Reducing LncRNA-5657 expression inhibits the brain inflammatory reaction in septic rats
Yi-An Zhan, Xin-Liang Qiu, Xu-Zhen Wang, Ning Zhao, Ke-Jian Qian,
Abstract Our preliminary study found that the long noncoding RNA (LncRNA)-5657 can reduce the expression of inflammatory factors during inflammatory reactions in rat glial cells. However, the role played by LncRNA-5657 during septic brain injury remains unclear. In the present study, rat models of septic encephalopathy were established by cecal ligation and puncture, and then the rats were treated with a hippocampal injection small hairpin RNA (shRNA) against LncRNA-5657 (sh-LnCRNA-5657). The sh-LncRNA-5657 treatment reduced the level of neuronal degeneration and necrosis in the rat hippocampus, reduced the immunoreactivities of aquaporin 4, heparanase, and metallopeptidase-9, and lowered the level of tumor necrosis factor-alpha. Glial cells were pre-treated with sh-LncRNA-5657 and then treated with 1 µg/mL lipopolysaccharide. Sh-LncRNA-5657 transfection decreased the expression of LncRNA-5657 in lipopolysaccharide-treated glial cells and decreased the mRNA and protein levels of tumor necrosis factor-alpha, interleukin-1β, and interleukin-6. These findings suggested that LncRNA-5657 expression can significantly reduce the inflammatory reaction during septic encephalopathy and induce protective effects against this disease. This study was approved by the Institutional Ethics Committee at the First Affiliated Hospital of Nanchang University of China (approval No. 2017-004) in 2017.
Key Words: brain injury; glial cells; inflammation; injury; lipopolysaccharide; long noncoding RNA; repair; sepsis
Introduction
The incidence rate of brain injury is approximately 8–70%among patients with severe sepsis (Chaudhry and Duggal,2014), presenting with clinical symptoms of delirium or deep coma. Brain injury caused by sepsis is a critical complication that affects the development and prognosis of patients with sepsis (Cardozo Júnior and Silva, 2014). Sepsis encephalopathy(SE) is an acute and diffuse brain injury that is a common complication of severe sepsis characterized by changes in cognition, arousal, and consciousness (Ziaja, 2013; Ren et al., 2020). SE is a leading cause of brain injury and death in the intensive care unit (Cardozo Júnior and Silva, 2014), and severe septicemia and septic shock remain the most common causes of death in the intensive care unit (Sun et al., 2017).The effective control of infection is a key component required to prevent and treat septic brain injuries and reduce mortality(Vincent et al., 2002). Despite currently available treatments designed to support organ function and maintain metabolic balance, effective interventions for septic brain injury remain lacking.
Long-chain noncoding RNA (LncRNA) is a type of RNA consisting of more than 200 base sequences that is transcribed by RNA polymerase II (Long et al., 2017). The structure of LncRNA is similar to that of mRNA, but LncRNA does not encode a protein due to the lack of an open reading frame (Yang et al., 2019). LncRNA is abnormally expressed in various diseases, promoting disease occurrence and maintaining various conditions associated with diseases (Zhou et al., 2017; Ding et al., 2020). LncRNA can regulate protein expression at the transcriptional and post-transcriptional levels and widely participates in various physiological and pathological processes of the body (Du et al., 2017; Zhao et al., 2020). To identify new targets for the treatment of SE,the role of LncRNA during the development of septic brain injury was explored. In our preliminary study, LncRNA-5657 was identified by high-throughput sequencing. We found that LncRNA-5657 expression reduced the expression of tumor necrosis factor-α (TNF-α) and other inflammatory factors during the inflammatory responses of rat microglia(unpublished data). However, the role played by LncRNA-5657 during SE development has not been investigated.
Cecal ligation and puncture (CLP) is a classical method for modeling SE, resulting in the development of septic symptoms in rats (Malkoç et al., 2020; Zhang et al., 2020). CLP-induced sepsis in rats is associated with hippocampal dysfunction and spatial memory impairment (Liu et al., 2014), and the hippocampus is a known region associated with cognition. In this study, the function of LncRNA-5657 in both septic rats and lipopolysaccharide (LPS)-treated microglia was determined in response to changes in LncRNA-5657 expression.
