-direct,and thus they require an active pathological process to target. Since there is no continuous cell death in a 6-OHDA model of PD, a stem cell has no cues to respond for migration and eventually cell replacement.
Instead, we concentrated on studying the behavior of our NPCs as treatment in sHW rats.The sHW mutant is a multigenetic, naturally occurring condition characterized by progressive death of Purkinje neurons in the cerebellum.Clinically, these spastic mutants develop fore limb tremor, rigidity in hind limbs, and balance instability – all motor symptoms of human ataxia.The motor deficits are progressive and culminate in an inability to move by day 60 of age. Our yet unpublished data also suggests that the mutants have a decreased number of dopaminergic neurons in substantia nigra as well. But the key quality of this model that brings it closer to human PD pathophysiology is continuous neuronal death. This same quality is also what we believe is required to elicit a cellular response similar to that observed in human PD cases.Rapid progression and easily defined severity of the disease in the sHW model allow for faster pre-clinical trials not requiring long-term aging studies.
Pre-clinical data:The hypothesis of undifferentiated stem cells responding to progressive disease was put to test in a number of studies published in the past four years. These completed studies examined both the relative efficacy of the delivery routes used and the success of NPC treatment.
Uhlendorf et al. (2017) compared the efficacy of carotid injections of cells to that of a unilateral intra-cerebellar injection. Even a unilateral cerebellar injection produced statistically significant improvements in motor scores. The cells not only migrated within the cerebellum but were distributed in the forebrain as well. Intracarotid delivery resulted in the majority of the cells being deposited in the lungs rather than the brain. There was some clinical improvement, but not statistically significantly different from dead NPC controls.
Later that year, Nuryyev et al. (2017) investigated the efficacy of injecting NPCs into the cerebellum of immunosuppressed 40-day-old sHW ratsvshippocampus. Bilateral cerebellum injections resulted in a statistically significant improvement in motor scores and longevity of the subjects.Histologically, the NPCs were seen migrating from the site of injection towards the degenerating Purkinje cell layer, theoretically to contribute to its repopulation. Hippocampal injections produced a few improvements but fell short of reaching a statistically significant difference compared to control mutants.
Taken together, these two studies provide evidence that undifferentiated cells were able to migrate in response to injury, but that the extent of such homing and migration is limited.When primary pathology is in the cerebellum,cells delivered via the carotid artery or into the forebrain had very little effect (Uhlendorf et al.,2017) .
The next question we attempted to answer was how much longer the human NPCs were able to survive in the rat cerebellum and continue to alleviate the sHW neurodegenerative deficits.Tierney et al. (2020) explored the longevity of NPC transplantation effects which demonstrated that mutants survived well past their normal lifespan, achieved motor scores similar to that of normal animals. They also examined whether the bilateral intra-cerebellar NPCs survived to the end of the experiment (100 days of age; 60 days post-transplant). Although some surviving human-derived NPCs were found in the cerebella of these animals, they were remarkably effective.Besides the significant improvements in motor behavior, the number of Purkinje cells found in the brains of these mutants receiving NPCs was significantly higher than that of 60-dayold untreated mutant controls. Thus, it appears that the NPCs migrated towards the Purkinje layer within 20 days post-transplantation and promoted either preservation of the host’s own Purkinje cells or possibly growth of new ones, culminating in the significant clinical improvements.
A recently carried out proteomics study (inhouse unpublished data) revealed that the NPCs secrete a number of neuronal factors that have been shown to either promote neuronal growth (Neuromodulin, Nestin) or have neuroprotective qualities (14-3-3 epsilon protein,insulin-like growth factor II, activity-dependent neuroprotective protein). It is likely that one or more of these proteins contributed to higher Purkinje cell numbers in NPC-treated animals.It would be interesting to investigate how the biochemical profile of these undifferentiated cells alters when influenced by age, location and possible disease state.