Materials and Methods
Animal models and LncRNA intervention
In total, 40 male Sprague-Dawley rats (4 months old, 250–300 g, specific-pathogen-free level) were provided by Hunan Shrek Jingda Experimental Animal Co., Ltd. (license No. SCXK(Xiang) 2016-0002). The animal protocols were approved by the Animal Ethics Committee of the First Affiliated Hospital of Nanchang University (approval No. 2017-004) in 2017.
A rat sepsis model was induced by performing CLP, as previously described (Kafa et al., 2010). Briefly, 25 rats were anesthetized by isoflurane inhalation (5%; RWD, Shenzhen, China). The head was fixed with a rat-toothed hook. The limbs were fixed on a foam operation board using rubber bands, and the median abdominal area was cleaned with iodine and 75% ethanol. A 2-cm incision was made in the lateral abdomen of rats to fully expose the cecum. Then, 1/3 of the cecum was ligated with a 5-0 suture and punctured twice with a 20-G needle. The cecum was squeezed gently to induce fecal residue overflow from the puncture hole, which was allowed to flow into the abdominal cavity. The cecum was returned to the abdominal cavity. The inner layer was sutured with a 5-0 suture, and the outer layer was sutured with a 3-0 suture. After surgery, the animals were raised in a specific pathogen-free environment, which was maintained at a temperature of 23 ± 2°C (humidity of 45–65%)under a controlled 12-hour light/dark cycle.
The neurobehavioral score was evaluated based on the scores for the auricular reflex, corneal reflex, tail reflex, escape reflex, and righting reflex, which were evaluated 8 hours after modeling, as previously described (Kafa et al., 2010).In each reflex test, no reflex was scored as 0; mild reflex was counted as 1, and normal reflex was scored as 2. The highest possible score was 10 points. After modeling, eight animals died, and seven animals did not meet the criteria for SE (total score of fewer than 5 points). The success rate of our model was approximately 62.5%, which was comparable to the rate reported by a previous publication (Bozza et al., 2010).The 25 rats with successful model induction were divided into two groups: SE (n= 10) and sh-LncRNA-5657 (SE + sh-LncRNA-5657,n= 15) groups. Normal rats that underwent surgical procedures without ligation and puncture were used as a sham control group (n= 10).
At 24 hours after modeling, the rats were anesthetized with isoflurane and fixed on a brain stereotaxic instrument (RWD).The rats in the control, SE, and sh-LncRNA-5657 groups were intrahippocampally injected with normal saline (10 µL), empty lentivirus vector [10 µL, 1 × 109infection units (IFU)] (Santa Cruz Biotechnology, Dallas, TX, USA), and lentivirus encoding sh-LncRNA-5657 (10 µL, 1 × 109IFU), respectively.
Three different sh-LncRNA-5657 sequences were synthesized by Shanghai Sangon (Shanghai, China), of which sh-LncRNA-5657-3 was the most effective for reducing LncRNA-5657 expression and was packaged into a lentivirus vector, as previously described (Hutson et al., 2012; Xu et al.,2018).
One week after injection, the animals were anesthetized by isoflurane (5%; RWD, Shenzhen, China) and decapitated.Hippocampus tissues were also collected and stored at –80°C or fixed in 4% paraformaldehyde overnight at 4°C for the subsequent experiments.
Hematoxylin-eosin staining
After fixation in 4% paraformaldehyde overnight at 4°C, the brain tissues were washed with running water and underwent dehydration (70%, 80%, and 90% ethanol solution, and ethanol and xylene (1:1) for 15 minutes each, xylene for 15 minutes). Brain tissues were then embedded in paraffin for 50–60 minutes. Brain tissues were sectioned into 10-µm sections. The sections were immersed into hematoxylin and eosin for 5 minutes at room temperature in the dark.The slides were observed under a light microscope (BX41;Olympus, Tokyo, Japan).
Immunohistochemistry
The hippocampal slices were incubated with 3% hydrogen peroxide to block endogenous peroxidase activity (5 minutes at room temperature). The tissues were blocked in 5% bovine serum albumin for 2 hours at room temperature. Tissues were incubated with primary antibodies [aquaporin-4 (AQP4; rabbit polyclonal; 1:200; Cat# 16473-1-AP; Proteintech, Rosemont,IL, USA), heparanase (rabbit polyclonal; 1:200; Cat# 24529-1-AP; Proteintech), matrix metallopeptidase 9 (MMP-9;rabbit polyclonal; 1:100; Cat# AF-5228; Affinity, Cincinnati,OH, USA)] overnight at 4°C. The slides were then washed with phosphate-buffered saline and were then incubated with horseradish peroxidase-labeled goat anti-rabbit IgG(1:10,000; Cat# A16104SAMPLE; Thermo Fisher Scientific,Inc., Waltham, MA, USA) at room temperature for 30 minutes.The staining was visualized with 3,3′-diaminobenzidine chromogen for 3 minutes at room temperature. The nucleus was counterstained with hematoxylin for 3 minutes at room temperature. In each animal, the immunohistochemistry was conducted in three sections. Images were obtained under a microscope (magnification 200×; CX41; Olympus Corporation,Tokyo, Japan). At least four fields were blindly selected from each image by a professional pathologist. The gray density of the hippocampus was calculated using ImageProPlus software(Media Cybernetics, Inc., Rockville, MD, USA) in a blinded manner. The relative expression levels of target proteins were normalized against the levels obtained for the negative control(without primary antibody).
Enzyme-linked immunosorbent assay
After treatment, all animals were decapitated, and the hippocampi were isolated on ice. TNF-α levels in fresh hippocampal tissues were detected using enzyme-linked immunosorbent assay, according to the manufacturer’s instructions (MM-0132M2; Shanghai Shenghong Biotech,Shanghai, China). The optical density was measured at a 450 nm wavelength using an automatic microplate reader(Shanghai Meilian, Shanghai, China).
Cell transfection
Microglia were purchased from the Chinese Academy of Sciences, Shanghai, China (Cat# ac340723) and cultured in Dulbecco’s modified Eagle medium (DMEM, GIBCO, Shanghai,China) containing 10% fetal bovine serum (Hyclone, Shanghai,China). The cells were passaged when they reached 70%confluence. The cells were divided into four groups, including the control, LPS group, LPS + vector group (scrambled control),and LPS + sh-LncRNA-5657 groups.
Prior to transfection, the cell media in all groups were replaced with serum-free DMEM (GIBCO). In a sterilized Eppendorf (EP) tube (Eppendorf, Shanghai, China), 125 µL Opti-modified Eagle medium (GIBCO) was combined with 5µL Lipofectamine 3000 (Thermo Scientific, Shanghai, China).In a separate EP tube, 12.5 µL sh-LncRNA-5657-3 (shRNA dry powder was dissolved in diethyl pyrocarbonate-treated water at 125 µL/optical density). Then, the contents of the two EP tubes were mixed gently and incubated at room temperature for 60 minutes. The mixture was added to the cells (1 µg/mL),and the cells were returned to the incubator. After 4 hours of transfection, 1 mL DMEM, containing 20% fetal bovine serum,was added to each plate. The cells were collected and 48 hours after transfection for use in subsequent experiments.
Following transfection for 48 hours, the cells in all groups except the control group were treated with LPS (1 µg/mL;Sigma, Shanghai, China) for 6, 12, and 24 hours. The cells were collected for subsequent experiments at the end of the LPS incubation.