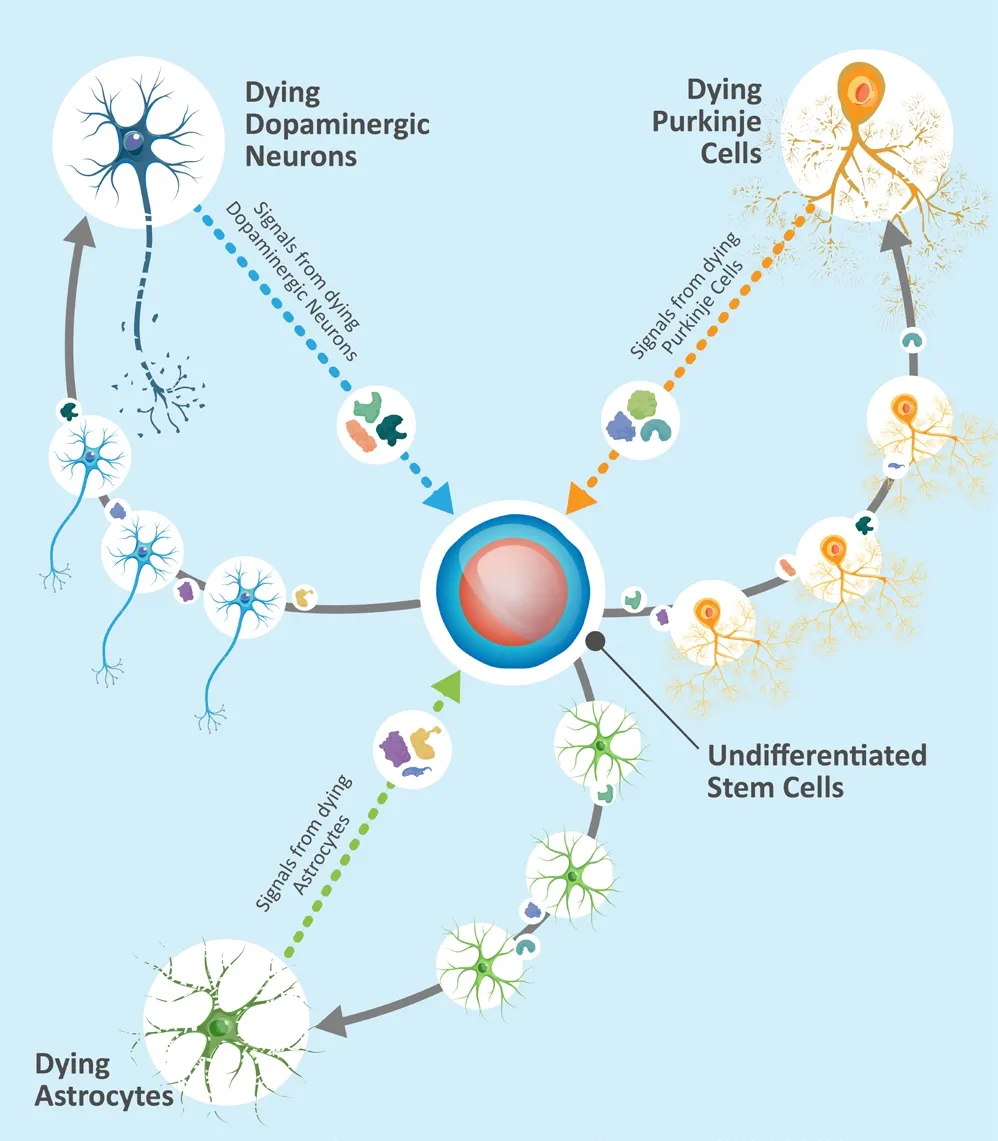
Figure 1|Undifferentiated stem cell respond.
Clinical data:Response to microenvironmental cues as demonstrated in being able to alleviate a Purkinje cells depletion is just one part of our evidence supporting NPCs as treatment. Another part is the ability to respond to different cues.As a part of first in human pilot trial, Madrazo et al. (2019) implanted the same undifferentiated cells into putamina of eight patients with PD of varying severity. Four years later, none of the patients developed tumors or any other stem cell related side effect. Most of these patients demonstrate better motor scores and take less dopamine supplementation four years after than before the treatment (Madrazo et al., 2019).Of special interest is that not only none of the patients developed dyskinesia – a common side effect of implantation of pre-differentiated dopaminergic neurons, but several patients with pre-existing dyskinesia had it resolve or decrease in severity. It appears, the same undifferentiated NPC appears to have the capability to positively affect both Purkinje and dopaminergic cell deficiency. This speaks to the ability of these undifferentiated NPCs to adapt themselves in different environments and possibly align their secretome to the pathology of the host.
Conclusions:At this point we can only hypothesize about the mechanism of action of undifferentiated NPCs. Because of their inherent flexibility, it is very likely that there is more than one mechanism involved in treating any given disease. Not being able to find transplanted cells a month or longer after transplantation impedes the search for the mechanism, but also raises additional questions in itself. Do cells undergo apoptosis because they served their purpose and are no longer needed? Or are they, being a xenotransplant, not able to survive despite the immunosuppression after they are able to produce a clinical effect? Transplanted predifferentiated cells have been shown to survive in the brains of PD patients for 7 years or longer.There is no reason to think that undifferentiated NPCs, that are known to be immuno-privileged,would fare any worse in an allotransplant.However, until we are able to examine long term surviving transplants or the biochemistry of xenotransplants, the mechanism(s) of action are likely to remain a mystery.
Of course, that is not necessarily a problem in itself. Precise pathogenesis of PD is still far from being clear, and the parts of the PD puzzle that are known are too numerous as to be able to target all of them. Plasticity of undifferentiated NPCs makes this lack of knowledge on our part more or less irrelevant. Without controlled studies (i.e., Food and Drug Administration Phase II clinical trials) of allotransplants, it is impossible to be certain whether undifferentiated cells act with a form of intelligent self-targeting. However,there is enough evidence to pursue this direction further. A single, dose-independent treatment that tailors itself to the patient’s condition is attractive enough. The fact that it potentially can be used for conditions with poorly understood or unknown multifactorial pathogenesis is even more attractive.
Alex Kopyov*, Toni L. Uhlendorf,Randy W. Cohen*
Celavie Biosciences LLC, Oxnard, CA, USA(Kopyov A)
Department of Biology, California State University,Northridge, CA, USA (Uhlendorf TL, Cohen RW)
*Correspondence to:Alex Kopyov, BS,akopyov@celavie.com; Randy W. Cohen, PhD,randy.cohen@csun.edu.
https://orcid.org/0000-0003-0420-138x(Alex Kopyov)
https://orcid.org/0000-0002-3945-7169(Randy W. Cohen)
Date of submission:June 19, 2020
Date of decision:August 1, 2020
Date of acceptance:September 16, 2020
Date of web publication:December 7, 2020
https://doi.org/10.4103/1673-5374.300990
How to cite this article:Kopyov A, Uhlendorf TL,Cohen RW (2021) Why use pre-differentiated cells to address complex multi-factorial neurodegenerative diseases? Neural Regen Res 16(7):1413-1414.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Clusterin: a multifaceted protein in the brain
- Positive effects of music therapist’s selected auditory stimulation on the autonomic nervous system of patients with disorder of consciousness: a randomized controlled trial
- Transcranial pulse current stimulation improves the locomotor function in a rat model of stroke
- Comparative transcriptomic analysis of rat versus mouse cerebral cortex after traumatic brain injury
- Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke
- Extremely low frequency electromagnetic fields promote cognitive function and hippocampal neurogenesis of rats with cerebral ischemia