Fluorescence quantitative polymerase chain reaction
Target gene expression was detected by fluorescence quantitative polymerase chain reaction. Briefly, total RNA was extracted from cells using the TRIzol Reagent (CW0580S;CWBIO, Beijing, China). Complementary DNA was synthesized according to the instructions provided by the reverse transcription kit (CW2569M; CWBIO, Beijing, China). The expression levels of LncRNA-5657, TNF-α, interleukin-1β (IL-1β), and interleukin-6 (IL-6) were determined by fluorescence quantitative polymerase chain reaction using complementary DNA as the template. The primers used are listed inTable 1.Target gene expression levels were calculated using the 2–∆∆Ctmethod (Livak and Schmittgen, 2001) and were normalized against the expression level of glyceraldehyde 3-phosphate dehydrogenase.
Western blot assay
Cytoplasmic proteins were isolated from treated cells.After determining the protein concentration using the bicinchoninic acid method (Song et al., 2019), proteins were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transferring onto a polyvinylidene difluoride membrane, nonspecific staining was blocked with 5% non-fat milk. The membrane was incubated with rabbit polyclonal anti-IL-1β (1:500; Cat# ab9722; Abcam, Cambridge,MA, USA), rabbit polyclonal anti-TNF-α (1:500; Cat# bs-2018R; Bioss, Wuhan, China), and mouse monoclonal anti-IL-6 (1:500, Cat# ab9324; Abcam) at 4°C overnight. The membranes were then probed with horseradish peroxidaseconjugated anti-mouse IgG (1:1000; Cat# 31430; Thermo Fisher Scientific, Inc.) or horseradish peroxidase-conjugated anti-rabbit IgG (1:1000; Cat# 31460; Thermo Fisher Scientific,Inc.) at room temperature for 2 hours. The membranes were visualized using a gel imaging system (Bio-Rad Laboratories,Inc., Hercules, CA, USA). Densitometry was performed using Quantity One version 1.4.6 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are expressed as the mean ± standard deviation (SD)and analyzed by SPSS 19.0 (IBM, Armonk, NY, USA). Significant differences were analyzed by one-way analysis of variance followed by the Newman-Keulspost hoctest.P< 0.05 was considered significant.
Results
Sh-LncRNA-5657 ameliorates the pathological changes in the septic rat hippocampus
Initially, three shRNA sequences were designed to reduce LncRNA-5657 expression. As shown inFigure 1, the expression level of LncRNA-5657 decreased significantly in the sh-LncRNA-5657-1 and sh-LncRNA-5657-3 groups compared with the control group (P< 0.05). Sh-LncRNA-5657-3 appeared to have the most potent reduction effect on LncRNA-5657 expression. Therefore, sh-LncRNA-5657-3 was packaged into a lentivirus for use in subsequent experiments.
The hematoxylin-eosin staining results showed cells with an orderly arrangement, featuring a dense stroma, no edema,and normal brain cell morphology in the hippocampal CA1 region of rats in the control group. The majority of neurons in the hippocampus of rats from the SE model group appeared seriously damaged, and the degenerated neurons presented features consistent with necrosis. However, the degree of degeneration and necrosis observed in the neurons was attenuated by treatment with the lentivirus encoding sh-LncRNA-5657 (Figure 2).

Table 1 |Primer sequences
Treatment with the lentivirus encoding sh-LncRNA-5657reduces the levels of AQP4, heparanase, and MMP-9 immunopositivity in the hippocampus of septic rats
Immunohistochemistry results indicated that the levels of AQP4, heparanase, and MMP-9 immunopositivity were significantly elevated in the sepsis model rat hippocampus compared with those in the control rat hippocampus (P<0.05). In contrast, the levels of AQP4, heparanase, and MMP-9 immunopositivity were significantly reduced in the lentiviral sh-LncRNA-5657-treated rat hippocampus compared with those in the sepsis model rat hippocampus (P< 0.05;Figure 3).
Treatment with the lentivirus encoding sh-LncRNA-5657 reduces hippocampal TNF-α levels in septic rats
On the basis of the enzyme-linked immunosorbent assay results, the expression level of TNF-α in the septic model rat hippocampus was significantly increased compared with that in the control rat hippocampus (P< 0.05). In contrast, the expression level of TNF-α in the lentiviral sh-LncRNA-5657-treated rat hippocampus was significantly decreased compared with that in the SE model rat hippocampus (P< 0.05;Figure 4).
Sh-LncRNA-5657 reduces LncRNA-5657 expression in LPS-treated microglia
The expression of LncRNA-5657 in microglia was detected by real-time polymerase chain reaction. The expression levels of LncRNA-5657 in the LPS group at 6, 12, and 24 hours were significantly increased compared with the expression levels of LncRNA-5657 at the same time points in the control group(P< 0.05 at each time point). In contrast, the expression level of LncRNA-5657 in the sh-LncRNA-5657 treated group was significantly decreased compared with that in the vector group (P< 0.05 at each time point;Figure 5). The expression of LncRNA-5657 was comparable in the LPS and vector groups.
Sh-LncRNA-5657 reduces TNF-α, IL-1β and IL-6 levels in LPS-treated microglia
Compared with the control group, the mRNA and protein expression levels of TNF-α, IL-1β and IL-6 increased significantly in the LPS group at all time points (P< 0.05). In contrast, the mRNA and protein expression levels of TNF-α,IL-1β and IL-6 in the sh-LncRNA-5657-treated group were significantly decreased compared with those in the vector group (P< 0.05;Figure 6). The expression of TNF-α, IL-1β, and IL-6 levels was comparable in LPS and vector groups.
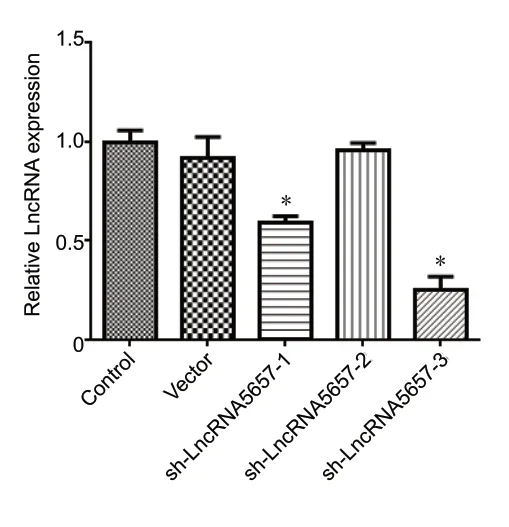
Figure 1|Effects of different sh-LncRNA-5657 sequences on LncRNA-5657 expression in normal microglia, as detected by fluorescence quantitative polymerase chainreaction.
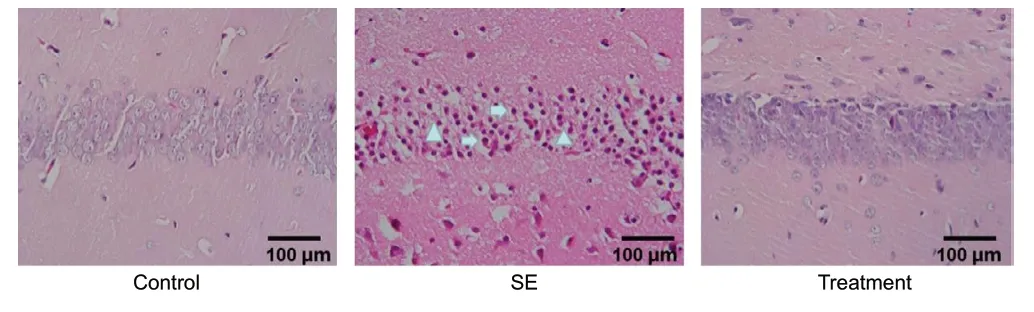
Figure 2|Effects of sh-LncRNA-5657 on the pathological changes in the hippocampus of septic rats (hematoxylin-eosin staining).

Figure 3|Effects of the lentivirus encoding sh-LncRNA-5657 on the levels of AQP4, HPA, and MMP-9 immunoreactivities in the hippocampus of septic model rats.

Figure 4|Effects of treatment with the lentivirus encoding sh-LncRNA-5657 on hippocampal tumor necrosis factor-α (TNF-α) levels in septic model rats,as detected by enzyme-linked immunosorbent assay.

Figure 5|Effects of sh-LncRNA-5657 on LncRNA-5657 expression in LPS-treated microglia detected by real-time polymerase chain reaction.

Figure 6|Effects of sh-LncRNA-5657 on TNF-α, IL-1β, and IL-6 levels in LPS-treated microglia.
Discussion
In this study, we found that treatment with a lentivirus encoding sh-LncRNA-5657 injected into the hippocampus ameliorated the morphological changes observed in hippocampal neurons, reduced the levels of AQP4,heparanase, and MMP-9 immunopositivity, and reduced TNF-α mRNA and protein levels in septic model rats compared with untreated septic model rats. Usingin vitrocultured microglia, we demonstrated that sh-LncRNA-5657 ameliorated the LPS-induced increase in LncRNA-5657 expression levels and the inflammatory response. These data suggested that LncRNA-5657 may serve as a novel therapeutic target for reducing the brain inflammatory response in SE.
SE can appear during the early stages of sepsis, even before other systemic symptoms appear. Patients with SE require more time on mechanical ventilation, tend to be hospitalized for longer, and have increased mortality (Ji et al., 2018). The incidence of long-term cognitive dysfunction in SE patients is significantly increased compared with non-SE patients, and SE patients are more likely to suffer from neurodegenerative diseases (Bedirli et al., 2018). The numbers of neurons in the brains of sepsis model rats decreased compared with control rats, and the neuronal morphology presented degenerative features (Ji et al., 2017). The hippocampus is a critical region of the brain that is responsible for memory formation (Zhu et al., 2018). The modulation of the pyramidal neuron cell structure in the hippocampus, especially Schaffer collateral-CA1 synapses, can impair synaptic plasticity and memory(Zhu et al., 2018). In this study, we found that neurons in the hippocampus of septic model rats were seriously damaged,characterized by degenerated neurons and features of necrosis. In contrast, rats treated with the lentivirus encoding sh-LncRNA-5657 presented repaired morphological damage in the hippocampus. LncRNAs are important regulators of microRNAs, which subsequently inhibit gene expression and participate in physiological function (Dykes and Emanueli,2017). The functions of the lung, kidney, and myocardia were shown to be modulated by LncRNAs in septic animal models(Chen et al., 2020; Wang et al., 2020). The present study suggested that LncRNA-5657 was elevated in septic model rats for the first time.
The acute inflammatory response is recognized as an essential mechanism underlying brain injury during sepsis (Sonneville et al., 2013). The role played by TNF-α in sepsis has been demonstrated in both experimental models of septic shock and SE (Schulte et al., 2013). TNF-α is thought to represent a potential therapeutic target for sepsis (Schulte et al., 2013).In this study, we also found that hippocampal TNF-α levels were elevated in CLP-induced septic model rats and reduced in sepsis model rats treated with the lentivirus encoding sh-LncRNA-5657.
AQP4 is an important member of the AQP family, primarily expressed in brain tissue, and directly contacts the capillary wall (Wang et al., 2017). AQP4 is a two-way water transport channel that regulates the passage of water molecules into and out of brain tissue. Under physiological conditions, AQP4 maintains the water balance of the brain and regulates the stability of the internal environment. AQP4 is also involved in the formation and elimination of various types of brain edema under pathological conditions (Popescu et al., 2017). Several studies have confirmed that brain edema, caused by trauma,infarction, hemorrhage, inflammation, and brain tumors, is related to the upregulated expression of AQP4 (Papadopoulos and Verkman, 2005; Kleffner et al., 2008; Karasu et al., 2010).Heparanase is an endo-β-glucuronidase that cleaves heparan sulfate side chains from their proteoglycans. Heparanase liberates highly potent circulating heparan sulfate fragments,which can trigger a fatal and excessive inflammatory response during sepsis (Martin et al., 2015). Additionally, MMPs play important roles in the pathogenesis of sepsis brain injury.During sepsis-induced brain injury, MMP-9 expression is upregulated (Kocaturk et al., 2016). MMP-9 expression has been positively correlated with the severity of sepsis-induced brain injury (Collazos et al., 2015). Our present study showed that AQP4, heparanase, and MMP-9 were highly expressed in the brain tissue of the sepsis rat model, which was also consistent with the above results. AQP4, heparanase, and MMP-9 expression were significantly decreased in brain tissue after treatment with the lentiviral sh-LncRNA-5657.
LPS can induce inflammation of the vascular endothelial system and immune system, leading to the release of TNF-α, which participates in the stress response and regulates inflammation (Chen et al., 2018b). The release of inflammatory mediators can aggravate brain damage, induce neuronal apoptosis, and amplify the inflammatory cascade(Pinheiro da Silva et al., 2013). Inhibiting overactive microglia in the brain and preventing the uncontrolled inflammatory mediator release are necessary to prevent and treat brain injury induced by sepsis. LncRNA participates in the pathogenrelated immune response and regulates the monocytemacrophage inflammatory response (Chen et al., 2018a).IL-1β and TNF-α can damage neurons and cause microglial activation or directly activate microglia, which in turn produce inflammatory mediators, in a vicious cycle that eventually results in septic brain damage (Zhao et al., 2015).
Septic brain injury can lead to the activation of endothelial cells in the brain, which produce inflammatory mediators,including TNF-α, IL-1β, and IL-6 (Aly et al., 2006). The concentrations of TNF-α and IL-6 increased in the sera and fresh hippocampal tissues of rats with brain injuries induced by sepsis (Aslankoc et al., 2018). The results of this study showed that the pathogenesis of sepsis-associated brain injury induced the expression of TNF-α, IL-1β, and IL-6. These results indicated that LncRNA-5657 is closely associated with the microglial inflammatory response and can inhibit the expression of TNF-α, IL-1β, IL-6, and other inflammatory factors induced by LPS.
Our study had some limitations. First, the structural and functional changes observed inin vitromicroglia were not verified in the hippocampus of septic model rats. Second,the molecular mechanisms that underlie the role played by LncRNA-5657 during microglia activation after CLP remain unclear. Third, because astrocytes are also associated with the inflammatory response in the hippocampus (Colombo and Farina, 2016), the potential roles played by LncRNA-5657 in astrocytes require further clarification. Finally, LncRNA functions through interactions with microRNA; however,because LncRNA-5657 is newly discovered, the downstream microRNAs targeted by this LncRNA remain to be identified in future experiments.
In conclusion, LncRNA-5657 is closely related to the inflammatory response of microglia. Sh-LncRNA-5657 can inhibit the inflammatory response induced by sepsis and reduce the damage experienced by hippocampal cells.
Author contributions:Study conception and design, and manuscript writing: YAZ, KJQ; experiment implementation: YAZ, XLQ, XZW, NZ. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 81660314, 82060345, and Jiangxi Provincial Natural Science Foundation of China, No. 20192BAB205057(both to YAZ). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University(approval No. 2017-004) in 2017.
Copyright license agreement:The Copyright License Agreement hasbeen signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Laura Romina Caltana, Universidad de Buenos Aires, Argentina; Guoqi Zhu, Key Laboratory of Xin’an Medicine, Ministry of Education, Anhui University of Chinese Medicine, China.
Additional files:
Additional file 1:Original data of the experiment.
Additional file 2:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Clusterin: a multifaceted protein in the brain
- Positive effects of music therapist’s selected auditory stimulation on the autonomic nervous system of patients with disorder of consciousness: a randomized controlled trial
- Transcranial pulse current stimulation improves the locomotor function in a rat model of stroke
- Comparative transcriptomic analysis of rat versus mouse cerebral cortex after traumatic brain injury
- Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke
- Extremely low frequency electromagnetic fields promote cognitive function and hippocampal neurogenesis of rats with cerebral ischemia

